Abstract
Primitive myxoid mesenchymal tumor of infancy is a rare sarcoma that preferentially affects infants. It can be locally aggressive and rarely metastasizes, but the long-term outcome of children with this tumor is mostly unknown. Histologically, it is characterized by primitive cells with abundant myxoid stroma. Internal tandem duplication of B-cell CLL/lymphoma 6 (BCL6)-interacting co-repressor (BCOR) exon 15 has recently been described in clear cell sarcoma of kidney, central nervous system high-grade neuroepithelial tumor with BCOR alteration, and primitive myxoid mesenchymal tumor of infancy. Herein, we report five cases of primitive myxoid mesenchymal tumor of infancy: three girls and two boys with mean age of 6.5 months. The tumors were located in the paraspinal region (n=3), back (n=1), or foot (n=1) and ranged in size from 2.5 to 10.2 cm. BCOR internal tandem duplication was confirmed by PCR and sequencing in all five cases. The minimally duplicated region consisted of nine residues, which is shorter than was previously reported in other BCOR-associated tumors. To assess the clinical value and specificity of the BCOR internal tandem duplication, a group of 11 ETV6-rearranged congenital infantile fibrosarcomas were evaluated and no BCOR internal tandem duplication was identified in any case. Though not detected in congenital infantile fibrosarcomas, BCOR and BCL6 immunoreactivity was present in >90% of the nuclei of tumor cells in each of the five primitive myxoid mesenchymal tumor of infancy. The presence of BCOR internal tandem duplication in all five primitive myxoid mesenchymal tumors of infancy provides evidence that it is a recurrent somatic abnormality and substantiates the concept that this tumor is a unique sarcoma of infancy. Our findings indicate that identification of BCOR internal tandem duplication and/or nuclear immunoreactivity for BCOR or BCL6 can aid in the diagnosis of primitive myxoid mesenchymal tumor of infancy and help to differentiate it from congenital infantile fibrosarcoma.
Similar content being viewed by others
Main
Primitive myxoid mesenchymal tumor of infancy, first described in 2006 by Allagio et al,1 is a primitive mesenchymal soft tissue sarcoma with no distinctive lineage of differentiation. This tumor typically affects young children, particularly infants, frequently presenting as a painless mass. Until recently, there were no known specific immunohistochemical markers or molecular alterations to distinguish primitive myxoid mesenchymal tumor of infancy from its histologic mimics, and the diagnosis was based solely on morphologic features and the absence of the ETV6–NTRK3 gene fusion that is typically present in congenital infantile fibrosarcoma.2 However, Kao et al3 have recently reported internal tandem duplication of the X-linked BCL6 co-repressor (BCOR) gene (BCOR internal tandem duplication) in six of the seven primitive myxoid mesenchymal tumor of infancy cases examined. BCOR internal tandem duplication has been previously identified as a recurrent somatic abnormality in approximately 85% of clear cell sarcomas of kidney4, 5, 6, 7 and in a subset of central nervous system high-grade neuroepithelial tumors with BCOR alterations.8
With only a limited number of cases reported in the literature, primitive myxoid mesenchymal tumor of infancy was not included in the most recent World Health Organization classification of tumors of the soft tissue (4th edition, February 2013).9 Based on available current data, the biological behavior of this mesenchymal soft tissue sarcoma in considered to be intermediate due to the fact that it can be locally aggressive and rarely metastasizes, similar to congenital infantile fibrosarcoma. Nevertheless, the outcome and long-term survival in children with primitive myxoid mesenchymal tumor of infancy is mostly unknown.10, 11, 12, 13, 14, 15, 16, 17, 18 This study aimed to evaluate the clinical utility of testing for the BCOR internal tandem duplication and the use of BCOR and B-cell CLL/lymphoma 6 (BCL6) immunohistochemistry as a diagnostic marker for this rare soft tissue sarcoma. Specifically, we wished to evaluate the effectiveness of these tests to differentiate primitive myxoid mesenchymal tumor of infancy from congenital infantile fibrosarcoma.
