Abstract
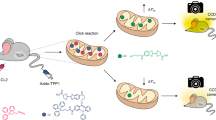
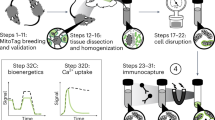
ATP, the energy exchange factor that connects anabolism and catabolism, is required for major reactions and processes that occur in living cells, such as muscle contraction, phosphorylation and active transport. ATP is also the key molecule in extracellular purinergic signaling mechanisms, with an established crucial role in inflammation and several additional disease conditions. Here, we describe detailed protocols to measure the ATP concentration in isolated living cells and animals using luminescence techniques based on targeted luciferase probes. In the presence of magnesium, oxygen and ATP, the protein luciferase catalyzes oxidation of the substrate luciferin, which is associated with light emission. Recombinantly expressed wild-type luciferase is exclusively cytosolic; however, adding specific targeting sequences can modify its cellular localization. Using this strategy, we have constructed luciferase chimeras targeted to the mitochondrial matrix and the outer surface of the plasma membrane. Here, we describe optimized protocols for monitoring ATP concentrations in the cytosol, mitochondrial matrix and pericellular space in living cells via an overall procedure that requires an average of 3 d. In addition, we present a detailed protocol for the in vivo detection of extracellular ATP in mice using luciferase-transfected reporter cells. This latter procedure may require up to 25 d to complete.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Burnstock, G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87, 659–797 (2007).
Burnstock, G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol. Rev. 58, 58–86 (2006).
Lazarowski, E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal 8, 359–373 (2012).
Di Virgilio, F. & Adinolfi, E. Extracellular purines, purinergic receptors and tumor growth. Oncogene 36, 293–303 (2017).
DeLuca, M. & McElroy, W.D. Kinetics of the firefly luciferase catalyzed reactions. Biochemistry 13, 921–925 (1974).
Ignowski, J.M. & Schaffer, D.V. Kinetic analysis and modeling of firefly luciferase as a quantitative reporter gene in live mammalian cells. Biotechnol. Bioeng. 86, 827–834 (2004).
Jouaville, L.S., Pinton, P., Bastianutto, C., Rutter, G.A. & Rizzuto, R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA 96, 13807–13812 (1999).
Porcelli, A.M. et al. Targeting of reporter molecules to mitochondria to measure calcium, ATP, and pH. Methods Cell. Biol. 65, 353–380 (2001).
Torrano, V. et al. The metabolic co-regulator PGC1α suppresses prostate cancer metastasis. Nat. Cell. Biol. 18, 645–656 (2016).
Patergnani, S. et al. Methods to monitor and compare mitochondrial and glycolytic ATP production. Methods Enzymol. 542, 313–332 (2014).
Gould, S.G., Keller, G.A. & Subramani, S. Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J. Cell. Biol. 105, 2923–2931 (1987).
Manfredi, G., Yang, L., Gajewski, C.D. & Mattiazzi, M. Measurements of ATP in mammalian cells. Methods 26, 317–326 (2002).
Michels, A.A., Nguyen, V.T., Konings, A.W., Kampinga, H.H. & Bensaude, O. Thermostability of a nuclear-targeted luciferase expressed in mammalian cells. Destabilizing influence of the intranuclear microenvironment. Eur. J. Biochem. 234, 382–389 (1995).
Wright, R.H. et al. ADP-ribose-derived nuclear ATP synthesis by NUDIX5 is required for chromatin remodeling. Science 352, 1221–1225 http://dx.doi.org/10.1126/science.aad93 (2016).
Pellegatti, P., Falzoni, S., Pinton, P., Rizzuto, R. & Di Virgilio, F. A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol. Biol. Cell 16, 3659–3665 (2005).
Beigi, R., Kobatake, E., Aizawa, M. & Dubyak, G.R. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am. J. Physiol. 276, C267–C278 (1999).
Michaud, M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 (2011).
Pellegatti, P. et al. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One 3, e2599 (2008).
Pietrocola, F. et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160 (2016).
Weber, F.C. et al. Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J. Exp. Med. 207, 2609–2619 (2010).
Wilhelm, K. et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 16, 1434–1438 (2010).
Miller, D.S. & Horowitz, S.B. Intracellular compartmentalization of adenosine triphosphate. J. Biol. Chem. 261, 13911–13915 (1986).
Abrahams, J.P., Leslie, A.G., Lutter, R. & Walker, J.E. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370, 621–628 (1994).
Boyer, P.D. The ATP synthase--a splendid molecular machine. Annu. Rev. Biochem. 66, 717–749 (1997).
Bianchi, G. et al. ATP/P2X7 axis modulates myeloid-derived suppressor cell functions in neuroblastoma microenvironment. Cell Death Dis. 5, e1135 (2014).
Loo, J.M. et al. Extracellular metabolic energetics can promote cancer progression. Cell 160, 393–406 (2015).
