Abstract
Development of targeted agents for the treatment of diffuse large B-cell lymphoma includes clinical evaluation of enzastaurin, an agent that suppresses signaling through protein kinase C-β and AKT pathways. To determine whether protein kinase C-β expression has prognostic significance for diffuse large B-cell lymphoma patients treated with immunochemotherapy, we analyzed the expression of protein kinase C-β II, BCL-2 and cell of origin immunohistochemically from pretreatment samples of 95 diffuse large B-cell lymphoma patients. All patients received rituximab with CHOP or CHOEP. According to Kaplan–Meier analyses, overall survival at 3 years was better among the patients with low than high protein kinase C-β II protein levels (94 vs 76%, P=0.036). The prognostic value of protein kinase C-β II expression on survival was seen in the patients with low and high International Prognostic Index risk groups, and in all molecular entities. Gene expression data from an independent set of 233 diffuse large B-cell lymphoma patients treated with a combination of rituximab and CHOP-like chemotherapy was analyzed in comparison. Accordingly, a better 3-year overall survival was observed among the subgroup with low protein kinase C-β II mRNA levels (84 vs 68%, P=0.005). In multivariate analysis with cell of origin, protein kinase C-β II mRNA expression remained as an independent predictor for overall survival. Together, the data show that protein kinase C-β II expression has prognostic significance in diffuse large B-cell lymphoma patients treated with immunochemotherapy.
Similar content being viewed by others
Main
Diffuse large B-cell lymphoma is the most common lymphoma subtype comprising 30–40% of all non-Hodgkin lymphomas. It is an aggressive disease, of which only 50% of the patients can be cured with anthracyclin-based chemotherapy. However, the concurrent administration of CD20 antibody, rituximab, with different chemotherapies has resulted in a significant improvement of survival compared to chemotherapy alone.1, 2, 3, 4, 5
Diffuse large B-cell lymphoma is a heterogeneous disease both clinically and biologically. In estimating the prognosis of diffuse large B-cell lymphoma, clinically based International Prognostic Index has been the most important tool in classifying chemotherapy- and immunochemotherapy-treated patients into low- and high-risk groups.6 However, biological analyses have shown that the outcome within individual risk subgroups can vary considerably.7, 8, 9 Therefore, there is a need for a ‘Biological Prognostic Index’ that could be used to improve the prediction of outcome of diffuse large B-cell lymphoma patients.
On the basis of gene expression profiles, diffuse large B-cell lymphoma can be classified into distinct molecular subtypes. Two major diffuse large B-cell lymphoma entities, showing germinal center B-cell and activated B-cell-like signatures, have been identified. The subgroups have significantly different outcome in response to chemotherapy and immunochemotherapy.7, 8, 10, 11, 12 However, the prognostic function of the immunohistochemically defined cell of origin distinction has remained unresolved in the immunochemotherapy era. In separate gene expression studies, other genes with additional prognostic information for diffuse large B-cell lymphoma patients have been found.9, 13, 14, 15 As an example of these, protein kinase C-β (PKC-β) is an interesting example, as it represents a target for a novel biological drug, enzastaurin that is being used in a randomized clinical trial of the patients with primary diffuse large B-cell lymphoma.
The protein kinase C family includes several important isoforms of serine/threonine kinases, which are key members in tumor development and growth.16 Of these, PKC-β I and PKC-β II are the two major isoforms expressed in B lymphocytes. The adverse prognostic impact of PKC-β II expression on the survival of diffuse large B-cell lymphoma patients was initially shown by gene expression profiling and subsequently confirmed immunohistochemically.9, 17, 18, 19, 20 However, in these studies, the patients received chemotherapy without rituximab. The aim of this study was to assess whether expression of PKC-β II remains to have prognostic value in the post-rituximab era of lymphoma therapies.
