Abstract
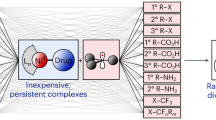
This protocol describes the aerobic oxidation of aromatic anilines to aromatic azo compounds using gold (Au) nanoparticles supported on TiO2 as a catalyst. Yields above 98% are achieved under a few bars of oxygen pressure. It should be noted that the use of stoichiometric amounts of environmentally unfriendly reagents, e.g., transition metals and nitrites, commonly used in current syntheses of azo compounds, is avoided using this approach. The protocol is illustrated with the synthesis of parent azobenzene from aniline, and this reaction takes 22 h. Au on TiO2 can also be used as a hydrogenation catalyst, making it possible to prepare azo compounds directly from nitroaromatics through a two-step (hydrogenation followed by aerobic oxidation), one-pot, one-catalyst reaction. In addition, the catalytic process is efficient for the synthesis of symmetric and a range of asymmetric aromatic azo compounds from the mixtures of two anilines substituted with electron-donor and electron-acceptor substituents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Catino, S.C. & Farris, E. Concice Encyclopedia of Chemical Technology (Wiley, New York, 1985).
Egli, R., Peter, A.P. & Freeman, H.S. The Design and Synthesis of Organic Dyes and Pigments, Chap. VII (Springer, London, 1991).
Venkataraman, K. Chemistry of Synthetic Dyes, Chap. VI (Academic Press, London, 1970).
Grirrane, A., Corma, A. & Garcia, H. Gold-catalyzed synthesis of aromatic azo compounds from anilines and nitroaromatics. Science 322, 1661–1664 (2008).
Corma, A. & Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 37, 2096–2126 (2008).
Corma, A. & Serna, P. Preparation of substituted anilines from nitro compounds by using supported gold catalysts. Nat. Protoc. 1, 2590–2595 (2006).
Baer, E. & Tosoni, A.L. Formation of symmetric azo-compounds from primary aromatic amines by lead tetraacetate. J. Am. Chem. Soc. 78, 2857–2858 (1956).
Firouzabadi, H. & Mostafavipoor, Z. Barium manganate—a versatile oxidant in organic-synthesis. Bull. Chem. Soc. Jpn 56, 914–917 (1983).
Clark, J.H. Chemistry of Waste Minimization (Chapman & Hall, London, 1995).
March, J. Advanced Organic Chemistry: Reactions, Mechanisms and Structures, 3rd ed. (McGraw Hill, New York, 1993).
Dabbagh, H.A., Teimouri, A. & Chermahini, A.N. Green and efficient diazotization and diazo coupling reactions on clays. Dyes Pigm. 73, 239–244 (2007).
Wang, M.X., Funabiki, K. & Matsui, M. Synthesis and properties of bis(hetaryl)azo dyes. Dyes Pigm. 57, 77–86 (2003).
Sheldon, R.A. & van Santen, R.A. Catalytic Oxidation: Principles and Applications (World Scientific, Singapore, 1995).
Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 36, 153–166 (1997).
Haruta, M. et al. Low-temperature oxidation of carbon monoxide over gold supported on titanium dioxide, ferric oxide, and cobalt tetraoxide. J. Catal. 144, 175–192 (1993).
Abad, A., Corma, A. & Garcia, H. Catalyst parameters determining activity and selectivity of supported gold nanoparticles for the aerobic oxidation of alcohols: the molecular reaction mechanism. Chem. Eur. J. 14, 212–222 (2008).
Corma, A. & Serna, P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science 313, 332–334 (2006).
Acknowledgements
Financial support by the Spanish MICINN (CTQ2009-11583) is gratefully acknowledged. We are also thankful to La Fundación Areces for generous funding in this project. A.G. thanks CSIC for a JAE contract as research associate.
Author information
Authors and Affiliations
Contributions
A.C. and H.G. designed the research work. A.G. carried out the experimental work and synthetic protocol. All authors discussed the results and commented on the manuscript.
Corresponding authors
Rights and permissions
About this article
Cite this article
Grirrane, A., Corma, A. & Garcia, H. Preparation of symmetric and asymmetric aromatic azo compounds from aromatic amines or nitro compounds using supported gold catalysts. Nat Protoc 5, 429–438 (2010). https://doi.org/10.1038/nprot.2009.242
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.242
This article is cited by
-
A new approach to the synthesis of azobenzenes based on the oxidative N—N coupling of anilines under the action of electrogenerated NiO(OH), NaOCl, and NaOBr
Russian Chemical Bulletin (2023)
-
Removal of Basic Yellow 51 Dye by Using Ion Exchange Resin Obtained by Modification of Byproduct Sugar Beet Pulp
Sugar Tech (2023)
-
Palladium Nanoparticles on Silica Nanospheres for Switchable Reductive Coupling of Nitroarenes
Catalysis Letters (2020)
-
Cu(II)-catalyzed aerobic oxidative amidation of azoarenes with amides
Science China Chemistry (2018)
-
Tuning Catalytic Selectivity in Cascade Reactions by Light Irradiation
Catalysis Letters (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.