Abstract
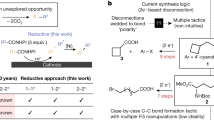
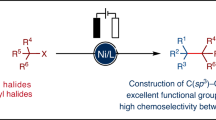
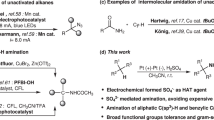
The importance of constructing Csp2–Csp3 bonds has motivated the development of electrochemical, photochemical and thermal activation methods to reductively couple abundant aryl and alkyl electrophiles. However, these methodologies are limited to couplings of very specific substrate classes and require specialized sets of catalysts and reaction set-ups. Here we show a consolidation of these myriad strategies into a single set of conditions that enable reliable alkyl–aryl couplings, including those that were previously unknown. These reactions rely on the discovery of unusually persistent organonickel complexes that serve as stoichiometric platforms for C(sp2)–C(sp3) coupling. Aryl, heteroaryl or vinyl complexes of Ni can be inexpensively prepared on a multigram scale by mild electroreduction from the corresponding C(sp2) electrophile. Organonickel complexes can be isolated and stored or telescoped directly to reliably diversify drug-like molecules. Finally, the procedure was miniaturized to micromole scales by integrating soluble battery chemistries as redox initiators, enabling a high-throughput exploration of substrate diversity.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All experimental data, copies of spectra and crystallographic data are available in the Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition no. CCDC 2290873 (complex 1). Copies of the data can be obtained free of charge at https://www.ccdc.cam.ac.uk/structures/.
References
Dombrowski, A. W. et al. Expanding the medicinal chemist toolbox: comparing seven C(sp2)-C(sp3) cross-coupling methods by library synthesis. ACS Med. Chem. Lett. 11, 597–604 (2020).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Wang, X., Dai, Y. & Gong, H. Nickel-catalyzed reductive couplings. Top. Curr. Chem. 374, 43 (2016).
Le, C., Chen, T. Q., Liang, T., Zhang, P. & MacMillan, D. W. C. A radical approach to the copper oxidative addition problem: trifluoromethylation of bromoarenes. Science 360, 1010–1014 (2018).
Cho, E. J. et al. The palladium-catalyzed trifluoromethylation of aryl chlorides. Science 328, 1679–1681 (2010).
Hamby, T. B., LaLama, M. J. & Sevov, C. S. Controlling Ni redox states by dynamic ligand exchange for electroreductive Csp3-Csp2 coupling. Science 376, 410–416 (2022).
Weix, D. J. Methods and mechanisms for cross-electrophile coupling of Csp2 halides with alkyl electrophiles. Acc. Chem. Res. 48, 1767–1775 (2015).
Palkowitz, M. D. et al. Overcoming limitations in decarboxylative arylation via Ag-Ni electrocatalysis. J. Am. Chem. Soc. 144, 17709–17720 (2022).
Harwood, S. J. et al. Modular terpene synthesis enabled by mild electrochemical couplings. Science 375, 745–752 (2022).
Salgueiro, D. C., Chi, B. K., Guzei, I. A., Garc¡a-Reynaga, P. & Weix, D J. Control of redox-active ester reactivity enables a general cross-electrophile approach to access arylated strained rings. Angew. Chem. Int. Ed 61, e202205673 (2022).
Zuo, Z. et al. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp3-carbons with aryl halides. Science 345, 437–440 (2014).
Twitty, J. C. et al. Diversifying amino acids and peptides via deaminative reductive cross-couplings leveraging high-throughput experimentation. J. Am. Chem. Soc. 145, 5684–5695 (2023).
Yi, J., Badir, S. O., Kammer, L. M., Ribagorda, M. & Molander, G. A. Deaminative reductive arylation enabled by nickel/photoredox dual catalysis. Org. Lett. 21, 3346–3351 (2019).
Martin-Montero, R., Yatham, V. R., Yin, H., Davies, J. & Martin, R. Ni-catalyzed reductive deaminative arylation at sp3 carbon centers. Org. Lett. 21, 2947–2951 (2019).
Yue, H. et al. C-N bond activation: activated primary amines as alkylating reagents in reductive cross-coupling. Chem. Sci. 10, 4430–4435 (2019).
Douthwaite, J. L. et al. Formal cross-coupling of amines and carboxylic acids to form sp3–sp2 carbon–carbon bonds. J. Am. Chem. Soc. 145, 10930–10937 (2023).
Kennedy, S. H., Dherange, B. D., Berger, K. J. & Levin, M. D. Skeletal editing through direct nitrogen deletion of secondary amines. Nature 593, 223–227 (2021).
Holst, D. E., Wang, D. J., Kim, M. J., Guzei, I. A. & Wickens, Z. K. Aziridine synthesis by coupling amines and alkenes via an electrogenerated dication. Nature 596, 74–79 (2021).
Ruffoni, A., Hampton, C., Simonetti, M. & Leonori, D. Photoexcited nitroarenes for the oxidative cleavage of alkenes. Nature 610, 81–86 (2022).
Uehling, M. R., King, R. P., Krska, S. W., Cernak, T. & Buchwald, S. L. Pharmaceutical diversification via palladium oxidative addition complexes. Science 363, 405–408 (2019).
Corcoran, E. B. et al. Aryl amination using ligand-free Ni(II) salts and photoredox catalysis. Science 353, 279–283 (2016).
Yan, M., Lo, J. C., Edwards, J. T. & Baran, P. S. Radicals: reactive intermediates with translational potential. J. Am. Chem. Soc. 138, 12692–12714 (2016).
