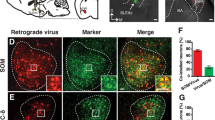
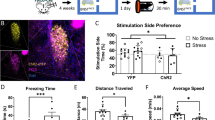
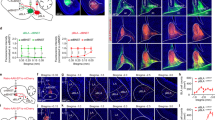
Abstract
Behavioural states in mammals, such as the anxious state, are characterized by several features that are coordinately regulated by diverse nervous system outputs, ranging from behavioural choice patterns to changes in physiology (in anxiety, exemplified respectively by risk-avoidance and respiratory rate alterations)1,2. Here we investigate if and how defined neural projections arising from a single coordinating brain region in mice could mediate diverse features of anxiety. Integrating behavioural assays, in vivo and in vitro electrophysiology, respiratory physiology and optogenetics, we identify a surprising new role for the bed nucleus of the stria terminalis (BNST) in the coordinated modulation of diverse anxiety features. First, two BNST subregions were unexpectedly found to exert opposite effects on the anxious state: oval BNST activity promoted several independent anxious state features, whereas anterodorsal BNST-associated activity exerted anxiolytic influence for the same features. Notably, we found that three distinct anterodorsal BNST efferent projections—to the lateral hypothalamus, parabrachial nucleus and ventral tegmental area—each implemented an independent feature of anxiolysis: reduced risk-avoidance, reduced respiratory rate, and increased positive valence, respectively. Furthermore, selective inhibition of corresponding circuit elements in freely moving mice showed opposing behavioural effects compared with excitation, and in vivo recordings during free behaviour showed native spiking patterns in anterodorsal BNST neurons that differentiated safe and anxiogenic environments. These results demonstrate that distinct BNST subregions exert opposite effects in modulating anxiety, establish separable anxiolytic roles for different anterodorsal BNST projections, and illustrate circuit mechanisms underlying selection of features for the assembly of the anxious state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
LeDoux, J. E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000)
Davis, M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 15, 353–375 (1992)
LeDoux, J. E., Iwata, J., Cicchetti, P. & Reis, D. J. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 8, 2517–2529 (1988)
Viviani, D. et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333, 104–107 (2011)
Kessler, R. C. et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602 (2005)
Singewald, N., Salchner, P. & Sharp, T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol. Psychiatry 53, 275–283 (2003)
Dong, H. W., Petrovich, G. D. & Swanson, L. W. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res. Brain Res. Rev. 38, 192–246 (2001)
Dabrowska, J. et al. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology 36, 1312–1326 (2011)
Walker, D. L., Miles, L. A. & Davis, M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1291–1308 (2009)
Duvarci, S., Bauer, E. P. & Paré, D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J. Neurosci. 29, 10357–10361 (2009)
Yassa, M. A., Hazlett, R. L., Stark, C. E. L. & Hoehn-Saric, R. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J. Psychiatr. Res. 46, 1045–1052 (2012)
Straube, T., Mentzel, H.-J. & Miltner, W. H. R. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage 37, 1427–1436 (2007)
Carola, V., D’Olimpio, F., Brunamonti, E., Mangia, F. & Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 134, 49–57 (2002)
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010)
Martin, B. The assessment of anxiety by physiological behavioral measures. Psychol. Bull. 58, 234–255 (1961)
Suess, W. M., Alexander, A. B., Smith, D. D., Sweeney, H. W. & Marion, R. J. The effects of psychological stress on respiration: a preliminary study of anxiety and hyperventilation. Psychophysiology 17, 535–540 (1980)
Terreberry, R. R., Oguri, M. & Harper, R. M. State-dependent respiratory and cardiac relationships with neuronal discharge in the bed nucleus of the stria terminalis. Sleep 18, 139–144 (1995)
Dong, H.-W. & Swanson, L. W. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J. Comp. Neurol. 468, 277–298 (2004)
Dunn, J. D. & Williams, T. J. Cardiovascular responses to electrical stimulation of the bed nucleus of the stria terminalis. J. Comp. Neurol. 352, 227–234 (1995)
Dunn, J. D. Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain Res. 407, 327–331 (1987)