Materials and methods
Patients and Treatments
This is a population-based retrospective analysis for diffuse large B-cell lymphoma patients treated with immunochemotherapy. Initially, 95 CD20-positive de novo diffuse large B-cell lymphoma patients treated during 2002–2006 at the University Hospitals of Helsinki and Lund were selected for the immunohistochemistry group. The patients were eligible if they had received a combination of rituximab (R) and CHOP regimen (cyclophosphamide, doxorubicin, etoposide, vincristine, and prednisone) with or without etoposide (E), if paraffin-embedded lymphoma tissue was available for immunohistochemical stainings, and if the sample had been taken before any treatment. Patients were excluded if they were HIV positive, had central nervous system involvement at presentation, or had evidence of transformation from an indolent lymphoma. The baseline clinical characteristics were collected, and the risk stratification performed according to the International Prognostic Index.6 To confirm immunohistochemistry, we used a microarray data generated by Lymphoma/Leukemia Molecular Profiling Project.11 The data set contains mRNA expression data from de novo 233 diffuse large B-cell lymphoma patients treated with combination of rituximab and CHOP-like chemotherapy. Two probe sets were annotated as PKC-β II. The protocol and sampling were approved by the institutional review boards in Helsinki and Lund, and the Finnish National Authority for Medicolegal Affairs.
Immunohistochemistry
Immunohistochemical stainings were performed on formalin-fixed, paraffin-embedded 4-μm tissue sections from samples taken at the time of diagnosis. Immunohistochemistry was centralized to the University of Helsinki. For the determination of the molecular subgroups, stainings for CD10, Bcl-6, MUM1, FOXP1, and BCL-2 were carried out as previously described.21, 22, 23 Both Hans algorithm and modified activated B-cell-like classification were used. According to the Hans algorithm,24 the cases were assigned to germinal center phenotype, if CD10 alone or both CD10 and Bcl-6 were positive. If both CD10 and Bcl-6 were negative, the case was considered to belong to the non-germinal center subgroup. MUM1/IRF4 expression determined the subgroup in cases where CD10 was negative, and Bcl-6 positive: a MUM1/IRF4-positive case was assigned to non-germinal center subgroup whereas a MUM1/IRF4-negative case belonged to the germinal center subgroup. In the modified activated B-cell-like classification,23 the cases expressing MUM1/IRF4 or FOXP1 were considered to belong to the activated B-cell-like subgroup, and the rest of the cases were assigned as others.
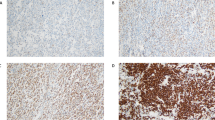
For PKC-β II staining, the samples were dehydrated and deparaffinized according to standard procedures. Heat-induced antigen retrieval was performed in an autoclave in sodium citrate and then washed with phosphate-buffered saline. The slides were incubated overnight with a monoclonal antibody to PKC-β II (Serotec, Oxford, UK) in a 1:200 dilution. The immunoreactions were visualized with avidin–biotin–peroxidase method and sections stained with Mayer hematoxylin. Immunoreactivity was determined without knowledge of the clinical data. The PKC-β II-positive cells were counted per high-power fields ( × 1000 magnification) as absolute cell numbers with Olympus BH-2 brightfield microscope (Olympus America Inc, Melville, NY, USA). Ten fields were counted on each slide. The counting results were averaged. The staining was even in most cases. In cases with uneven staining the areas showing higher positivity were analyzed.
Statistical Analysis
A χ2-test was performed to evaluate the differences in the frequency for the prognostic factors. Survival rates were estimated using the Kaplan–Meier method and the differences between the subgroups were compared with the log-rank test. Overall survival was determined from the time of diagnosis to death or date of last follow-up. Failure-free survival was calculated as the period between diagnosis and date of relapse or death of any cause. Both univariate and multivariate analysis were performed according to the Cox proportional hazards regression model. All P-values were two-tailed. A significant level of probability was considered as less than 0.05. The statistical analyses were carried out with SPSS 16.0 for Windows (SPSS, Chicago, IL, USA).
Results
Associations Between Clinical Characteristics, PKC-β II Expression, and Cell of Origin
The baseline characteristics of the 95 patients in the immunohistochemistry group are shown in Table 1. The median age of the cohort was 61 years (range, 18–84 years). All patients received R-CHOP or R-CHOEP regimen. The median follow-up of the surviving patients was 43 months (range, 16–75 months). In total, 17 patients had died at the time of last follow-up. The predicted 3-year overall and failure-free rates for the entire group were 84 and 76%, respectively.
