Abstract
MYC oncogene rearrangements (MYC-R) negatively affect survival in patients with Ann Arbor stage III–IV diffuse large B-cell lymphoma (DLBCL), but their impact in limited stage (LS) I–II is unclear. Therefore, we assessed the impact of MYC-R on progression-free survival (PFS) and overall survival (OS) in LS DLBCL patients at the population level. We identified 1,434 LS DLBCL patients with known MYC-R status diagnosed between 2014 and 2020, who received R-CHOP(-like) regimens using the Netherlands Cancer Registry, with survival follow-up until February 2022. Stage I patients with (n = 83, 11%) and without (n = 650, 89%) a MYC-R had similar 2-years PFS (89% and 93%, p = 0.63) and OS (both 95%, p = 0.22). Conversely, stage II DLBCL patients with a MYC-R (n = 90, 13%) had inferior survival outcomes compared to stage II patients without a MYC-R (n = 611, 87%) (PFS 70% vs. 89%, p = 0.001; OS 79% vs. 94%, p < 0.0001). Both single MYC-R (single hit, n = 36) and concurrent BCL2 and/or BCL6 rearrangements (double/triple hit, n = 39) were associated with increased mortality and relapse risk. In conclusion, in stage II DLBCL a MYC-R is negatively associated with survival. In stage I DLBCL, however, survival outcomes are excellent irrespective of MYC-R status. This challenges the diagnostic assessment of MYC-R in stage I DLBCL patients.

Similar content being viewed by others
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma and is generally treated with immunochemotherapy R-CHOP (rituximab, cyclofosphamide, doxorubicin, vincristine, and prednisone) [1,2,3]. However, the clinical outcomes of DLBCL patients are heterogeneous, which can at least partially be attributed to the variety in the genetic landscape in DLBCL patients [4,5,6]. A translocation of the MYC oncogene, detected with fluorescent in situ hybridization (FISH) in approximately 10–15% of all DLBCL cases, is one of the genetic aberrations associated with inferior prognosis [7].
The 5-year overall survival (OS) in patients with a MYC rearrangement (MYC-R) ranges from 35–55% as compared to 72% in patients without a MYC-R [8, 9]. Five-year progression-free survival (PFS) is lower in patients with a MYC-R (31–55%) compared to patients without a MYC-R (66%) [8, 9]. However, these associations are mainly observed in patients with advanced-stage DLBCL (Ann Arbor stage III–IV) [8, 9].
Impaired survival is most prominent in patients with a MYC-R combined with a rearrangement of the BCL2 and/or BCL6 gene (so-called ‘double hit’ [DH] and/or ‘triple hit’ [TH] high-grade B-cell lymphoma (DH/TH HGBL), especially when the fusion partner of MYC is the IgH locus [9]. To improve survival outcomes, advanced-stage DH/TH HGBL patients are usually treated with more intensive immunochemotherapeutic regimens, such as DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), or R-CHOP plus lenalidomide, although randomized controlled trials supporting this are lacking [10, 11]. Notably, in the aforementioned studies, stage II patients were grouped together with stage III and IV. As a result, the distinct prognosis for stage II patients remains unexplored. Besides, it remains unclear whether differences in clinical course between limited (stage I and II) and advanced-stage DLBCL should be attributed to early disease detection or to distinct biologic features, including the prognostic impact of a MYC-R [12].
Two previous studies where limited-stage (LS) DLBCL was defined as stage I or stage II, showed that the complete remission (CR) rate in DH patients was lower, but survival rates were similar as compared to patients without a DH (2-year PFS 74% and 78%, and 2-year OS 81% and 86%, respectively) [13, 14]. A third study reported an inferior relapse-free survival in DH/TH compared to non-DH/TH DLBCL [15]. However, due to the limited number of patients with a MYC-R without any distinction between stage I and stage II DLBCL, the impact of MYC-R on survival in stage I and stage II separately remains uncertain. Therefore, the aim of this study was to assess the impact of an MYC-R on survival outcomes for stage I and stage II DLBCL patients in the Netherlands.