Woods, J. H., Katz, J. L. & Winger, G. Benzodiazepines: use, abuse, and consequences. Pharmacol. Rev. 44, 151–347 (1992)
Koob, G. F. A role for brain stress systems in addiction. Neuron 59, 11–34 (2008)
Dong, H.-W. & Swanson, L. W. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J. Comp. Neurol. 494, 142–178 (2006)
Dong, H. W., Petrovich, G. D., Watts, A. G. & Swanson, L. W. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J. Comp. Neurol. 436, 430–455 (2001)
Hakvoort Schwerdtfeger, R. M. & Menard, J. L. The lateral hypothalamus and anterior hypothalamic nucleus differentially contribute to rats’ defensive responses in the elevated plus-maze and shock-probe burying tests. Physiol. Behav. 93, 697–705 (2008)
Chamberlin, N. L. & Saper, C. B. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J. Neurosci. 14, 6500–6510 (1994)
Sartor, G. C. & Aston-Jones, G. Regulation of the ventral tegmental area by the bed nucleus of the stria terminalis is required for expression of cocaine preference. Eur. J. Neurosci. 36, 3549–3558 (2012)
Georges, F. & Aston-Jones, G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J. Neurosci. 22, 5173–5187 (2002)
Poulin, J.-F., Arbour, D., Laforest, S. & Drolet, G. Neuroanatomical characterization of endogenous opioids in the bed nucleus of the stria terminalis. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1356–1365 (2009)
Adhikari, A., Topiwala, M. A. & Gordon, J. A. Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron 71, 898–910 (2011)
Acknowledgements
We thank M. Davis, D. Walker, D. Paré, D. Rainnie, H. Shin, K. Thompson, P. Anikeeva, T. Davidson, I. Goshen, A. Andalman, L. Gunaydin, A. Bryant, C. Lee, J. Mirzabekov and the entire Deisseroth laboratory for discussions. Supported by a Samsung Scholarship (to S.-Y.K.), the US National Institute of Mental Health (NIMH; to R.C.M.), and a Berry Fellowship (to A.A.). K.D. and M.R.W. are NARSAD grant awardees, and K.D. was supported by the Wiegers Family Fund, the NIMH, the US National Institute on Drug Abuse (NIDA), the DARPA REPAIR Program, the Keck Foundation, the McKnight Foundation, the Gatsby Charitable Foundation, the Snyder Foundation, the Woo Foundation, the Tarlton Foundation, and the Albert Yu and Mary Bechman Foundation. All tools and methods are distributed and supported freely (http://www.optogenetics.org).
Author information
Authors and Affiliations
Contributions
S.-Y.K., A.A. and K.D. designed the study, interpreted results and wrote the paper. S.-Y.K. coordinated the experiments. S.-Y.K., A.A., S.Y.L., C.S.M., M.R.W. and K.M.T. performed optogenetic behaviour and electrophysiology experiments. S.-Y.K., M.L., S.P. and J.M. performed immunohistochemistry. J.H.M. and C.K.K. performed calcium imaging. B.K.L., R.C.M. and R.N. provided viruses. All authors contributed to editing. K.D. supervised all aspects of the project.
Corresponding author
Ethics declarations
Competing interests
S.-Y.K., A.A. and K.D. have disclosed these findings to the Stanford Office of Technology Licensing regarding the possible use of the findings and methods in identifying new treatments for anxiety. All materials, methods and reagents remain freely available for academic and non-profit research in perpetuity through the Deisseroth optogenetics website (http://www.optogenetics.org).
Supplementary information
Supplementary Information
This file contains Supplementary Statistical Analysis, Supplementary Methods and Data, Supplementary References and Supplementary Figures 1-24. (PDF 18863 kb)
Rights and permissions
About this article
Cite this article
Kim, SY., Adhikari, A., Lee, S. et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–223 (2013). https://doi.org/10.1038/nature12018
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12018
This article is cited by
-
Chemogenetic activation of corticotropin-releasing factor-expressing neurons in the anterior bed nucleus of the stria terminalis reduces effortful motivation behaviors
Neuropsychopharmacology (2024)
-
An ensemble recruited by α2a-adrenergic receptors is engaged in a stressor-specific manner in mice
Neuropsychopharmacology (2023)
-
Topographic representation of current and future threats in the mouse nociceptive amygdala
Nature Communications (2023)
-
A sex-specific role for the bed nucleus of the stria terminalis in proactive defensive behavior
Neuropsychopharmacology (2023)
-
Defensive responses: behaviour, the brain and the body
Nature Reviews Neuroscience (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.