The patients were further divided into two subgroups based on immunohistochemically defined PKC-β II expression in their diffuse large B-cell lymphoma tissue (Table 1). The immunohistochemical analysis of PKC-β II showed variation from complete absence to intense reactivity. With respect to intracellular compartmentalization, PKC-β II immunoreactivity was localized to cell membrane and cytoplasm. The median level for PKC-β II-positive lymphoma cells per high power field was 16 (range 0–76). The number for the lowest tertile was 13 and the lowest quartile 11. If the positivity was determined as a percentage of PKC-β II-positive cells from all lymphoma cells, the values corresponding to median, the lowest tertile, and the lowest quartile were 15, 13, and 11%, respectively. When the lowest tertile was used to divide the patients into PKC-β II-low (median 9, range 0–13) and PKC-β II-high (median 22, range 13–76) groups, no significant differences were observed in the frequency of clinical characteristics between the groups (Table 1). However, the PKC-β II-low group tended to contain more females than males. Conversely, the PKC-β II-high group tended to have more males.
In Table 1, the patient characteristics are also presented in relation to molecular subgroups. The distributions of germinal center and non-germinal center phenotypes according to Hans algorithm, modified activated B-cell-like classification, and BCL-2 positivity were as previously reported.21, 22, 23, 24 Furthermore, no association between these molecular features and PKC-β II expression was found.
Survival Analyses
A significant inverse relationship of PKC-β II expression and prognosis was observed. In Kaplan–Meier analyses, the lowest tertile (33%), rather than the lowest quartile (25%) or the median (50%), values were found to be the best discriminator between the subgroups with different outcomes (Table 2). The 3-year overall survival was 94% among the patients who had a diffuse large B-cell lymphoma with low PKC-β II expression (≤33%, n=32), and 76% when the expression was high (>33%, n=63; P=0.036; Figure 1a, Table 2). Likewise, the failure-free survival rates for the patients with low and high PKC-β II expression were 84 and 70% (P=0.108), respectively (Figure 1b, Table 2). Together, the results suggest that the expression of PKC-β II in the lymphoma tissue correlates with the survival of diffuse large B-cell lymphoma patients treated with rituximab and chemotherapy.
Survival rates for diffuse large B-cell lymphoma patients according to PKC-β II expression. (a) Overall survival of all patients according to low (n≤33%; n=32) and high (n>33%; n=63) PKC-β II expression level. (b) Failure-free survival of the patients according to low and high PKC-β II expression level.
The estimated survival proportions according to the International Prognostic Index scores are shown in Figure 2a. The 3-year overall survival for the patients with low International Prognostic Index (0–2) at diagnosis was 88%, and for the ones with high scores (3–5) 76% (P=0.029). To determine whether PKC-β II expression could improve the prognostic value of the International Prognostic Index, we estimated the PKC-β II-related overall survival separately for the patients with low and high scores. After stratification, PKC-β II expression retained its prognostic impact on the survival (P=0.074; Figure 2b and c), but the difference was more evidently seen in the subgroup of patients with high International Prognostic Index.
Survival rates according to PKC-β II expression and International Prognostic Index (IPI) scores. (a) Overall survival according to low and high IPI scores (0–2 vs 3–5). (b) Overall survival as a function of PKC-β II status in IPI low (0–2) subgroup. (c) Overall survival as a function of PKC-β II status in the IPI high (3–5) subgroup.
Survival analyses according to modified activated B-cell classification showed 3-year overall survival of 93% for the patients with activated B-cell-like phenotype in comparison to 77% for the others (P=0.064; Figure 3a). In comparison, no difference in survival was observed between germinal and non-germinal center phenotypes when Hans classifier was used (P=0.947). The results are compatible with our previous data.21, 23 When the PKC-β II-associated outcome was adjusted for the activated B-cell classification, the difference in overall survival rates was seen in both activated B-cell-like and other patients (P=0.030; Figure 3b and c).