Methods
Registry and study population
The nationwide population-based Netherlands Cancer Registry (NCR) is maintained and hosted by the Netherlands Comprehensive Cancer Organization (IKNL) and has nationwide coverage of at least 95% of all malignancies since 1989 [16]. The NCR relies on comprehensive case notification through the Nationwide Histopathology and Cytopathology Data Network and the Nationwide Registry of Hospital Discharges (i.e., inpatient and outpatient discharges). Information on topography and morphology, hospital, type of diagnosis, the World Health Organization (WHO) performance score, LDH level, and presence of MYC, BCL2, and/or BCL6 rearrangements, and first-line therapy is routinely recorded by trained registrars of the NCR through retrospective medical records review. Information on the last known vital status for all patients (i.e., alive, dead, or emigration) is obtained through annual linkage with the Nationwide Population Registries Network that holds vital statistics on all residents of the Netherlands.
Patients aged ≥18 years with de novo stage I(E) or stage II DLBCL diagnosed between January 1st, 2014 and December 31st, 2020 were identified in the Netherlands Cancer Registry (NCR), using the International Coding System of Disease—Oncology (ICD-O) of the WHO, morphology code 9680/3. Stages I and II were defined according to the Ann Arbor staging system, determined by physician assessment. The increased use of FDG-PET and CT for the staging of aggressive lymphoma helps to more accurately distinguish those patients who truly have stage I from those who have stage II disease from 2014 onwards. However, it was not until 2018 that FDG-PET and CT were implemented as diagnostic tools at the national level. Patients with an unknown MYC-R status (n = 1792, 53%), and patients who received treatment other than R-CHOP-like regimens (n = 184, Supplementary Table 1) were excluded, leaving 1434 patients for all analyses (Fig. 1).
In the current study, patients with only a MYC-R were categorized as single hit (SH) B-cell lymphoma, whereas patients with a BCL2 or BCL6 rearrangements in addition to MYC-R were defined as double hit (DH) HGBL and patients with MYC, BCL2 and BCL6 rearrangements as triple hit (TH) HGBL according to the revised 4th edition of the WHO classification (2016) [17].
Patients were divided into 4 R-CHOP(-like) treatment groups: 1) 6–8 cycles R-CHOP, 2) abbreviated (3 cycles) R-CHOP plus radiotherapy (RT), 3) less intensive R-CHOP-like regimens, such as R-miniCHOP, rituximab combined with etoposide instead of doxorubicine (R-CEOP) or without etoposide or doxorubicin (R-CVP), and 4) more intensive R-CHOP-like regimens, such as rituximab combined with cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisolone (R-CHOEP), and dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R).
According to the Central Committee on Research Involving Human Subjects in the Netherlands (CCMO), this type of observational study does not require the approval of an ethics committee. The use of anonymized data for this study has been approved by the NCR Privacy Review Board.
Endpoints
The primary endpoint of this study was overall survival (OS) defined as the time between the date of diagnosis and death by any cause. Secondary endpoints were progression-free survival (PFS) and best response. PFS was defined as the time between initial diagnosis and relapse or death by any cause. The best response, i.e., complete remission (CR), or stable/progressive disease (SD/PD) to first-line treatment, was determined through physician assessment and routinely collected by trained registrars of the NCR through retrospective medical record review.
Statistical analysis
Analyses were performed separately for patients with stage I and stage II disease, as stage I patients more commonly receive abbreviated chemotherapy with RT. Comparisons between patients with and without a MYC-R were made using the Pearson χ2 test and the Kruskall–Wallis test for categorical and continuous variables, respectively. Kaplan–Meier estimates were used to analyze OS and PFS between patients with and without a MYC-R. Survival differences between patients with and without a MYC-R were performed using a log-rank test. Survival follow-up was cut off on February 1st, 2022. Patients diagnosed between 2014 and 2018 were actively followed for the occurrence of relapse, while patients diagnosed in 2019 or 2020 were not. As a consequence, only relapses within 1 year post-diagnosis were identified for patients diagnosed in 2019 or 2020. Therefore, patients diagnosed in 2019 or 2020 and alive without relapse were censored at 1 year of follow-up.