Materials and methods
Patients
This study was approved by our Institutional Review Board. A retrospective review of our institutional records was performed. Five children with low-grade primitive sarcoma diagnosed as primitive myxoid mesenchymal tumor of infancy were identified and all available clinicopathologic data, hematoxylin and eosin (H&E)-stained slides, immunohistochemistry-stained slides, and interphase fluorescent in situ hybridization results were reviewed. All five cases of primitive myxoid mesenchymal tumor of infancy identified in our files were pathology consultations, with treatment and follow-up performed at an outside institution.
DNA Extraction and PCR Analysis
Genomic DNA was extracted from formalin-fixed paraffin-embedded tissue by using a Maxwell 16 formalin-fixed paraffin-embedded Plus LEV DNA purification kit (Promega) in accordance with the manufacturer’s instructions. Targeted PCR amplification of the duplicated segment of the BCOR gene (exon 15) was performed on genomic DNA by using GoTaq DNA polymerase (Promega) and BCOR internal tandem duplication primers previously described.6 PCR products were sequenced using BigDye Terminator v3.1 Sequencing Kit (Applied Biosystems). The sequence results were analyzed using 3730xl DNA Analyzer (Life Technologies) in conjunction with CLC Workbench 6.0 (CLCBio). All tandem duplications detected were confirmed by independent replicate analyses. Negative (normal tissue) and positive (10 cases of clear cell sarcomas of kidney) controls were used to validate the detection assay. In addition to the primitive myxoid mesenchymal tumor of infancy cases, 11 cases of ETV6-rearranged congenital infantile fibrosarcoma were also evaluated for BCOR internal tandem duplication.
Immunohistochemical Staining
Both primitive myxoid mesenchymal tumors of infancy and congenital infantile fibrosarcoma cases were assessed for expression of BCOR and BCL6 by immunohistochemistry. Formalin-fixed paraffin-embedded tissue (4 μm in thickness) from primitive myxoid mesenchymal tumors of infancy, congenital infantile fibrosarcomas, and appropriated negative and positive controls were subjected to immunohistochemistry staining for BCL6 using a BenchMark ULTRA automated slide stainer (Ventana Medical Systems) and immunohistochemistry staining for BCOR using a Dako Omnis automated staining platform in accordance with the manufacturers’ recommended procedure. In brief, immunohistochemistry for BCL6 was performed after deparaffinization and antigen retrieval using cell conditioning solution (CC1) for 64 min, followed by 32 min incubation at 37 °C with mouse monoclonal anti-BCL6 antibody (Bond ready-to-use primary antibody BCL-6 [LN22]; Leica Biosystems, catalog number PA0204). Immunohistochemistry for BCOR was also performed following deparaffinization and antigen retrieval using EnVision FLEX high pH Target Retrieval Solution (Dako) for 30 min. The slides were incubated with mouse monoclonal IgG1 BCOR antibody, which targets the N terminus of BCOR protein (1:100 dilution, incubated for 20 min at 36 °C; clone C-10, catalog number SC-514576, Santa Cruz Biotechnology). An OptiView DAB immunohistochemistry detection kit (OptiView, Ventana Medical Systems) was used as an indirect biotin-free system for signal detection. Before light microscopy examination, the slides were counterstained with hematoxylin.
Results
Clinicopathologic Characteristics of Primitive Myxoid Mesenchymal Tumor of Infancy
We identified five cases of primitive myxoid mesenchymal tumor of infancy in our records. The mean age at diagnosis of the three girls and two boys was 6.5 months (range, 1 week to 13 months). The tumors were located in the paraspinal region (three cases), back (one case), or foot (one case). The tumor size was available for three patients and ranged from 2.5 to 10.2 cm (mean, 5.3 cm) in the largest dimension. At initial diagnosis, all patients had localized disease with no evidence of metastasis. However, one patient in whom primitive myxoid mesenchymal tumor of infancy was initially diagnosed at 1 week of age (primitive myxoid mesenchymal tumor of infancy—case 1) experienced local recurrence after 6 months. A concise summary of the clinicopathologic features is presented in Table 1.
