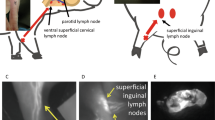
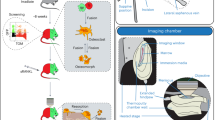
Abstract
Chronic imaging windows in mice have been developed to allow intravital microscopy of many different organs and have proven to be of paramount importance in advancing our knowledge of normal and disease processes. A model system that allows long-term intravital imaging of lymph nodes would facilitate the study of cell behavior in lymph nodes during the generation of immune responses in a variety of disease settings and during the formation of metastatic lesions in cancer-bearing mice. We describe a chronic lymph node window (CLNW) surgical preparation that allows intravital imaging of the inguinal lymph node in mice. The CLNW is custom-made from titanium and incorporates a standard coverslip. It allows stable longitudinal imaging without the need for serial surgeries while preserving lymph node blood and lymph flow. We also describe how to build and use an imaging stage specifically designed for the CLNW to prevent (large) rotational changes as well as respiratory movement during imaging. The entire procedure takes approximately half an hour per mouse, and subsequently allows for longitudinal intravital imaging of the murine lymph node and surrounding structures for up to 14 d. Small-animal surgery experience is required to successfully carry out the protocol.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jeong, H.S. et al. Investigation of the lack of angiogenesis in the formation of lymph node metastases. J. Natl. Cancer Inst. 107 djv155 (2015).
Van den Broeck, W., Derore, A. & Simoens, P. Anatomy and nomenclature of murine lymph nodes: descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J. Immunol. Methods 312, 12–19 (2006).
von Andrian, U.H. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation 3, 287–300 (1996).
Miller, M.J. et al. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296, 1869–1873 (2002).
Stoll, S. et al. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science 296, 1873–1876 (2002).
Mora, J.R. et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424, 88–93 (2003).
Mempel, T.R., Henrickson, S.E. & Von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
Schramm, R. et al. The cervical lymph node preparation: a novel approach to study lymphocyte homing by intravital microscopy. Inflamm. Res. 55, 160–167 (2006).
Ito, K. et al. Unexpected dissemination patterns in lymphoma progression revealed by serial imaging within a murine lymph node. Cancer Res. 72, 6111–6118 (2012).
Jafarnejad, M. et al. Modeling lymph flow and fluid exchange with blood vessels in lymph nodes. Lymphat. Res. Biol. 13, 234–247 (2015).
Gonzalez, S.F. et al. The role of innate immunity in B cell acquisition of antigen within LNs. Adv. Immunol. 106, 1–19 (2010).
Brown, E. et al. In vivo imaging of tumors. Cold Spring Harb. Protoc. http://dx.doi.org/10.1101/pdb.prot5452 (2010).
Padera, T.P. et al. Conventional and high-speed intravital multiphoton laser scanning microscopy of microvasculature, lymphatics, and leukocyte-endothelial interactions. Mol. Imaging 1, 9–15 (2002).
Vakoc, B.J. et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 15, 1219–1223 (2009).
Luo, W. et al. Optical biopsy of lymph node morphology using optical coherence tomography. Technol. Cancer Res. Treat. 4, 539–548 (2005).
Nolan, R.M. et al. Intraoperative optical coherence tomography for assessing human lymph nodes for metastatic cancer. BMC Cancer 16, 144 (2016).
Kedrin, D. et al. Intravital imaging of metastatic behavior through a mammary imaging window. Nat. Methods 5, 1019–1021 (2008).
Shan, S., Sorg, B. & Dewhirst, M.W. A novel rodent mammary window of orthotopic breast cancer for intravital microscopy. Microvasc. Res. 65, 109–117 (2003).
Leunig, M. et al. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer Res. 52, 6553–6560 (1992).
Endrich, B. et al. Technical report—a new chamber technique for microvascular studies in unanesthetized hamsters. Res. Exp. Med. 177, 125–134 (1980).
Yuan, F. et al. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 54, 4564–4568 (1994).
Farrar, M.J. et al. Chronic in vivo imaging in the mouse spinal cord using an implanted chamber. Nat. Methods 9, 297–302 (2012).