The impact of MYC status on risk of mortality and relapse was evaluated by using a multivariable Cox proportional hazard regression analysis, thereby evaluating age per year increment, sex, serum LDH, WHO performance score, number of extranodal sites, and treatment received as covariables. The results from the Cox regression analyses produced hazard ratios (HRs) with associated 95% confidence intervals (Cl). The proportional hazard assumption was tested based on the Schoenfeld residuals. All variables were introduced in the multivariable regression model simultaneously, thereby using a backward selection method to exclude stepwise covariables with a p-value below 0.05.
To investigate the potential benefit of intensive chemotherapy regimens in MYC-R patients, we performed a sensitivity analysis including stage II patients who were treated with R-CHOP (n = 57) or treated with more intensive chemotherapy regimens (n = 22).
p-values < 0.05 were considered statistically significant. All statistical analyses were performed in R versions 4.0.3 and 4.2.2.
Results
In total, 1,434 LS DLBCL patients with known MYC-R status, diagnosed between 2014 and 2020, and treated with R-CHOP(-like) regimens, were identified in the NCR including 733 (51%) stage I patients and 701 (49%) stage II patients (Fig. 1).
Clinical characteristics of stage I DLBCL patients
In stage I patients, 83 (11%) had a MYC-R of whom 36 (43%) were DH/TH HGBL and 18 (22%) had unknown BCL2 and BCL6 status (Fig. 1 and Table 1). MYC-R patients were more often male (p < 0.01), but other baseline characteristics did not significantly differ between patients with and without a MYC-R (Table 1).
Out of the 83 MYC-R stage I patients, 40 patients received 3 cycles of R-CHOP plus RT (49%), and 35 received 6–8 cycles of R-CHOP (42%, Supplementary Fig. 1A and Supplementary Table 2). Three patients received less intensive chemotherapy (4%) and five patients received more intensive chemotherapy regimens (6%). Among the 650 patients without an MYC-R, similar treatment distributions were observed: 326 received 3 cycles of R-CHOP plus RT (50%), 275 patients received 6–8 cycles of R-CHOP (42%), and the remaining patients received less intensive chemotherapy (48,7%), or a more intensive chemotherapy regimen (n = 1; 0.2%).
Outcome of stage I DLBCL patients
For stage I patients, CR rates were both 89% for patients with and without a MYC-R (p = 0.58).
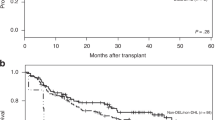
Median follow-up was similar between patients with (41 months) and without a MYC-R (47 months, p = 0.24). The 2-year PFS in patients with stage I disease with and without a MYC-R was similar (89% (95% CI 82–96%) and 93% (95% CI 91–95%), respectively, p = 0.63; Fig. 2A). Two-year OS was 95% (95% CI 93–97%) for MYC-R patients as well as for patients without a MYC-R (95% CI 92-100%, p = 0.22; Fig. 2B).
In a multivariable analysis, where we assessed the impact of MYC and BCL2 and/or BCL6 rearrangements on the risk of mortality and relapse, SH and DH/TH were not associated with risk of mortality (Supplementary Table 3) or risk of relapse (Supplementary Table 4) compared to patients without a MYC-R.
Older age, elevated LDH, WHO performance score 2–4, and male gender were associated with a higher mortality risk (Supplementary Table 3) and relapse risk (Supplementary Table 4). Treatment with three cycles of R-CHOP plus RT was associated with a lower relapse risk as compared to 6–8 cycles of R-CHOP (Supplementary Table 4).
Clinical characteristics of stage II DLBCL patients
In stage II patients, 90 (13%) had a MYC-R of whom 39 (43%) were DH/TH HGBL and 15 (17%) had unknown BCL2 and BCL6 status (Fig. 1 and Table 2). Baseline characteristics did not differ between patients with and without MYC-R patients (Table 2).