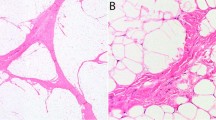
Histologically, all five tumors displayed a diffuse growth pattern, a vaguely multinodular appearance, and variable degrees of cellularity. Round-to-oval or slightly spindle-shaped primitive cells were distributed in a loose myxoid matrix. A well-developed vasculature was noted. The nuclei were mostly ovoid, with fine chromatin and discreet nucleoli (Figures 1a and b). The mean mitotic rate was three mitoses per 10 high-power fields. Only typical mitoses were identified. Areas with hemangiopericytoma-like vascular pattern (Case 3, Figure 1c), prominent myxoid background (Case 4, Figure 1d), and focal mild inflammatory infiltrate were also noted (Case 5).
Histomorphology of primitive myxoid mesenchymal tumor of infancy. (a) Primitive cell morphology with delicate vessels and myxoid stroma (primitive myxoid mesenchymal tumor of infancy—case 1, × 200); (b) tumor cells show uniform round-to-oval nuclei with inconspicuous nucleoli (primitive myxoid mesenchymal tumor of infancy—case 1, × 400); (c) areas with hemangiopericytoma-like vascular pattern (primitive myxoid mesenchymal tumor of infancy—case 3, × 200) and (d) prominent myxoid background were also noted (primitive myxoid mesenchymal tumor of infancy—case 4, × 400). Hematoxylin and eosin.
A summary of all the different immunohistochemistry stains performed on the primitive myxoid mesenchymal tumor of infancy cases are presented in Figure 2a. In four cases in which immunohistochemistry staining for CD99 was performed, all were diffusely positive, and all five primitive myxoid mesenchymal tumor of infancy were negative for S-100. Interphase fluorescence in situ hybridization analysis to detect ETV6 rearrangement, as seen in congenital infantile fibrosarcoma, was negative in all five primitive myxoid mesenchymal tumors of infancy cases (Table 1).
(a) Immunohistochemical staining and interphase fluorescence in situ hybridization results for five cases of primitive myxoid mesenchymal tumor of infancy. Green, negative; red, positive; gray, not tested. (b) Diagram of predicted BCOR protein sequences from BCOR internal tandem duplication-associated tumors. BCOR wild-type protein sequence is presented on top followed by three cases from our cohort (primitive myxoid mesenchymal tumor of infancy—cases 1, 2, and 4). A homologous sequence of nine residues is seen (red box). The common duplicated region identified in the primitive myxoid mesenchymal tumors of infancy by Kao et al,3 clear cell sarcomas of kidney by Roy et al,6 and central nervous system high-grade neuroepithelial tumors with BCOR alterations by Sturm et al8 includes 20, 14, and 13 residues, respectively. BCL6, B-cell CLL/lymphoma 6; CD, cluster of differentiation; CK, cytokeratin; EMA, epithelial membrane antigen; ETV6, ETS variant gene 6 (TEL oncogene); EWSR-1, Ewing sarcoma breakpoint region 1; INI1, SMARCB1 SWI/SNF-related, matrix-associated, actin-dependent; SALL4, spalt-like transcription factor 4; SMA, smooth muscle actin; Synap, synaptophysin; SYT, synovial sarcoma translocation.