Diccianni, J., Lin, Q. & Diao, T. Mechanisms of nickel-catalyzed coupling reactions and applications in alkene functionalization. Acc. Chem. Res. 53, 906–919 (2020).
Ting, S. I. et al. 3d-d excited states of Ni(II) complexes relevant to photoredox catalysis: spectroscopic identification and mechanistic implications. J. Am. Chem. Soc. 142, 5800–5810 (2020).
Lin, Q. & Diao, T. Mechanism of Ni-catalyzed reductive 1,2-dicarbofunctionalization of alkenes. J. Am. Chem. Soc. 141, 17937–17948 (2019).
Piszel, P. E. et al. Protodemetalation of (bipyridyl)Ni(II)–aryl complexes shows evidence for five-, six- and seven-membered cyclic pathways. J. Am. Chem. Soc. 145, 8517–8528 (2023).
Terrett, J. A., Cuthbertson, J. D., Shurtleff, V. W. & MacMillan, D. W. C. Switching on elusive organometallic mechanisms with photoredox catalysis. Nature 524, 330–334 (2015).
Martin, R. et al. Late-stage C(sp2)-C(sp3) diversification via nickel oxidative addition complexes. Preprint at https://chemrxiv.org/engage/chemrxiv/article-details/64cca70469bfb8925a551908 (2023).
Hansen, E. C. et al. New ligands for nickel catalysis from diverse pharmaceutical heterocycle libraries. Nat. Chem. 8, 1126–1130 (2016).
Zhang, P., Le, C. C. & MacMillan, D. W. C. Silyl radical activation of alkyl halides in metallaphotoredox catalysis: a unique pathway for cross-electrophile coupling. J. Am. Chem. Soc. 138, 8084–8087 (2016).
Perkins, R. J., Pedro, D. J. & Hansen, E. C. Electrochemical nickel catalysis for sp2-sp3 cross-electrophile coupling reactions of unactivated alkyl halides. Org. Lett. 19, 3755–3758 (2017).
Truesdell, B. L., Hamby, T. B. & Sevov, C. S. General C(sp2)-C(sp3) cross-electrophile coupling reactions enabled by overcharge protection of homogeneous electrocatalysts. J. Am. Chem. Soc. 142, 5884–5893 (2020).
Prieto Kullmer, C. N. et al. Accelerating reaction generality and mechanistic insight through additive mapping. Science 376, 532–539 (2022).
Wang, J. et al. Formation of C(sp2)-C(sp3) bonds instead of amide C-N bonds from carboxylic acid and amine substrate pools by decarbonylative cross-electrophile coupling. J. Am. Chem. Soc. 145, 9951–9958 (2023).
Kariofillis, S. K. et al. Using data science to guide aryl bromide substrate scope analysis in a Ni/photoredox-catalyzed cross-coupling with acetals as alcohol-derived radical sources. J. Am. Chem. Soc. 144, 1045–1055 (2022).
Kutchukian, P. S. et al. Chemistry informer libraries: a chemoinformatics enabled approach to evaluate and advance synthetic methods. Chem. Sci. 7, 2604–2613 (2016).
Tcyrulnikov, S. et al. Dissection of alkylpyridinium structures to understand deamination reactions. ACS Catal. 11, 8456–8466 (2021).
West, M. S., Gabbey, A. L., Huestis, M. P. & Rousseaux, S. A. L. Ni-catalyzed reductive cross-coupling of cyclopropylamines and other strained ring NHP esters with (hetero)aryl halides. Org. Lett. 24, 8441–8446 (2022).
Rein, J. et al. Unlocking the potential of high-throughput experimentation for electrochemistry with a standardized microscale reactor. ACS Cent. Sci. 7, 1347–1355 (2021).
Gütz, C., Klöckner, B. & Waldvogel, S. R. Electrochemical screening for electroorganic synthesis. Org. Process Res. Dev. 20, 26–32 (2016).
Winsberg, J., Hagemann, T., Janoschka, T., Hager, M. D. & Schubert, U. S. Redox-flow batteries: from metals to organic redox-active materials. Angew. Chem. Int. Ed. 56, 686–711 (2017).
Sevov, C. S., Fisher, S. L., Thompson, L. T. & Sanford, M. S. Mechanism-based development of a low-potential, soluble and cyclable multielectron anolyte for nonaqueous redox flow batteries. J. Am. Chem. Soc. 138, 15378–15384 (2016).
Chen, S.-J. et al. Accessing three-dimensional molecular diversity through benzylic C-H cross-coupling. Nat. Synth 2, 998–1008 (2023).
Acknowledgements
We thank the National Institutes of Health (NIH R35 GM138373) and the Alfred P. Sloan Research Fellowship for supporting this work.
Author information
Authors and Affiliations
Contributions
L.P.D., T.B.H. and C.S.S. conceived the work and designed the experiments. L.P.D., H.F.S., T.B.H., M.J.L. and D.K. performed all experiments and collected all data. L.P.D. and H.F.S. performed all high-throughput reactions. L.P.D. and C.Q.H. performed parameterization studies. All authors analysed the data. C.S.S. wrote the paper, and all authors provided revisions.
Corresponding authors
Ethics declarations
Competing interests
L.P.D., T.B.H., H.F.S. and C.S.S. have filed patent applications directed to the technology described here. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24, X-ray crystal structure, all synthetic procedures and NMR spectra.
Supplementary Data 1
Crystallographic data for complex 1; CCDC reference 2290873.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dinh, L.P., Starbuck, H.F., Hamby, T.B. et al. Persistent organonickel complexes as general platforms for Csp2–Csp3 coupling reactions. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01528-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01528-7