Out of the 90 MYC-R stage I patients, 57 patients received 6–8 cycles of R-CHOP (63%). The remaining MYC-R patients received 3 cycles of R-CHOP plus RT (n = 3; 3%), less intensive chemotherapy (n = 8; 9%), or more intensive chemotherapy regimens (n = 22; 24%, Supplementary Fig. 1B and Supplementary Table 2). Among the 611 patients without a MYC-R, similar treatment distributions were observed, with 543 patients (89%) receiving 6–8 cycles of R-CHOP, and the remaining patients R-CHOP plus RT (n = 22; 3%), less intensive chemotherapy (n = 43; 7%) or more intensive chemotherapy regimens (n = 3; 1%).
Outcome of stage II DLBCL patients
CR rate was lower in stage II patients with a MYC-R compared to patients without a MYC-R (81% vs. 89%, respectively; p = 0.02). Median follow-up was similar between patients with (42 months) and without (33 months, p = 0.12) a MYC-R.
The 2-year PFS was lower in MYC-R patients compared to patients without a MYC-R (70% (95% CI 60–81%) and 89% (95% CI 86–91%), respectively, p = 0.0012; Fig. 3A).
The 2-year OS was also lower in MYC-R patients compared to patients without a MYC-R (79% (95% CI 70–88%) vs. 94% (95% CI 92–96%), respectively, p < 0.0001; Fig. 3B).
Within MYC-R patients, SH and DH/TH patients had a comparable 2-year PFS (60% (95% CI 43–84%) and 70% (95% CI 56% and 87%), respectively, p = 0.83, Supplementary Fig. 2A) and OS (78% (95% CI 65–94%) and 73% (95% CI 60–89%), p = 0.57, Supplementary Fig. 2B).
In a multivariable analysis, SH and DH/TH were associated with a higher risk of mortality (HR 2.27, 95% CI 1.12–4.59, p = 0.02, and HR 3.55, 95% CI 1.90–6.65, p < 0.01, respectively, Supplementary Table 5) and risk of relapse (HR 2.20, 95% CI 1.17–4.14, p = 0.01, and HR 2.08, 95% CI 1.14–3.79, p = 0.02, respectively, Supplementary Table 6) compared to patients without a MYC-R.
Furthermore, involvement of ≥1 extranodal site was associated with a higher mortality risk and older age was associated with a higher mortality and relapse risk (Supplementary Tables 5 and 6).
Outcome of stage II DLBCL MYC-R patients treated with more intensive chemotherapy
Given the inferior outcomes of stage II MYC-R DLBCL patients, we aimed to investigate the potential benefit of intensive chemotherapy regimens in MYC-R patients in a sensitivity analysis. Survival outcomes in MYC-R patients treated with more intensive treatment regimens (n = 22) were compared to patients treated with 6–8 cycles of R-CHOP (n = 57). Median follow-up time was similar between the two groups (Table 3). The 2-year PFS and 2-year OS did not differ between the two treatment groups (p = 0.82, Fig. 4A, and p = 0.69, Fig. 4B, respectively). In DH/TH patients (n = 18 in both treatment groups, Table 4), the 2-year PFS and 2-year OS did not differ between the two treatment groups either (p = 0.96 Fig. 4C, and p = 0.88, Fig. 4D, respectively).
A, B Progression-free survival (A) and overall survival (B) analyses in stage II MYC-R DLBCL patients including single hit and double/triple hit lymphoma treated with R-CHOP (gray, n = 57) or more intensive chemotherapy regimens (red, n = 22), using Kaplan–Meier survival analysis. C, D Progression-free survival (C) and overall survival (D) analyses in stage II DLBCL double hit patients treated with R-CHOP (gray, n = 18) or more intensive chemotherapy regimens (red, n = 18), using Kaplan–Meier survival analysis.
Discussion
In this large population-based study, we demonstrated that a MYC-R is a negative prognostic factor for survival outcomes in stage II, but not in stage I DLBCL.