Recurrent Somatic BCOR Internal Tandem Duplication in Primitive Myxoid Mesenchymal Tumor of Infancy
To determine whether the BCOR internal tandem duplication could be used clinically to distinguish primitive myxoid mesenchymal tumor of infancy from congenital infantile fibrosarcoma, we compared a cohort of 11 ETV6-rearranged congenital infantile fibrosarcomas to our cohort of primitive myxoid mesenchymal tumors of infancy for the presence of the BCOR internal tandem duplication using targeted polymerase chain reaction for the duplicated region of BCOR (exon 15) on DNA extracted from formalin-fixed paraffin-embedded tumor tissue. Clear cell sarcomas of kidney and normal brain were used as positive and negative controls of the assay, respectively. All five cases of primitive myxoid mesenchymal tumor of infancy demonstrated the BCOR internal tandem duplication, which was confirmed by sequencing. In contrast, the BCOR internal tandem duplication was not detected in any of the 11 congenital infantile fibrosarcoma cases (Table 1).
BCOR and BCL6 Expression in Primitive Myxoid Mesenchymal Tumor of Infancy and Congenital Infantile Fibrosarcoma
To determine whether BCL6 or BCOR immunoreactivity could be used as a surrogate of the BCOR internal tandem duplication and help distinguish primitive myxoid mesenchymal tumor of infancy and congenital infantile fibrosarcoma, we performed immunohistochemistry staining for both BCL6 and BCOR on our cohort of tumors. All 10 congenital infantile fibrosarcoma cases tested for BCL6 expression were negative, and only rare intratumoral inflammatory cells were immunoreactive for BCL6. BCOR immunostaining was completely negative in all seven congenital infantile fibrosarcomas examined. In contrast, each of the primitive myxoid mesenchymal tumors of infancy examined demonstrated nuclear positivity for both BCOR and BCL6 in over 90% of the tumor cells (Figure 3).
Primitive myxoid mesenchymal tumor of infancy in contrast with congenital infantile fibrosarcoma. (a–r) Five cases of primitive myxoid mesenchymal tumor of infancy, all negative for ETV6 rearrangement by interphase fluorescent in situ hybridization and positive for BCOR internal tandem duplication (first column, H&E, × 400); immunohistochemistry staining for BCL6 (second column) and BCOR (third column) show diffuse nuclear positivity in all primitive myxoid mesenchymal tumor of infancy, but negative staining in an ETV6-rearranged congenital infantile fibrosarcoma (congenital infantile fibrosarcoma—case 6), immunohistochemistry stains, × 400. ETV6, ETS variant gene 6 (TEL oncogene); BCOR, B-cell CLL/lymphoma 6 (BCL6)-interacting co-repressor.
Discussion
The main differential diagnosis of primitive myxoid mesenchymal tumor of infancy is congenital infantile fibrosarcoma, which also presents as a soft tissue mass and most commonly affects infants. The presence of recurrent ETV6–NTRK3 fusion is a diagnostic feature of congenital infantile fibrosarcoma that is absent in cases of primitive myxoid mesenchymal tumor of infancy. Various primary sites have been reported, but primitive myxoid mesenchymal tumor of infancy has a slight predilection for involving deep soft tissue, especially around the spinal cord.12, 13 Indeed, three of our five cases involved a paraspinal location. Both primitive myxoid mesenchymal tumor of infancy and congenital infantile fibrosarcoma frequently recur locally if not completely excised and rarely metastasize.1, 2 Nevertheless, based on the currently available data, primitive myxoid mesenchymal tumor of infancy appears to be more aggressive than congenital infantile fibrosarcoma, exhibiting frequent local recurrence, poor response to chemotherapy, and the potential to transform into an undifferentiated sarcoma with marked cellular atypia.17
Before the identification of BCOR internal tandem duplication, ultrastructural, molecular, and cytogenetic analysis of published cases of primitive myxoid mesenchymal tumor of infancy failed to demonstrate any specific alteration. Electron microscopic analysis revealed primitive mesenchymal features and the presence of abundant dilated rough endoplasmic reticulum in the cytoplasm, but found no distinctive features other than an apparent fibroblastic line of differentiation.1, 16, 18
