Abstract
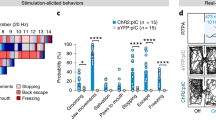
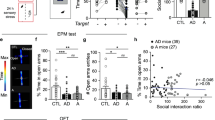
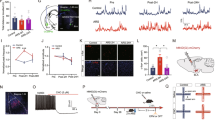
The bed nucleus of the stria terminalis (BNST) is a critical mediator of stress responses and anxiety-like behaviors. Neurons expressing protein kinase C delta (BNSTPKCδ) are an abundant but understudied subpopulation implicated in inhibiting feeding, but which have conflicting reports about their role in anxiety-like behaviors. We have previously shown that expression of PKCδ is dynamically regulated by stress and that BNSTPKCδ cells are recruited during bouts of active stress coping. Here, we first show that in vivo activation of this population is mildly aversive. This aversion was insensitive to prior restraint stress exposure. Further investigation revealed that unlike other BNST subpopulations, BNSTPKCδ cells do not exhibit increased cfos expression following restraint stress. Ex vivo current clamp recordings also indicate they are resistant to firing. To elucidate their afferent control, we next used rabies tracing with whole-brain imaging and channelrhodopsin-assisted circuit mapping, finding that BNSTPKCδ cells receive abundant input from affective, arousal, and sensory regions including the basolateral amygdala (BLA) paraventricular thalamus (PVT) and central amygdala PKCδ-expressing cells (CeAPKCδ). Given these findings, we used in vivo optogenetics and fiber photometry to further examine BNSTPKCδ cells in the context of stress and anxiety-like behavior. We found that BNSTPKCδ cell activity is associated with increased anxiety-like behavior in the elevated plus maze, increases following footshock, and unlike other BNST subpopulations, does not desensitize to repeated stress exposure. Taken together, we propose a model in which BNSTPKCδ cells may serve as threat detectors, integrating exteroceptive and interoceptive information to inform stress coping behaviors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Macatee RJ, Albanese BJ, Schmidt NB, Cougle JR. Attention bias towards negative emotional information and its relationship with daily worry in the context of acute stress: an eye-tracking study. Behav Res Ther. 2017;90:96.
Egan LJ, Dennis-Tiwary TA. Dynamic measures of anxiety-related threat bias: links to stress reactivity. Motiv Emot. 2018;42:546.
Frisch JU, Häusser JA, Mojzisch A. The trier social stress test as a paradigm to study how people respond to threat in social interactions. Front Psychol. 2015;6:14.
Timmers I, Quaedflieg CWEM, Hsu C, Heathcote LC, Rovnaghi CR, Simons LE. The interaction between stress and chronic pain through the lens of threat learning. Neurosci Biobehav Rev. 2019;107:641.
Lebow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 2016;21:450–63.
Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–35.
Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharm. 2003;463:199–16.
Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog NeuroPsychopharmacol Biol Psychiatry. 2009;33:1291–308.
Avery SN, Clauss JA, Blackford JU. The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology. 2016;41:126–41.
Ju G, Swanson LW. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. J Comp Neurol. 1989;280:587–602.
Ju G, Swanson LW, Simerly RB. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. J Comp Neurol. 1989;280:603–21.
Lesur A, Gaspar P, Alvarez C, Berger B. Chemoanatomic compartments in the human bed nucleus of the stria terminalis. Neuroscience. 1989;32:181–94.
Walter A, Mai JKK, Lanta L, Görcs T. Differential distribution of immunohistochemical markers in the bed nucleus of the stria terminalis in the human brain. J Chem Neuroanat. 1991;4:281–98.
Welch JD, Kozareva V, Ferreira A, Vanderburg C, Martin C, Macosko EZ. Single-cell multi-omic integration compares and contrasts features of brain cell identity. Cell. 2019;177:1873–87.e17.
Rodriguez-Romaguera J, Ung RL, Nomura H, Otis JM, Basiri ML, Namboodiri VMK, et al. Prepronociceptin-expressing neurons in the extended amygdala encode and promote rapid arousal responses to motivationally salient stimuli. Cell Rep. 2020;33:108362.