Survival rates according to PKC-β II expression and cell of origin. (a) Overall survival according to modified activated B-cell-like classification. (b) Overall survival according to PKC-β II expression in the activated B-cell-like lymphomas. (c) Overall survival according to PKC-β II expression in the non-activated B-cell lymphomas.
To further investigate the prognostic impact of PKC-β II expression, it was entered into a multivariate survival analysis together with the International Prognostic Index. In this model, both factors remained borderline significant predictors for overall survival (International Prognostic Index, risk ratio=2.45; 95% CI 0.93–6.47; P=0.070; PKC-β II expression, risk ratio=3.77; 95% CI 0.86–16.62; P=0.079).
To find support for the immunohistochemical data, we analyzed the prognostic significance of PKC-β II mRNA expression from the Lymphoma/Leukemia Molecular Profiling Project microarray data set.11 The median follow-up of the surviving patients was 33 months (range, 2–123 months). In total, 60 patients (26%) had died at the time of last follow-up. The predicted 3-year overall survival for the entire group was 73%. Consistent with the protein analyses, the cutoff level for the lowest tertile (33%) rather than the median (50%), or the lowest quartile (25%) was found to best discriminate the outcomes between the low and high PKC-β II subgroups (Table 2). The 3-year overall survival for the patients with low PKC-β II mRNA levels (≤33%, n=77) was 84% compared with 68% of those with higher levels (>33%, n=156; P=0.005; Figure 4, Table 2).
To study the association of PKC-β II mRNA expression with germinal center and activated B-cell molecular entities, we excluded the patients with unclassified molecular subtypes according to their gene expression profiles from the analyses. Accordingly, the overall survival rates in the remaining 200 patients for the low and high PKC-β II groups were 84 and 64% (P=0.002). When the PKC-β II-associated survival was stratified for the molecular subtypes, the difference in overall survival rates was seen both in the germinal center and activated B-cell subgroups (P=0.024). In multivariate analysis with cell of origin, both factors had independent prognostic value for overall survival (PKC-β II transcripts (low vs high), risk ratio=2.22; 95% CI, 1.102–4.463; P=0.026; Cell of origin (germinal center vs activated B-cell), risk ratio=2.90; 95% CI 1.600–5.244; P<0.001).
Discussion
In this study, we show that PKC-β II expression has prognostic impact on the survival of immunochemotherapy-treated diffuse large B-cell lymphoma patients. Immunohistochemically defined low PKC-β II protein levels allowed us to select a subgroup of diffuse large B-cell lymphoma patients with extremely favorable prognosis. Gene expression analysis of an independent patient cohort supported our immunohistochemical findings. All together, the data show that PKC-β II expression predicts outcome both at protein and mRNA levels and in two independent patient cohorts. This encourages us to believe that a novel prognostic factor for immunochemotherapy-treated diffuse large B-cell lymphoma patients has been identified. The study has the limitation of being retrospective in nature. If confirmed in a prospective clinical trial, the findings would have both clinical and economical value, because they could be used to direct biological therapy only to the subgroups of diffuse large B-cell lymphoma patients.
To date, International Prognostic Index is the strongest prognostic factor in diffuse large B-cell lymphoma.6 The patients with high International Prognostic Index scores have poor prognosis, as approximately half of them relapse and ultimately die for the disease even if they have received rituximab-containing therapies. Nevertheless, the clinical outcome of high-risk patients is heterogeneous, and includes patients whose outcome is comparable with low-risk patients. Those are mainly cases displaying favorable molecular features, such as BCL-2 negativity and germinal center phenotype.11, 12, 15, 22, 23 Unfortunately, efforts to validate the prognostic function of the gene expression-based cell of origin classification immunohistochemically in the rituximab era have provided contradictory findings, and the issue has remained unresolved.21, 23, 25, 26 In this study, we found a new indicator for a favorable prognosis in the patients treated with immunochemotherapy. The patients with low PKC-β II expression had a significantly better survival than the patients with high PKC-β II levels. Only 16% of diffuse large B-cell lymphoma patients with low PKC-β II protein levels relapsed during 43 months follow-up in comparison to 30% of the patients in the high PKC-β II group. The difference was seen in both International Prognostic Index low- and high-risk patients and in all studied molecular subgroups.