The BCOR gene, located on the short arm of chromosome X (ch Xp11.4), encodes a protein that selectively interacts with BCL6 as a co-repressor. Although somatic alterations in the BCOR gene have been reported in different types of human neoplasm, particularly undifferentiated round cell sarcomas of the bone that harbor a BCOR-CCNB3 translocation19, 20 and, most recently, clear cell sarcomas of kidney, in which BCOR internal tandem duplication was found in 85% of the cases analyzed,4, 5, 6, 7 BCOR germline mutation recognized in patients with X-linked oculofacialcardiodental syndrome is not considered a cancer predisposing condition.21, 22, 23, 24
Until recently, the diagnosis of primitive myxoid mesenchymal tumor of infancy was based exclusively on morphologic features and the absence of ETV6–NTRK3 gene fusion; hence, the identification of BCOR internal tandem duplication in six of the seven cases studied by Kao et al3 and in all five of our cases of primitive myxoid mesenchymal tumor of infancy indicates that BCOR internal tandem duplication has a diagnostic utility in this tumor. Kao et al have also shown that BCOR immunohistochemistry stain was diffuse and strongly positive in 11 out of 14 BCOR internal tandem duplication positive tumors (78%), moderately positive in two cases (14%) and negative in one patient (7%).25 Even though they have shown that a large number of variable sarcomas are negative for BCOR immunostaining, congenital infantile fibrosarcomas were not among those tested.25 In our hands, both immunohistochemistry staining for BCL6 and BCOR demonstrated diffuse nuclear positivity in more than 90% of tumor cells in all five of the primitive myxoid mesenchymal tumors of infancy examined, whereas it was negative in all congenital infantile fibrosarcomas tested (Fisher’s exact test, P<0.01). Therefore, the diffuse nuclear expression of BCOR and BCL6 observed in primitive myxoid mesenchymal tumor of infancy appears useful in differentiating it from congenital infantile fibrosarcoma, particularly when molecular testing for BCOR internal tandem duplication and/or ETV6–NTRK3 is unavailable.
Five distinct types of in-frame BCOR internal tandem duplication mutants with varying lengths of internal tandem duplication (in base pairs, bp) were identified in a cohort of clear cell sarcomas of kidney: type I, 96 bp (five cases); type II, 93 bp (three cases); type III, 90 bp (one case); type IV, 87 bp (one case); and type V, 114 bp (one case), all located within the C-terminal coding region.6 In our five cases of primitive myxoid mesenchymal tumor of infancy, the internal tandem duplication ranged from 66 to 96 bp (Table 1). The length of the minimally duplicated region was nine amino acid residues, which is shorter than what was previously reported in BCOR internal tandem duplication positive primitive myxoid mesenchymal tumor of infancy,3 clear cell sarcomas of kidney,6 and central nervous system high-grade neuroepithelial tumors with BCOR alterations8 (Figure 2b). The significance of the insert size and/or length of the homologous region in positive BCOR internal tandem duplication tumors are still unknown. The molecular characterization of a larger number of primitive myxoid mesenchymal tumors of infancy is required to establish the clinical, biological, and prognostic implications of BCOR gene alteration, and BCOR and BCL6 protein overexpression. Supplementary molecular analysis and methylation studies would be beneficial for characterizing this distinctive tumor.
Central nervous system high-grade neuroepithelial tumors with BCOR alterations8 also share some histologic similarities with primitive myxoid mesenchymal tumor of infancy and clear cell sarcomas of kidney. Although the phenotypic resemblance among these three BCOR internal tandem duplication positive tumors does not necessarily indicate that they have similar histogenesis, it does cause us to speculate whether these three embryonal tumors constitute another ‘triad of tumors’ involving soft tissue, kidney, and CNS. This is similar to the triad of extrarenal rhabdoid tumor of soft tissue, rhabdoid tumor of the kidney, and atypical teratoid rhabdoid tumor of the brain, which share similar morphologic features and, in almost all cases, a common genetic alteration affecting the SMARCB1 locus on the long arm of chromosome 22 that results in the loss of SMARCB1 (INI1) protein expression.26, 27, 28 Moreover, primitive myxoid mesenchymal tumor of infancy and clear cell sarcoma of kidney share morphological features and similar molecular aberration analogous to congenital infantile fibrosarcoma of soft tissue and the cellular variant of mesoblastic nephroma of the kidney, which have identical histology and share a common t(12;15)(p13;q25) translocation that results in an ETV6–NTRK3 gene fusion.