Kash TL, Pleil KE, Marcinkiewcz CA, Lowery-Gionta EG, Crowley N, Mazzone C, et al. Neuropeptide regulation of signaling and behavior in the BNST. Mol Cells. 2015;38:1–13.
Ch’ng S, Fu J, Brown RM, Mcdougall SJ, Lawrence AJ, Ch S, et al. The intersection of stress and reward: BNST modulation of aversive and appetitive states. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87:108–25.
Flanigan ME, Kash TL. Coordination of social behaviors by the bed nucleus of the stria terminalis. Eur J Neurosci. 2022;55:2404–20.
Fetterly TL, Basu A, Nabit BP, Awad E, Williford KM, Centanni SW, et al. α2A-adrenergic receptor activation decreases parabrachial nucleus excitatory drive onto BNST CRF neurons and reduces their activity in vivo. J Neurosci. 2019;39:472–84.
Ye J, Veinante P. Cell-type specific parallel circuits in the bed nucleus of the stria terminalis and the central nucleus of the amygdala of the mouse. Brain Struct Funct. 2019;224:1067–95.
Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci. 2014;17:1240–8.
Wilson TD, Valdivia S, Khan A, Ahn HS, Adke AP, Gonzalez SM, et al. Dual and opposing functions of the central amygdala in the modulation of pain. Cell Rep. 2019;29:332–46.e5.
Venniro M, Russell TI, Ramsey LA, Richie CT, Lesscher HMB, Giovanetti SM, et al. Abstinence-dependent dissociable central amygdala microcircuits control drug craving. PNAS. 2020;117:8126–34.
Domi E, Xu L, Toivainen S, Nordeman A, Gobbo F, Venniro M, et al. A neural substrate of compulsive alcohol use. Sci Adv. 2021;7:eabg9045.
Dilly GA, Kittleman CW, Kerr TM, Messing RO, Mayfield RD. Cell-type specific changes in PKC-delta neurons of the central amygdala during alcohol withdrawal. Transl Psychiatry. 2022;12:289.
Moscarello JM, Penzo MA. The central nucleus of the amygdala and the construction of defensive modes across the threat-imminence continuum. Nat Neurosci. 2022;25:999–1008.
Griessner J, Pasieka M, Böhm V, Grössl F, Kaczanowska J, Pliota P, et al. Central amygdala circuit dynamics underlying the benzodiazepine anxiolytic effect. Mol Psychiatry. 2021;26:534–44.
Whittle N, Fadok J, MacPherson KP, Nguyen R, Botta P, Wolff SBE, et al. Central amygdala micro-circuits mediate fear extinction. Nat Commun. 2021;12:4156.
Jaramillo AA, Williford KM, Marshall C, Winder DG, Centanni SW. BNST transient activity associates with approach behavior in a stressful environment and is modulated by the parabrachial nucleus. Neurobiol Stress. 2020;13:100247.
Luchsinger JR, Fetterly TL, Williford KM, Salimando GJ, Doyle MA, Maldonado J, et al. Delineation of an insula-BNST circuit engaged by struggling behavior that regulates avoidance in mice. Nat Commun. 2021;12:3561.
Wang Y, Kim J, Schmit MB, Cho TS, Fang C, Cai H. A bed nucleus of stria terminalis microcircuit regulating inflammation-associated modulation of feeding. Nat Commun. 2019;10:2769.
Wang X, Zhang Y, Wang X, Dai J, Hua R, Zeng S, et al. Anxiety-related cell-type-specific neural circuits in the anterior-dorsal bed nucleus of the stria terminalis. Sci Bull. 2020;65:1203–16.
Ueda S, Hosokawa M, Arikawa K, Takahashi K, Fujiwara M, Kakita M, et al. Distinctive regulation of emotional behaviors and fear-related gene expression responses in two extended amygdala subnuclei with similar molecular profiles. Front Mol Neurosci. 2021;14:186.
Folkes OM, Báldi R, Kondev V, Marcus DJ, Hartley ND, Turner BD, et al. An endocannabinoid-regulated basolateral amygdala–nucleus accumbens circuit modulates sociability. J Clin Invest. 2020;130:1728.