White, E.H., Rapaport, E.E., Seliger, H.H. & Hopkins., T.A. The chemi- and bioluminescence of firefly luciferin: an efficient chemical production of electronically excited states. Bioorg. Chem. 1, 92–122 (1971).
Harwood, K.R., Mofford, D.M., Reddy, G.R. & Miller, S.C. Identification of mutant firefly luciferases that efficiently utilize aminoluciferins. Chem. Biol. 18, 1649–1657 (2011).
Evans, M.S. et al. A synthetic luciferin improves bioluminescence imaging in live mice. Nat. Methods 11, 393–395 (2014).
Shinde, R., Perkins, J. & Contag, C.H. Luciferin derivatives for enhanced in vitro and in vivo bioluminescence assays. Biochemistry 45, 11103–11112 (2006).
Bhaumik, S. & Gambhir, S.S. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc. Natl. Acad. Sci. USA 99, 377–382 (2002).
Luker, K.E. et al. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc. Natl. Acad. Sci. USA 101, 12288–12293 (2004).
McNabb, D.S., Reed, R. & Marciniak, R.A. Dual luciferase assay system for rapid assessment of gene expression in Saccharomyces cerevisiae. Eukaryot. Cell. 4, 1539–1549 (2005).
Day, J.C., Tisi, L.C. & Bailey, M.J. Evolution of beetle bioluminescence: the origin of beetle luciferin. Luminescence 19, 8–20 (2004).
Helenius, M., Jalkanen, S. & Yegutkin, G. Enzyme-coupled assays for simultaneous detection of nanomolar ATP, ADP, AMP, adenosine, inosine and pyrophosphate concentrations in extracellular fluids. Biochim. Biophys. Acta 1823, 1967–1975 (2012).
Shapiro, A.B., Hajec, L. & Gao, N. High-throughput, homogeneous, fluorescence intensity-based measurement of adenosine diphosphate and other ribonucleoside diphosphates with nanomolar sensitivity. Anal. Biochem. 415, 190–196 (2011).
Ando, T. et al. Visualization and measurement of ATP levels in living cells replicating hepatitis C virus genome RNA. PLoS Pathog. 8, e1002561 (2012).
Imamura, H. et al. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc. Natl. Acad. Sci. USA 106, 15651–15656 (2009).
Kotera, I., Iwasaki, T., Imamura, H., Noji, H. & Nagai, T. Reversible dimerization of Aequorea victoria fluorescent proteins increases the dynamic range of FRET-based indicators. ACS Chem. Biol. 5, 215–222 (2010).
Nakano, M., Imamura, H., Nagai, T. & Noji, H. Ca(2)(+) regulation of mitochondrial ATP synthesis visualized at the single cell level. ACS Chem. Biol. 6, 709–715 (2011).
Liemburg-Apers, D.C. et al. Quantitative glucose and ATP sensing in mammalian cells. Pharm. Res. 28, 2745–2757 (2011).
Berg, J., Hung, Y.P. & Yellen, G. A genetically encoded fluorescent reporter of ATP:ADP ratio. Nat. Methods 6, 161–166 (2009).
Tantama, M., Martinez-Francois, J.R., Mongeon, R. & Yellen, G. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat. Commun. 4, 2550 (2013).
Tantama, M. & Yellen, G. Imaging changes in the cytosolic ATP-to-ADP ratio. Methods Enzymol. 547, 355–371 (2014).
Llaudet, E., Hatz, S., Droniou, M. & Dale, N. Microelectrode biosensor for real-time measurement of ATP in biological tissue. Anal. Chem. 77, 3267–3273 (2005).
Corriden, R., Insel, P.A. & Junger, W.G. A novel method using fluorescence microscopy for real-time assessment of ATP release from individual cells. Am. J. Physiol. Cell Physiol. 293, C1420–C1425 (2007).
Schneider, S.W. et al. Continuous detection of extracellular ATP on living cells by using atomic force microscopy. Proc. Natl. Acad. Sci. USA 96, 12180–12185 (1999).
Hayashi, S., Hazama, A., Dutta, A.K., Sabirov, R.Z. & Okada, Y. Detecting ATP release by a biosensor method. Sci. STKE 2004, pl14 (2004).
Inoue, Y. et al. Comparison of subcutaneous and intraperitoneal injection of D-luciferin for in vivo bioluminescence imaging. Eur. J. Nucl. Med. Mol. Imaging 36, 771–779 (2009).
Hong, S. & Pedersen, P.L. ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas. Microbiol. Mol. Biol. Rev. 72, 590–641 (2008).
Bonora, M. et al. Subcellular calcium measurements in mammalian cells using jellyfish photoprotein aequorin-based probes. Nat. Protoc. 8, 2105–2118 (2013).
Zhuo, H. et al. Tumor imaging and interferon-γ-inducible protein-10 gene transfer using a highly efficient transferrin-conjugated liposome system in mice. Clin. Cancer Res. 19, 4206–4217 (2013).