While a rearrangement of the MYC oncogene has been widely recognized as a negative prognostic factor in advanced-stage (III–IV) DLBCL, its impact in limited-stage (I–II) DLBCL remains unclear. Previous studies evaluating the impact of MYC on survival include a limited number of patients and did not distinguish stage I and stage II disease, or focused mainly on DH patients [13, 14]. In this study, separate analyses were performed for stage I and stage II patients. This approach allowed us to dissect the relevance of a MYC-R and its clinical consequences for each disease stage.
Our results demonstrate that stage I MYC-R patients, including those with DH/TH disease, have an excellent survival outcome comparable to patients without a MYC rearrangement, which is in line with a previous report involving limited-stage DH DLBCL [14]. Besides, the excellent outcome of stage I MYC-R patients in our cohort is similar to outcomes of limited-stage DLBCL patients treated with R-CHOP(-like) regimens as described in earlier cohorts [18, 19]. This challenges the necessity to perform MYC-R screening by FISH in patients with stage I DLBCL disease. This should be carefully streamlined on a per-hospital basis since the Ann Arbor stage is not always known prior to biopsy. In stage II (and beyond), screening for a MYC-R is inevitable due to its prognostic significance.
The excellent outcome of stage I MYC-R DLBCL patients in this study does not justify treatment with more intensive immunochemotherapy regimens. Instead, we propose the inclusion of stage I MYC-R DLBCL patients in potential future studies aiming to reduce treatment intensity.
In contrast, in stage II DLBCL, we found inferior survival outcomes in MYC-R patients. Multivariable analyses showed that SH and DH/TH both negatively affected mortality risk and relapse risk. However, due to the limited number of patients in the SH and DH/TH subgroups, no definitive conclusions can be made regarding survival differences between these groups.
The treatment landscape of DH/TH has evolved over time, resulting in more intensive chemotherapeutic regimens available [10].
In retrospective studies on MYC-R patients, intensification of immunochemotherapy treatment with regimens such as DA-EPOCH-R or R-CODOX-M/R-IVAC increased PFS [20] and OS, regardless of disease stage [21]. In a recent French retrospective cohort among patients with advanced-stage DH/TH lymphoma, intensive chemotherapy increased PFS, but not OS [22]. In a small subgroup of stage II MYC-R patients in our cohort, treatment with more intensive chemotherapeutic regimens did not seem to improve OS or PFS. Despite small patient numbers, this suggests that new treatment strategies for stage II MYC-R patients are needed.
The main strength of this study is the use of a large nationwide population-based cancer registry. In the absence of prospective randomized trials in limited-stage DLBCL patients, national registries offer the best data for population-based analysis of treatment outcomes. Our cohort represents the general DLBLC population, as older age, male gender, and characteristics of high disease burden (elevated LDH, involvement of ≥1 extranodal site, WHO performance score 2–4) were associated with a higher mortality or relapse risk in limited-stage DLBCL, as expected [23,24,25,26].
Besides, a nationwide, diagnostic screening assessment of the MYC rearrangement using FISH was implemented in the Netherlands [27], resulting in access to comprehensive clinical data on MYC status. Due to the increased recognition of MYC as a potential prognostic factor, the proportion of FISH analyses has increased over the years in stage I and II DLBCL from 18% in 2014 to 83% in 2020. Nevertheless, 1792 patients had to be excluded due to missing MYC status. Given the better prognosis of limited-stage disease compared to advanced-stage disease, FISH testing may be less frequently performed in limited-stage patients. We cannot rule out the possibility that excluding patients with missing MYC status has introduced some selection bias. Another limitation of this study is that the diagnostic criteria employed align with the revised 4th edition of the WHO classification [17]. Therefore, patients with MYC and BCL6 rearrangements were classified as DH HGBL, instead of the DLBCL-NOS subtype.