Brown JA, Petersen N, Centanni SW, Jin AY, Yoon HJ, Cajigas SA, et al. An ensemble recruited by α2a-adrenergic receptors is engaged in a stressor-specific manner in mice. Neuropsychopharmacology. 2022;2022:1–11.
Salimando GJ, Hyun M, Boyt KM, Winder DG. BNST GluN2D-containing NMDA receptors influence anxiety- and depressive-like behaviors and modulate cell-specific excitatory/inhibitory synaptic balance. J Neurosci. 2020;40:3949.
Park YG, Sohn CH, Chen R, McCue M, Yun DH, Drummond GT, et al. Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat Biotechnol. 2019;37:73.
Dean KM, Roudot P, Welf ES, Danuser G, Fiolka R. Deconvolution-free subcellular imaging with axially swept light sheet microscopy. Biophys J. 2015;108:2807–15.
Hedde PN, Gratton E. Selective plane illumination microscopy with a light sheet of uniform thickness formed by an electrically tunable lens. Microsc Res Tech. 2018;81:924–8.
Paré WP, Glavin GB. Restraint stress in biomedical research: a review. Neurosci Biobehav Rev. 1986;10:339–70.
Glavin GB, Paré WP, Sandbak T, Bakke H-KK, Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev. 1994;18:223–49.
Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: recent developments. Neurosci Biobehav Rev. 2009;33:1089–98.
Kovács LÁ, Füredi N, Ujvári B, Golgol A, Gaszner B. Age-dependent FOSB/ΔFOSB response to acute and chronic stress in the extended amygdala, hypothalamic paraventricular, habenular, centrally-projecting edinger-westphal, and dorsal raphe nuclei in male rats. Front Aging Neurosci. 2022;14:403.
Egli RE, Winder DG. Dorsal and ventral distribution of excitable and synaptic properties of neurons of the bed nucleus of the stria terminalis. J Neurophysiol. 2003;90:405–14.
Silberman Y, Matthews RT, Winder DG. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J Neurosci. 2013;33:950–60.
LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2003;23:155–84.
Šimić G, Tkalčić M, Vukić V, Mulc D, Španić E, Šagud M, et al. Understanding emotions: origins and roles of the amygdala. Biomolecules. 2021;11:823.
Kirouac GJ. The paraventricular nucleus of the thalamus as an integrating and relay node in the brain anxiety network. Front Behav Neurosci. 2021;15:21.
Zhao D, Wang D, Wang W, Dai J, Cui M, Wu M, et al. The altered sensitivity of acute stress induced anxiety-related behaviors by modulating insular cortex-paraventricular thalamus-bed nucleus of the stria terminalis neural circuit. Neurobiol Dis. 2022;174:105890.
Roberts GW, Woodhams PL, Polak JM, Crow TJ. Distribution of neuropeptides in the limbic system of the rat: the amygdaloid complex. Neuroscience. 1982;7:99–31.
Mccullough KM, Morrison FG, Hartmann J, Carlezon WA, Ressler KJ. Quantified coexpression analysis of central amygdala subpopulations. ENeuro. 2018;5:e0010-18..
Ahrens S, Wu MV, Furlan A, Hwang GR, Paik R, Li H, et al. A central extended amygdala circuit that modulates anxiety. J Neurosci. 2018;38:5567–83.
Pomrenze MB, Tovar-Diaz J, Blasio A, Maiya R, Giovanetti SM, Lei K, et al. A corticotropin releasing factor network in the extended amygdala for anxiety. J Neurosci. 2019;39:1030–43.
de Guglielmo G, Kallupi M, Pomrenze MB, Crawford E, Simpson S, Schweitzer P, et al. Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat Commun. 2019;10:1238.
Hogg S. A review of the validity and variability of the Elevated Plus-Maze as an animal model of anxiety. Pharm Biochem Behav. 1996;54:21–30.
Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–10.
Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–205.
Kraeuter AK, Guest PC, Sarnyai Z. The elevated plus maze test for measuring anxiety-like behavior in rodents. Methods Mol Biol. 2019;1916:69–74.