Cool, S.K., Breyne, K., Meyer, E., De Smedt, S.C. & Sanders, N.N. Comparison of in vivo optical systems for bioluminescence and fluorescence imaging. J. Fluoresc. 23, 909–920 (2013).
Sambrook, J. & Russell, D.W. Calcium-phosphate-mediated transfection of eukaryotic cells with plasmid DNAs. CSH Protoc. 2006 http://dx.doi.org/10.1101/pdb.prot3871 (2006).
Robb-Gaspers, L.D. et al. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 17, 4987–5000 (1998).
Lazarowski, E.R., Boucher, R.C. & Harden, T.K. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 64, 785–795 (2003).
Suadicani, S.O., Brosnan, C.F. & Scemes, E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 26, 1378–1385 (2006).
Spray, D.C., Ye, Z.C. & Ransom, B.R. Functional connexin 'hemichannels': a critical appraisal. Glia 54, 758–773 (2006).
Zhang, Z. et al. Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 9, 945–953 (2007).
Di Virgilio, F., Pinton, P. & Falzoni, S. Assessing extracellular ATP as danger signal in vivo: the pmeLuc system. Methods Mol. Biol. 1417, 115–129 (2016).
Ozalp, V.C., Nielsen, L.J. & Olsen, L.F. An aptamer-based nanobiosensor for real-time measurements of ATP dynamics. Chembiochem 11, 2538–2541 (2010).
Bhatt, D.P., Chen, X., Geiger, J.D. & Rosenberger, T.A. A sensitive HPLC-based method to quantify adenine nucleotides in primary astrocyte cell cultures. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 889–890, 110–115 (2012).
Lee, D.H., Kim, S.Y. & Hong, J.I. A fluorescent pyrophosphate sensor with high selectivity over ATP in water. Angew. Chem. Int. Ed. Engl. 43, 4777–4780 (2004).
Gajewski, C.D., Yang, L., Schon, E.A. & Manfredi, G. New insights into the bioenergetics of mitochondrial disorders using intracellular ATP reporters. Mol. Biol. Cell 14, 3628–3635 (2003).
Jager, L. et al. A rapid protocol for construction and production of high-capacity adenoviral vectors. Nat. Protoc. 4, 547–564 (2009).
Clark, P.R. et al. Polycations and cationic lipids enhance adenovirus transduction and transgene expression in tumor cells. Cancer Gene Ther. 6, 437–446 (1999).
Acknowledgements
P.P. is grateful to C. degli Scrovegni for continuous support. P.P. is supported by the Italian Ministry of Education, University and Research (COFIN no. 20129JLHSY_002, FIRB no. RBAP11FXBC_002 and Futuro in Ricerca no. RBFR10EGVP_001), the Italian Cystic Fibrosis Research Foundation (no. 19/2014) and the Telethon Foundation (no. GGP15219/B). P.P. and C.G. are supported by local funds from the University of Ferrara and the Italian Ministry of Health, as well as by the Italian Association for Cancer Research (AIRC nos. IG-18624 and MFAG-13521). F.D.V. is supported by grants from the Italian Association for Cancer Research (no. IG 2016 Id.18581), the Ministry of Health of Italy (no. RF-2011–02348435) and EU-COST Action (no. BM 1406). L.R. is funded by 'Cinque per mille dell'IRPEF–Finanziamento della ricerca sanitaria'.
Author information
Authors and Affiliations
Contributions
G.M., A.C.S., S. Marchi, S. Missiroli, S.F., L.R., V.P., C.G., F.D.V. and P.P. contributed extensively to the writing of this paper. G.M. and A.C.S. performed the experiments, analyzed the data and generated the figures.
Corresponding authors
Ethics declarations
Competing interests
F.D.V. serves as a member of the Scientific Advisory Board of Biosceptre International, a UK-based R&D company developing therapeutic applications of P2X7-receptor-targeting Abs.
Supplementary information
Supplementary Data
Supplementary Data. Nucleotide sequences of luciferases. (PDF 273 kb)
Rights and permissions
About this article
Cite this article
Morciano, G., Sarti, A., Marchi, S. et al. Use of luciferase probes to measure ATP in living cells and animals. Nat Protoc 12, 1542–1562 (2017). https://doi.org/10.1038/nprot.2017.052
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.052
This article is cited by
-
P2RX7 promotes osteosarcoma progression and glucose metabolism by enhancing c-Myc stabilization
Journal of Translational Medicine (2023)
-
Engineering sulfonate group donor regeneration systems to boost biosynthesis of sulfated compounds
Nature Communications (2023)
-
Energy matters: presynaptic metabolism and the maintenance of synaptic transmission
Nature Reviews Neuroscience (2022)
-
Optimized HPLC method to elucidate the complex purinergic signaling dynamics that regulate ATP, ADP, AMP, and adenosine levels in human blood
Purinergic Signalling (2022)
-
P2X7 is a cytotoxic receptor….maybe not: implications for cancer
Purinergic Signalling (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.