Abstract
Subclassification of intraductal papillary mucinous neoplasms of the pancreas (IPMNs), based on morphological features and immunohistochemical profiles, has been proposed. Intestinal-type IPMNs frequently show moderate to severe dysplasia. Regenerating islet-derived family, member 4 (REG4) is associated with the adenoma-carcinoma sequence in colon cancer and it is also associated with intestinal phenotype. Therefore, to identify REG4 expression in IPMNs may be helpful to detect high-grade IPMNs. We also investigated REG4 expression and CDX2 expression in IPMNs. To investigate the expressions of REG4 and CDX2 in IPMNs and in invasive ductal adenocarcinoma derived from IPMN, we used immunohistochemical staining and microdissection-based quantitative real-time reverse transcription-polymerase chain reaction. Among 125 IPMNs, 43 (34%) were positive for REG4 and most of the intestinal-type IPMNs showed its expression (35/38). The positive ratio of REG4 expression in colloid carcinoma (5/7) was significantly higher than that in tubular carcinoma (1/17; P=0.003). Most of CDX2-positive cases (31/33) expressed REG4 protein, whereas only 12 of 92 CDX2-negative cases did (P<0.001). The levels of REG4 mRNA in intestinal-type IPMN were significantly higher compared to those in gastric-type IPMN or to normal pancreatic ductal epithelium (P=0.005, P=0.004, respectively). REG4 expression was observed more frequently in borderline lesions (14/28) and carcinoma (21/45) compared to adenoma (8/52). Using the Ki-67 labeling index, REG4 expression was significantly correlated with proliferative activity in borderline lesions. We conclude that REG4 is involved in the ‘intestinal’ pathway of carcinogenesis in IPMN.
Similar content being viewed by others
Main
Intraductal papillary mucinous neoplasms (IPMNs) show a wide spectrum of histological differentiation from hyperplasia, adenoma and borderline neoplasm to carcinoma, and the existence of an adenoma-carcinoma sequence is documented.1, 2, 3 IPMNs are considered to be precursors of invasive cancer of either tubular ductal adenocarcinoma or colloid (mucinous noncystic) carcinoma.4, 5, 6, 7
Recently, subclassification of IPMNs, based on morphological features and immunohistochemical profiles, has been proposed. Intestinal-type IPMNs frequently show moderate to severe dysplasia and the intestinal-type associated invasive cancer tends to be colloid carcinoma.8, 9, 10, 11, 12 Meanwhile, most gastric-type IPMNs show low-grade dysplasia and rarely associated with invasive cancer.8, 9, 10 IPMNs arising from branch ducts often show gastric-type differentiation and have a less aggressive clinical course.1, 9 Pancreatobiliary-type IPMNs show severe atypia corresponding to in situ carcinoma and is often associated with an invasive tubular type of ductal adenocarcinoma. These findings suggest that each type of IPMN follows a different pathway of carcinogenesis. Adsay et al suggested that the intestinal-type IPMN to colloid carcinoma sequence is a distinct pathway of carcinogenesis involving intestinal-related genes CDX2 and MUC2, and they described this pathway as the ‘intestinal’ pathway of carcinogenesis.4, 5, 10
Regenerating islet-derived gene family, member 4 (REG4), recently isolated from a complementary DNA library of ulcerative colitis tissues,13 is a member of the Reg gene family and encodes secreted protein. REG4 is also expressed in gastrointestinal carcinoma.14, 15, 16, 17 REG4 is involved in the carcinogenesis of colon cancer and is associated with proliferative function.15, 18 REG4 is also detected in goblet cells of intestinal metaplasia of the stomach, which is associated with CDX2.14 The proliferative activity of the goblet cells is higher in the intestinal metaplasia of the stomach compared to those in the small intestine and colon.19
The biological and clinical significance of the occurrence of the ‘intestinal’ pathway of carcinogenesis of IPMN remains controversial. Of particular note is whether gastric-type IPMN is capable of progressing to intestinal or to other types of IPM borderline (IPMB) and IPM carcinoma (IPMC).9, 10 Therefore, identifying the involvement of REG4, an intestinal differentiation-related gene, in gastric-type IPMN may be helpful to understand its tendency to progress to the intestinal-type IPMB and IPMC.