Brodnick, S.K. et al. A chronic window imaging device for the investigation of in vivo peripheral nerves. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2014, 1985–1988 (2014).
Ritsma, L. et al. Surgical implantation of an abdominal imaging window for intravital microscopy. Nat. Protoc. 8, 583–594 (2013).
Brown, E.B. et al. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat. Med. 7, 864–868 (2001).
Hoshida, T. et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 66, 8065–8075 (2006).
Acknowledgements
This work was supported by the NIH under award nos. R00CA137167 (T.P.P.), DP2OD008780 (T.P.P.), R01CA163528 (B.J.V.) and R01HL128168 (T.P.P.). Research reported in this publication was supported in part by the Center for Biomedical OCT Research and Translation through grant no. P41EB015903 (B.J.V.), awarded by the National Institute of Biomedical Imaging and Bioengineering of the NIH. National Cancer Institute Federal Share of Proton Income grant CA059267 (T.P.P., B.J.V.) and the Massachusetts General Hospital Executive Committee on Research ISF (T.P.P.) also supported this work. We thank J. Kahn, E. Beech and C. Cui for outstanding technical support.
Author information
Authors and Affiliations
Contributions
E.F.J.M and H.-S.J. developed the protocol. E.F.J.M. and T.P.P. wrote the manuscript. E.F.J.M. and E.R.P. performed the experiments, including supplementary video acquisition. C.B. and B.J.V. performed intravital imaging and processing of optical coherence tomography. E.F.J.M., E.R.P. and C.B. prepared in-text figures and supplementary videos. T.A.R. designed, developed and prepared the schematics presented in the supplementary figures. T.P.P. supervised all authors who participated in this project. All authors contributed intellectually to, and reviewed, the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Titanium frame schematics
The CLNW frame with a bend where the mouse leg is positioned. Made from titanium for its known biocompatibility and favorable strength to weight properties.
Supplementary Figure 2 Titanium ring schematics
The CLNW ring, with a precise fit to the CLNW frame. The tension C-ring is inserted into this ring, keeping the custom-made 11.7mm glass cover slip in place.
Supplementary Figure 3 CLNW assembled
This schematic shows the CLNW titanium frame, CLNW titanium ring and tension C-ring combined.
Supplementary Figure 4 Characterization of immune cell populations after CLNW implantation
Characterization of immune cell populations after CLNW implantation. 16 BALB/c male mice (~30g, 8-10 weeks old) were randomly distributed to four groups for inguinal lymph node removal at day 0 (no CLNW implantation), 2, 7 or 14 days after CLNW implantation. One lymph node was resected per mouse resulting in four lymph nodes per time point. A) Images of lymph nodes removed from CLNW after the noted times show enlargement in the size of these lymph nodes with time. B) However, no significant changes in total number of cells per lymph node was observed (ANOVA). Results are presented as Mean ± SEM, n = 16, 1 lymph node per mouse. C) Flow cytometry data showing no significant changes in cell populations through day 7. On day 14 there were differences in the total number of CD45+ cells as well as various CD45+ cell populations. Results are presented as mean ± SEM.
Lymph nodes were mechanically dissociated using a 40μm filter and cells were stained for 30 minutes on ice in staining buffer (cat. no. 00-4222-26, eBioscience) containing Fc block (cat. no. 16-0161-86, eBioscience) and data were collected on an 8 Laser FACSAria Fusion Flow Cytometer (Becton Dickinson). Data were analyzed using the FlowJo software. The following antibodies were used for staining: CD45 APCAlexa700-A (clone 30-F11), CD3 FITC (clone 17A2), CD4 PE (clone RM4-5), CD25 APC Cy7 (clone PC61), CD19 PerCPCy5.5 (clone 6D5), CD11b PE Cy7 (clone M1/70), CD11c APC Cy7 (clone N418), F480 PerCPCy5.5 (clone BM8), Gr1 FITC (clone R86-8C5), Ly6C PE (clone AL-21) (all from BD Biosciences). All antibodies were used at a concentration of 0.25μg per 106 cells in 100μl staining buffer. Gating Statistics for each subpopulation identified: Percentage of CD45+ lymphocytes was based on the total number of viable events, by gating for single cells based on the forward scatter (FSC) and side scatter (SSC) parameters. Percentage of CD3+, CD19+, CD11b, CD11c lymphocytes was based on the CD45+ population. Percentage of CD25+, CD4+, CD8+ lymphocytes was based on the CD3+ population. Percentage of F480+ and Gr1+ cells was based on CD11b+ population. Percentage of Ly6C+ cells was based on Gr1+ population. All antibodies were stored at 4°C protected from light, as all antibodies used were conjugated to a fluorophore. These experiments were approved by the IACUC at MGH.