La-Vu M, Tobias BC, Schuette PJ, Adhikari A. To approach or avoid: an introductory overview of the study of anxiety using rodent assays. Front Behav Neurosci. 2020;14:145.
Palmiter RD. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 2018;41:280–93.
Jaramillo AA, Brown JA, Winder DG. Danger and distress: parabrachial-extended amygdala circuits. Neuropharmacology. 2021;198:108757.
Shimada S, Inagaki S, Kubota Y, Kito S, Funaki H, Takagi H. Light and electron microscopic studies of calcitonin gene-related peptide-like immunoreactive terminals in the central nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Exp Brain Res. 1989;77:217–20.
Dobolyi A, Irwin S, Makara G, Usdin TB, Palkovits M. Calcitonin gene-related peptide-containing pathways in the rat forebrain. J Comp Neurol. 2005;489:92–119.
Flavin SA, Matthews RT, Wang Q, Muly EC, Winder DG. α2A-adrenergic receptors filter parabrachial inputs to the bed nucleus of the stria terminalis. J Neurosci. 2014;34:9319–31.
Gozzi A, Jain A, Giovanelli A, Bertollini C, Crestan V, Schwarz AJ, et al. A neural switch for active and passive fear. Neuron. 2010;67:656–66.
Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–76.
Chastrette N, Pfaff DW, Gibbs RB. Effects of daytime and nighttime stress on Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus, the habenula, and the posterior paraventricular nucleus of the thalamus. Brain Res. 1991;563:339–44.
Bhatnagar S, Dallman MF. The paraventricular nucleus of the thalamus alters rhythms in core temperature and energy balance in a state-dependent manner. Brain Res. 1999;851:66–75.
Kim JH, Kromm GH, Barnhill OK, Sperber J, Heuer LB, Loomis S, et al. A discrete parasubthalamic nucleus subpopulation plays a critical role in appetite suppression. Elife. 2022;11:e75470.
McKinley MJ, Pennington GL, Ryan PJ. The median preoptic nucleus: a major regulator of fluid, temperature, sleep, and cardiovascular homeostasis. Handb Clin Neurol. 2021;179:435–54.
Yu S, François M, Huesing C, Münzberg H. The hypothalamic preoptic area and body weight control. Neuroendocrinology. 2018;106:187.
Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1207-28.
Andersson B, Larsson B. Influence of local temperature changes in the preoptic area and rostral hypothalamus on the regulation of food and water intake. Acta Physiol Scand. 1961;52:75–89.
Dong H, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J. Comp. Neurol. 2004;468:277–98.
Mcdonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–32.
Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Rev. 2001;38:192–46.
Jolkkonen E, Miettinen R, Pitkänen A. Projections from the amygdalo-piriform transition area to the amygdaloid complex: a PHA-l study in rat. J Comp Neurol. 2001;432:440–65.
Wilson DA, Kadohisa M, Fletcher ML. Cortical contributions to olfaction: plasticity and perception. Semin Cell Dev Biol. 2006;17:462–70.
Comoli E, Ribeiro-Barbosa ÉR, Negrão N, Goto M, Canteras NS. Functional mapping of the prosencephalic systems involved in organizing predatory behavior in rats. Neuroscience. 2005;130:1055–67.
Kemppainen S, Jalkkonen E, Pitkänen A. Projections from the posterior cortical nucleus of the amygdala to the hippocampal formation and parahippocampal region in rat. Hippocampus. 2002;12:735–55.
Pavesi E, Canteras NS, Carobrez AP. Acquisition of pavlovian fear conditioning using β-adrenoceptor activation of the dorsal premammillary nucleus as an unconditioned stimulus to mimic live predator-threat exposure. Neuropsychopharmacology. 2011;36:926–39.
de Voogd LD, Hagenberg E, Zhou YJ, de Lange FP, Roelofs K. Acute threat enhances perceptual sensitivity without affecting the decision criterion. Sci Rep. 2022;12:9071.
Harris NA, Isaac AT, Günther A, Merkel K, Melchior J, Xu M, et al. Dorsal BNST alpha2A-adrenergic receptors produce HCN-dependent excitatory actions that initiate anxiogenic behaviors. J Neurosci. 2018;38:8922–42.
Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–64.
Vail G, Roepke TA. Membrane-initiated estrogen signaling via Gq-coupled GPCR in the central nervous system. Steroids. 2019;142:77.
Carrasquillo Y, Gereau RW. Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain. 2008;4:24.
Allen HN, Chaudhry S, Hong VM, Lewter LA, Sinha GP, Carrasquillo Y, et al. A parabrachial-to-amygdala circuit that determines hemispheric lateralization of somatosensory processing. Biol Psychiatry. 2023;93:370–81.
Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annu Rev Psychol. 1994;45:25–50.
Gorka AX, LaBar KS, Hariri AR. Variability in emotional responsiveness and coping style during active avoidance as a window onto psychological vulnerability to stress. Physiol Behav. 2016;158:90.
Spinhoven P, van Hemert AM, Penninx BWJH. Experiential avoidance and bordering psychological constructs as predictors of the onset, relapse and maintenance of anxiety disorders: one or many? Cogn Ther Res. 2017;41:867.
Hofmann SG, Hay AC. Rethinking avoidance: toward a balanced approach to avoidance in treating anxiety disorders. J Anxiety Disord. 2018;55:14.
Schlund MW, Carter H, Cudd G, Murphy K, Ahmed N, Dymond S, et al. Human social defeat and approach-avoidance: escalating social-evaluative threat and threat of aggression increases social avoidance. J Exp Anal Behav. 2021;115:157.
Ball TM, Gunaydin LA. Measuring maladaptive avoidance: from animal models to clinical anxiety. Neuropsychopharmacology. 2022;47:978.
Acknowledgements
We would like to thank Bruce Williams and Roger Williams in the Vanderbilt Scientific Instrumentation core for their assistance in the development and production of the RESTRAINT devices used. Microscopy was performed in part through the use of the Vanderbilt Cell Imaging Shared Resource and the assistance of Dr. Robert Matthews and Dr. Jenny Schafer. We also would like to thank Elana Milano, Bridget Morris, Laith Kayat, and Megan Altemus for assistance with mouse colony maintenance and genotyping, Elana Milano and Megan Altemus for keeping the lab running, and Megan Altemus for her assistance with daily acclimation of mice and other aspects of fiber photometry experiments when an extra set of hands was much needed.
Funding
This work was supported by the following funding sources: Howard Hughes Medical Institute Gilliam Fellowship (Grant No. GT11385 [KMW]), National Institute of Neurological Disorders and Stroke DSPAN Fellowship (Grant No 1F99NS120599-01 [KMW]), National Institute on Drug Abuse (Grant No. R01-DA042475-06A1 [DGW]).
Author information
Authors and Affiliations
Contributions
KMW and DGW designed the project and experiments, and DGW supervised the project. KMW ran all in vivo experiments, including optogenetics, GCaMP fiber photometry, and performed all surgical procedures. KMW analyzed and graphed all data. KMW ran all in vivo optogenetic experiments using equipment from SP. JRM ran all basal whole cell patch clamp electrophysiology. AT and DNA ran all PKCd+/− electrophysiological comparison experiments. AT ran all PVT and CeA channelrhodopsin-assisted circuit mapping recordings (CRACM). AT and DNA ran all BLA CRACM. JAB and SWC assisted in analysis of GCaMP fiber photometry. KMW, ES, and MDN ran and analyzed all immunohistochemistry experiments. HJY ran all footshock experiments, supervised by ESC. JRL assisted with preparation and imaging of some light sheet microscopy with equipment from RBS. JAB and MNB assisted with light sheet imaging and analysis, supervised by RBS. KMW wrote the first draft of manuscript with primary editing by DGW, and additional editing and reviewing by AT, JRM, HJY, ES, MDN, DNA, JAB, MNB, JRL, SWC, SP, ESC, and RBS. Funding was acquired by KMW and DGW.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Williford, K.M., Taylor, A., Melchior, J.R. et al. BNST PKCδ neurons are activated by specific aversive conditions to promote anxiety-like behavior. Neuropsychopharmacol. 48, 1031–1041 (2023). https://doi.org/10.1038/s41386-023-01569-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01569-5