In summary, we have confirmed the association between BCOR internal tandem duplication and primitive myxoid mesenchymal tumor of infancy and provided supporting evidence that BCOR internal tandem duplication is indeed a recurrent somatic abnormality in primitive myxoid mesenchymal tumor of infancy. Furthermore, we have demonstrated that BCOR internal tandem duplication identification and/or nuclear immunoreactivity for BCOR and/or BCL6 can have diagnostic utility and can help to differentiate primitive myxoid mesenchymal tumor of infancy from congenital infantile fibrosarcoma. Future studies are needed to elucidate the biologic role of the BCOR internal tandem duplication and BCOR/BLC6 protein overexpression in these rare mesenchymal tumors. Additional research into this area is necessary and may provide insight into the use of targeted therapeutics in such patients.
References
Alaggio R, Ninfo V, Rosolen A et al, Primitive myxoid mesenchymal tumor of infancy: a clinicopathologic report of 6 cases. Am J Surg Pathol 2006;30:388–394.
Knezevich SR, McFadden DE, Tao W et al, A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet 1998;18:184–187.
Kao YC, Sung YS, Zhang L et al, Recurrent BCOR internal tandem duplication and YWHAE-NUTM2B fusions in soft tissue undifferentiated round cell sarcoma of infancy: overlapping genetic features with clear cell sarcoma of kidney. Am J Surg Pathol 2016;40:1009–1020.
Astolfi A, Melchionda F, Perotti D et al, Whole transcriptome sequencing identifies BCOR internal tandem duplication as a common feature of clear cell sarcoma of the kidney. Oncotarget 2015;6:40934–40939.
Karlsson J, Valind A, Gisselsson D . BCOR internal tandem duplication and YWHAE-NUTM2B/E fusion are mutually exclusive events in clear cell sarcoma of the kidney. Genes Chromosomes Cancer 2015;55:120–123.
Roy A, Kumar V, Zorman B et al, Recurrent internal tandem duplications of BCOR in clear cell sarcoma of the kidney. Nat Commun 2015;6:8891.
Ueno-Yokohata H, Okita H, Nakasato K et al, Consistent in-frame internal tandem duplications of BCOR characterize clear cell sarcoma of the kidney. Nat Genet 2015;47:861–863.
Sturm D, Orr BA, Toprak UH et al, New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell 2016;164:1060–1072.
Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F . WHO Classification of Tumours of Soft Tissue and Bone, Fourth Edition. IARC: Lyon, 2013.
Lam J, Lara-Corrales I, Cammisuli S et al, Primitive myxoid mesenchymal tumor of infancy in a preterm infant. Pediatr Dermatol 2010;27:635–637.
Mulligan L, O'Meara A, Orr D et al, Primitive myxoid mesenchymal tumor of infancy: a report of a further case with locally aggressive behavior. Pediatr Dev Pathol 2011;14:75–79.
Gong Q, Wang Z, Li X et al, Primitive myxoid mesenchymal tumor of infancy: report of two cases and review of the literature. Pathol Int 2012;62:549–553.
Saito A, Taketani T, Kanai R et al, A case with sacrococcygeal primitive myxoid mesenchymal tumor of infancy: a case report and review of the literature. J Pediatr Hematol Oncol 2013;35:e280–e282.