Supplementary Figure 5 CLNW imaging stage schematic of stage pin
Mounted to the acryl top stage for attachment of the imaging plate. Made from stainless steel.
Supplementary Figure 6 CLNW imaging stage schematic of stage base
Acryl stage base is where the springs and acryl top stage will be attached.
Supplementary Figure 7 CLNW imaging stage schematic of the spring clamp
Two clamps are used to secure the CLNW on imaging stage. Made from spring steel.
Supplementary Figure 8 CLNW imaging stage schematic of top stage
Acryl top stage to which stage stand and stainless steel pin will be secured.
Supplementary Figure 9 CLNW imaging stage schematic of stage stand
Mounted to the acryl top stage on which the CLWN can rest during imaging. Made from acryl.
Supplementary Figure 10 CLNW imaging stage schematic of imaging plate
Contains predrilled holes which precisely fits the screws on CLNW. Also mounts the spring clamps. This imaging plate keeps the CLNW in position during imaging and prevents movement. Made from stainless steel.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10. (PDF 838 kb)
Supplementary Video 1. Short-term time-lapse intravital imaging using the CLNW.
The CLNW is used to visualize lymph node neutrophil and monocyte movement (green) in LysM-GFP transgenic mice. Intravenously injected tetramethylrhodamine-dextran dye is used to trace the vasculature (red). Tetramethylrhodamine-dextran extravasation is visible in the top left quadrant. Collagen (blue) is acquired by SHG imaging. The time-lapse MPM movie was taken over 90 min at 1× zoom, stack depth ~45 μm (z-projected using ImageJ software), with a 1.05 NA 25× objective, 512 × 512 pixels, 16-bit. Resolution not enhanced. Scale bar, 50 μm. This experiment was approved by the IACUC at MGH. (AVI 28420 kb)
Supplementary Video 2. Short-term time-lapse intravital imaging using the CLNW.
In this 30-min MPM movie, lymph node neutrophil and monocyte movement (green) is visualized in LysM-GFP transgenic mice. These cells can be seen flowing, rolling and extravasating in a lymph node high-endothelial venule (red), a specialized blood vessel in the lymph node used for lymphocyte migration. The blood vessel imaged is not directly (~130μ) below the cover slip, causing a reduced signal-to-noise ratio in the video but demonstrating the viability of MPM in deeper-situated regions of interest. Tetramethylrhodamine-dextran dye is used to trace the vasculature (red). Collagen (blue) is acquired by SHG imaging. Images acquired at 2× zoom, stack depth ~20 μm (z-projected using ImageJ software), with a 1.05 NA 25× objective, 256 × 256 pixels, 16-bit. Resolution not enhanced. Scale bar, 25 μm. This experiment was approved by the IACUC at MGH. (AVI 15141 kb)
Rights and permissions
About this article
Cite this article
Meijer, E., Jeong, HS., Pereira, E. et al. Murine chronic lymph node window for longitudinal intravital lymph node imaging. Nat Protoc 12, 1513–1520 (2017). https://doi.org/10.1038/nprot.2017.045
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.045
This article is cited by
-
Intravital imaging to study cancer progression and metastasis
Nature Reviews Cancer (2023)
-
Solid stress impairs lymphocyte infiltration into lymph-node metastases
Nature Biomedical Engineering (2021)
-
Intravital microscopy of dynamic single-cell behavior in mouse mammary tissue
Nature Protocols (2021)
-
T-cells produce acidic niches in lymph nodes to suppress their own effector functions
Nature Communications (2020)
-
Quantifying solid stress and elastic energy from excised or in situ tumors
Nature Protocols (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.