The considerable amount of missing WHO performance scores could have caused bias in the interpretation of the multivariable Cox regression analyses. However, the frequency of missing values was similar between MYC-R patients and patients without a MYC rearrangement in both stages. The lack of available data on toxicity and the cause of death prevents us from conclusively attributing survival differences in stage II MYC-rearranged patients vs. patients without a MYC rearrangement to either deficient treatment efficacy or an increased level of toxicity. Despite these limitations, national registries offer the best data for population-based analysis of treatment outcomes.
In conclusion, in stage II DLBCL a MYC-R is negatively associated with survival. MYC FISH is inevitable and should be used as guidance for new treatment strategies for these patients. In stage I DLBCL, however, survival outcomes of patients treated with R-CHOP(-like) regimens are excellent, irrespective of MYC-R status. This leaves no justification for more intensive treatment and challenges diagnostic MYC fluorescent in situ hybridization (FISH) in stage I DLBCL patients.
Data availability
The data that support the findings of this study are available via the Netherlands Comprehensive Cancer Organization (IKNL). These data are not publicly available, and restrictions apply to the availability of the data used for the current study. However, these data are available upon reasonable request and with the permission of IKNL.
References
Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36:1720–48. https://doi.org/10.1038/s41375-022-01620-2
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large B-cell lymphoma. N Engl J Med. 2002;346:235–42. https://doi.org/10.1056/NEJMoa011795
Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–5. https://doi.org/10.1182/blood-2010-03-276246
Reddy A, Zhang J, Davis NS, Moffitt AB, Love CL, Waldrop A, et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell. 2017;171:481–494.e415. https://doi.org/10.1016/j.cell.2017.09.027
Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378:1396–407. https://doi.org/10.1056/NEJMoa1801445
Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24:679–90. https://doi.org/10.1038/s41591-018-0016-8
Barrans S, Crouch S, Smith A, Turner K, Owen R, Patmore R, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol. 2010;28:3360–5. https://doi.org/10.1200/JCO.2009.26.3947
Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114:3533–7. https://doi.org/10.1182/blood-2009-05-220095
Rosenwald A, Bens S, Advani R, Barrans S, Copie-Bergman C, Elsensohn ME, et al. Prognostic significance of MYC rearrangement and translocation partner in diffuse large B-cell lymphoma: a study by the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol. 2019;37:3359–68. https://doi.org/10.1200/JCO.19.00743
Dunleavy K, Fanale MA, Abramson JS, Noy A, Caimi PF, Pittaluga S, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. The Lancet Haematology. 2018;5:e609–17. https://doi.org/10.1016/S2352-3026(18)30177-7
de Jonge AV, van Werkhoven E, Dinmohamed AG, Nijland M, Zwinderman AH, Bossuyt PM, et al. A non-randomized risk-adjusted comparison of lenalidomide + R-CHOP versus R-CHOP for MYC-rearranged DLBCL patients. Blood Cancer J. 2023;13. https://doi.org/10.1038/s41408-023-00854-2.