Su TC, Hwang MJ, Li CF et al, A rare malignant tumor of scalp in a 3-month-old Taiwanese infancy: case report of primitive myxoid mesenchymal tumor of infancy with molecular study. Med Mol Morphol 2013;46:109–113.
Cuthbertson DW, Caceres K, Hicks J et al, A cooperative approach to diagnosis of rare diseases: primitive myxoid mesenchymal tumor of infancy. Ann Clin Lab Sci 2014;44:310–316.
Cipriani NA, Ryan DP, Nielsen GP . Primitive myxoid mesenchymal tumor of infancy with rosettes: a new finding and literature review. Int J Surg Pathol 2014;22:647–651.
Guilbert MC, Rougemont AL, Samson Y et al, Transformation of a primitive myxoid mesenchymal tumor of infancy to an undifferentiated sarcoma: a first reported case. J Pediatr Hematol Oncol 2015;37:e118–e120.
Foster JH, Hicks J, Vasudevan S et al, Primitive myxoid mesenchymal tumor of infancy involving chest wall in an infant: a case report and clinicopathologic correlation. Pediatr Dev Pathol 2015;19:244–248.
Pierron G, Tirode F, Lucchesi C et al, A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet 2012;44:461–466.
Puls F, Niblett A, Marland G et al, BCOR-CCNB3 (Ewing-like) sarcoma: a clinicopathologic analysis of 10 cases, in comparison with conventional Ewing sarcoma. Am J Surg Pathol 2014;38:1307–1318.
Horn D, Chyrek M, Kleier S et al, Novel mutations in BCOR in three patients with oculo-facio-cardio-dental syndrome, but none in Lenz microphthalmia syndrome. Eur J Hum Genet 2005;13:563–569.
Davoody A, Chen IP, Nanda R et al, Oculofaciocardiodental syndrome: a rare case and review of the literature. Cleft Palate Craniofac J 2012;49:e55–e60.
Oberoi S, Winder AE, Johnston J et al, Case reports of oculofaciocardiodental syndrome with unusual dental findings. Am J Med Genet A 2005;136:275–277.
Jiang YH, Fang P, Adesina AM et al, Molecular characterization of co-occurring Duchenne muscular dystrophy and X-linked oculo-facio-cardio-dental syndrome in a girl. Am J Med Genet A 2009;149A:1249–1252.
Kao YC, Sung YS, Zhang L et al, BCOR overexpression is a highly sensitive marker in round cell sarcomas with BCOR genetic abnormalities. Am J Surg Pathol 2016;40:1670–1678.
Weeks DA, Beckwith JB, Mierau GW et al, Rhabdoid tumor of kidney. A report of 111 cases from the National Wilms' Tumor Study Pathology Center. Am J Surg Pathol 1989;13:439–458.
Kodet R, Newton WA Jr., Sachs N et al, Rhabdoid tumors of soft tissues: a clinicopathologic study of 26 cases enrolled on the Intergroup Rhabdomyosarcoma Study. Hum Pathol 1991;22:674–684.
Rorke LB, Packer R, Biegel J . Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood. J Neurooncol 1995;24:21–28.
Acknowledgements
The authors thank the pathologists and clinicians who submitted the cases in consultation, and the support from the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Santiago, T., Clay, M., Allen, S. et al. Recurrent BCOR internal tandem duplication and BCOR or BCL6 expression distinguish primitive myxoid mesenchymal tumor of infancy from congenital infantile fibrosarcoma. Mod Pathol 30, 884–891 (2017). https://doi.org/10.1038/modpathol.2017.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2017.12
This article is cited by
-
Small round cell sarcomas
Nature Reviews Disease Primers (2022)
-
Primary bone sarcoma with BCOR internal tandem duplication
Virchows Archiv (2020)
-
Primitive myxoid mesenchymal tumor of infancy with brain metastasis: first reported case
Child's Nervous System (2019)
-
Recent advances in the histological and molecular classification of endometrial stromal neoplasms
Virchows Archiv (2018)