In diffuse large B-cell lymphoma, PKC-β II expression has been associated with unfavorable prognosis in chemotherapy-treated patients.9, 17, 18, 19, 20 Our findings on immunochemotherapy-treated patients are consistent with previous studies, and further suggest that addition of rituximab to chemotherapy does not interfere with the PKC-β II-associated prognosis. In our study, both mRNA and protein levels of PKC-β II had predictive value. Furthermore, in both analyses the lowest tertile within the cohort was found to best discriminate the patients between favorable and adverse outcome. The findings are important in several ways. First, they show that it is possible to identify a cutoff level, which allows the comparison of PKC-β II-associated survival between different patient populations. A comparison of the data with previous immunohistochemical studies appears to be more difficult, because the cut points that best discriminate the low and high, or negative and positive subgroups have differed considerably (from 5 to 50%) in these series.17, 18, 20 For example, the lowest quartile and tertile in our cohort corresponding to positivity of 11 and 13% of all lymphoma cells is close to the 10% cutoff level used by Schaffel et al,20 but significantly lower than the 50% cut point reported by Hans et al.18 It seems obvious that different patient cohorts have intrinsic properties accounting for the outcome. Due to technical differences, a better standardization of the immunohistochemical methodology is also needed. Nevertheless, our findings provide one of the first biologically relevant prognostic factor, which can be measured both immunohistochemically and by gene expression-based methods. Finally, the data offer a rationale for clinical studies with PKC-β inhibitors in diffuse large B-cell lymphoma.
The mechanism by which PKC-β II expression impairs the survival of diffuse large B-cell lymphoma patients is currently unknown but likely to be related to its capacity to activate signaling pathways that are critical for proliferation and angiogenesis.27 In diffuse large B-cell lymphoma, the most compelling evidence for the function of PKC-β II in lymphomagenesis has been received from previous gene expression studies, showing that PKC-β II is among the genes differentially expressed in diffuse large B-cell lymphoma tissue of the patients who are not cured with standard chemotherapy.9 Further support for an adverse function for PKC-β II expression in diffuse large B-cell lymphoma has been obtained from in vitro studies showing that cultured diffuse large B-cell lymphoma cells with PKC-β II overexpression undergo apoptosis when treated with the PKC-β-selective inhibitor.28 Of note, the subsequent clinical study has showed that PKC-β inhibitor, enzastaurin, exhibits some single-agent activity in relapsed/refractory diffuse large B-cell lymphoma.29
In conclusion, immunohistochemical analyses of PKC-β II expression allowed us to distinguish diffuse large B-cell lymphoma patients with different outcomes after immunochemotherapy. This together with the reproducibility of the prognostic impact of PKC-β II expression at the mRNA level in an independent patient cohort provides, to our knowledge, one of the first candidates for a biomarker in the post-rituximab era of lymphoma therapies that can be measured both immunohistochemically and by gene expression-based methods. It appears that the outcome of the patients with low PKC-β II levels is outstanding in response to immunochemotherapy. In contrast, association of PKC-β II expression with poor outcome defines a group of patients who require additional treatments. Considering that PKC-β inhibitor, enzastaurin has advanced to phase III clinical trial of primary diffuse large B-cell lymphoma, it will be interesting to see whether this expression data will be relevant from a therapeutic standpoint.
References
Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235–242.
Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006;7:379–391.
Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008;9:105–116.
Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 2005;23:5027–5033.
Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 2006;24:3121–3127.
IPI-Project. A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993;329:987–994.
Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503–511.
Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937–1947.
Shipp MA, Ross KN, Tamayo P, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med 2002;8:68–74.
Jais JP, Haioun C, Molina TJ, et al. The expression of 16 genes related to the cell of origin and immune response predicts survival in elderly patients with diffuse large B-cell lymphoma treated with CHOP and rituximab. Leukemia 2008;22:1917–1924.
Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 2008;359:2313–2323.
Rimsza LM, Leblanc ML, Unger JM, et al. Gene expression predicts overall survival in paraffin-embedded tissues of diffuse large B-cell lymphoma treated with R-CHOP. Blood 2008;112:3425–3433.
Lossos IS, Czerwinski DK, Alizadeh AA, et al. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med 2004;350:1828–1837.
Monti S, Savage KJ, Kutok JL, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood 2005;105:1851–1861.
Malumbres R, Chen J, Tibshirani R, et al. Paraffin-based 6-gene model predicts outcome in diffuse large B-cell lymphoma patients treated with R-CHOP. Blood 2008;111:5509–5514.
Griner EM, Kazanietz MG . Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer 2007;7:281–294.
Espinosa I, Briones J, Bordes R, et al. Membrane PKC-beta 2 protein expression predicts for poor response to chemotherapy and survival in patients with diffuse large B-cell lymphoma. Ann Hematol 2006;85:597–603.
Hans CP, Weisenburger DD, Greiner TC, et al. Expression of PKC-beta or cyclin D2 predicts for inferior survival in diffuse large B-cell lymphoma. Mod Pathol 2005;18:1377–1384.
Li S, Phong M, Lahn M, et al. Retrospective analysis of protein kinase C-beta (PKC-beta) expression in lymphoid malignancies and its association with survival in diffuse large B-cell lymphomas. Biol Direct 2007;2:8.
Schaffel R, Morais JC, Biasoli I, et al. PKC-beta II expression has prognostic impact in nodal diffuse large B-cell lymphoma. Mod Pathol 2007;20:326–330.
Nyman H, Adde M, Karjalainen-Lindsberg ML, et al. Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B-cell lymphoma patients treated with immunochemotherapy. Blood 2007;109:4930–4935.
Nyman H, Jerkeman M, Karjalainen-Lindsberg ML, et al. Bcl-2 but not FOXP1, is an adverse risk factor in immunochemotherapy treated non-germinal center diffuse large B-cell lymphomas. Eur J Haematol 2009;82:364–372.
Nyman H, Jerkeman M, Karjalainen-Lindsberg ML, et al. Prognostic impact of activated B-cell focused classification in diffuse large B-cell lymphoma patients treated with R-CHOP. Mod Pathol 2009;22:1094–1101.
Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–282.
Fu K, Weisenburger DD, Choi WW, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol 2008;26:4587–4594.
Held G, Pfreundschuh M . Hematology: germinal center or nongerminal center DLBCL? Nat Rev Clin Onc 2009;6:188–190.
Graff JR, McNulty AM, Hanna KR, et al. The protein kinase Cbeta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res 2005;65:7462–7469.
Su TT, Guo B, Kawakami Y, et al. PKC-beta controls I kappa B kinase lipid raft recruitment and activation in response to BCR signaling. Nat Immunol 2002;3:780–786.
Robertson MJ, Kahl BS, Vose JM, et al. Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2007;25:1741–1746.
Acknowledgements
This work was supported by the grants from the Finnish Academy of Sciences, Finnish Cancer Societies, Sigrid Juselius Foundation, University of Helsinki, and Helsinki University Central Hospital to SL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Riihijärvi, S., Koivula, S., Nyman, H. et al. Prognostic impact of protein kinase C β II expression in R-CHOP-treated diffuse large B-cell lymphoma patients. Mod Pathol 23, 686–693 (2010). https://doi.org/10.1038/modpathol.2010.43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.43
Keywords
This article is cited by
-
Maintenance Therapy in Diffuse Large B Cell Lymphoma and Mantle Cell Lymphoma
Current Treatment Options in Oncology (2018)
-
Novel drug targets for personalized precision medicine in relapsed/refractory diffuse large B-cell lymphoma: a comprehensive review
Molecular Cancer (2015)
-
Emerging Therapeutic Targets in Diffuse Large B-Cell Lymphoma
Current Treatment Options in Oncology (2012)