Rojek AE, Smith SM. Evolution of therapy for limited stage diffuse large B-cell lymphoma. Blood Cancer J. 2022;12:33 https://doi.org/10.1038/s41408-021-00596-z
Torka P, Kothari SK, Sundaram S, Li S, Medeiros LJ, Ayers EC, et al. Outcomes of patients with limited-stage aggressive large B-cell lymphoma with high-risk cytogenetics. Blood Adv. 2020;4:253–62. https://doi.org/10.1182/bloodadvances.2019000875
Barraclough A, Alzahrani M, Ettrup MS, Bishton M, van Vliet C, Farinha P, et al. COO and MYC/BCL2 status do not predict outcome among patients with stage I/II DLBCL: a retrospective multicenter study. Blood Adv. 2019;3:2013–21. https://doi.org/10.1182/bloodadvances.2019000251
Grass GD, Mills MN, Ahmed KA, Liveringhouse CL, Montejo ME, Robinson TJ, et al. Radiotherapy for early stage diffuse large B-cell lymphoma with or without double or triple hit genetic alterations. Leuk Lymphoma. 2019;60:886–93. https://doi.org/10.1080/10428194.2018.1506586
Schouten LJ, Hoppener P, van den Brandt PA, Knottnerus JA, Jager JJ. Completeness of cancer registration in Limburg, The Netherlands. Int J Epidemiol. 1993;22:369–76. https://doi.org/10.1093/ije/22.3.369
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90. https://doi.org/10.1182/blood-2016-01-643569
Lamy T, Damaj G, Soubeyran P, Gyan E, Cartron G, Bouabdallah K, et al. R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood. 2018;131:174–81. https://doi.org/10.1182/blood-2017-07-793984
Persky DO, Li H, Stephens DM, Park SI, Bartlett NL, Swinnen LJ, et al. Positron emission tomography-directed therapy for patients with limited-stage diffuse large B-cell lymphoma: results of Intergroup National Clinical Trials Network Study S1001. J Clin Oncol. 2020;38:3003–11. https://doi.org/10.1200/JCO.20.00999
Petrich AM, Gandhi M, Jovanovic B, Castillo JJ, Rajguru S, Yang DT, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124:2354–61. https://doi.org/10.1182/blood-2014-05-578963
El-Sharkawi D, Sud A, Prodger C, Khwaja J, Shotton R, Hanley B, et al. A retrospective study of MYC rearranged diffuse large B-cell lymphoma in the context of the new WHO and ICC classifications. Blood Cancer J. 2023;13:54 https://doi.org/10.1038/s41408-023-00827-5
Laude MC, Lebras L, Sesques P, Ghesquieres H, Favre S, Bouabdallah K, et al. First-line treatment of double-hit and triple-hit lymphomas: survival and tolerance data from a retrospective multicenter French study. Am J Hematol. 2021;96:302–11. https://doi.org/10.1002/ajh.26068
International Non-Hodgkin’s Lymphoma Prognostic Factors P. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–94. https://doi.org/10.1056/NEJM199309303291402
Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–61. https://doi.org/10.1182/blood-2006-08-038257
Yildirim M, Kaya V, Demirpence O, Paydas S. The role of gender in patients with diffuse large B cell lymphoma treated with rituximab-containing regimens: a meta-analysis. Arch Med Sci. 2015;11:708–14. https://doi.org/10.5114/aoms.2015.53289
Eyre TA, Martinez-Calle N, Hildyard C, Eyre DW, Plaschkes H, Griffith J, et al. Male gender is an independent predictor for worse survival and relapse in a large, consecutive cohort of elderly DLBCL patients treated with R-CHOP. Br J Haematol. 2019;186:e94–e98. https://doi.org/10.1111/bjh.15927
Chamuleau MED. First Report on a successful screening program for MYC rearrangements and a prospective clinical trial based on MYC rearrangement in newly diagnosed DLBCL patients in the Netherlands. Blood. 2017;130:4144 https://doi.org/10.1182/blood.V130.Suppl_1.4144.4144
Acknowledgements
The authors would like to thank the registrars of the NCR for their dedicated data collection. The nationwide, population-based NCR is maintained and hosted by the Netherlands Comprehensive Cancer Organization (IKNL). The graphical abstract was created with Biorender.com.
Author information
Authors and Affiliations
Contributions
AVDJ, JAAB, MN, MEDC, and MB designed the research. AVDJ, JAAB, and DEFK analyzed the data and discussed the results with MN, MEDC, and MB. AVDJ, JAAB, and MB wrote the first draft of the manuscript; and all authors critically revised and modified the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
AVDJ, JAAB, DEFK, MB: nothing to disclose. MEDC: received research support from AbbVie, Genmab, BMS, and Gilead and has an advisory role ad AbbVie, Novartis, and Incyte. MN: received research support from Takeda and has an advisory role ad AbbVie.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Jonge, A.V., Bult, J.A.A., Karssing, D.F.E. et al. A MYC-rearrangement is a negative prognostic factor in stage II, but not in stage I diffuse large B-cell lymphoma. Blood Cancer J. 14, 2 (2024). https://doi.org/10.1038/s41408-023-00971-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-023-00971-y