In this study, we analyzed REG4 and CDX2 expressions in patients with IPMNs, focusing on both the grade of atypia and histological subtypes using immunohistochemistry and laser microdissection-based quantitative measurement of mRNA. We also investigated the function of REG4 in IPMNs using the Ki-67 labeling index (Ki-67 LI). The clinical course of IPMN limited to the branch ducts is considered to be less aggressive compared to that of main duct IPMNs (including the mixed type).20 Nonsurgical management with close observation is recommended for branch duct IPMNs without high-risk criteria such as mural nodules.1, 20, 21 However, there is a possibility that even branch duct IPMNs, without high-risk criteria, may include high-grade malignant lesions.22 We also analyzed REG4 expression according to IPMN location: branch limited, mixed and main duct type.
Materials and methods
Clinical Samples
A total of 125 patients with IPMN (including invasive carcinoma) and 40 patients with invasive ductal carcinomas (IDCs) without IPMN underwent surgical resection in our institute. We also analyzed pancreatic intraepithelial neoplasia (PanIN) lesions detected in these specimens. Histological grading of the tumors and diagnosis of PanINs were performed according to the WHO classification system.23 On the basis of the greatest degree of dysplasia present, IPMN lesions were classified as adenoma, borderline neoplasm or carcinoma. IPMNs were also classified into four groups, gastric, intestinal, pancreatobiliary and oncocytic types, in accordance with the recently suggested subclassification system.8 IPMNs that could not be categorized specifically into any above subtypes were segregated as unclassified type (n=8).
Immunohistochemical Procedures
The primary antibodies used were as follows: anti-REG4 (1:100 dilution; R&D Systems), anti-CDX2 (1:100 dilution; BioGenex), anti-MUC2 (1:200 dilution; Novocastra Laboratories) and anti-Ki-67 (1:50 dilution; R&D Systems). Sections were cut at 4-μm thickness from paraffin-embedded material, then deparaffinized in xylene and rehydrated through a graded series of ethanol. After inhibition of endogenous peroxidase and antigen retrieval (microwave irradiation in citrate buffer for both antibodies), sections were exposed to each primary antibody at 4°C overnight, and stained with a streptavidin-biotin-peroxidase kit (Nichirei, Tokyo, Japan). The sections were then finally reacted in 3,3′-diaminobenzidine, counterstained with hematoxylin and mounted. In this study, cytoplasmic and nuclear immunoreactivity was detected in tumor cells, endothelial cells around pancreatic cancer tissues and normal islet cells.
Evaluation of Immunohistochemical Staining
As IPMNs often show variability of epithelial dysplasia within a tumor, immunohistochemical staining was evaluated in the area showing the highest degree of dysplasia in each neoplasm. The proportion of REG4-positive cells was measured using the following scale according to the percentage of REG4-positive tumor cells: (negative, 0; <10%, 1+; 10–50%, 2+; >50%, 3+). The expression of REG4 in tumor cells was defined as positive when 10% or more tumor cells were stained (scores 2+ and 3+) and negative when less than 10% of cancer cells were stained (score 0 and 1+), as described previously.17 For CDX2, only nuclear staining was considered positive, as previously demonstrated.24 Positive MUC2 staining was determined, as described previously.5 All slides were evaluated independently by three investigators (KN, YM and AH) without any knowledge of the clinical features in each case.
Ki67-Labeling Index
In each case, the Ki-67 LI (percentage of Ki-67-positive cells) was calculated by counting Ki-67-positive cells among 300 pancreatic duct epithelial cells in normal pancreas and among 1000 tumor cells in IPMNs of the pancreas, as described previously.25
Microdissection-Based Quantitative Analysis of REG4 mRNA
Frozen tissue samples were cut into 8 μm sections. One section was stained with H&E for histological examination. IPMN lesions were isolated selectively with a laser microdissection and pressure catapulting system (P.A.L.M. Microlaser Technologies, Bernried, Germany) in accordance with the manufacturer’s protocols. After microdissection, total RNA was extracted from the selected cells and subjected to real-time RT-PCR for quantitative measurement of REG4 and CDX2 mRNA as described previously.26, 27
Quantitative Assessment of REG4 and CDX2 mRNA Levels by One-Step qRT-PCR
We designed specific primers as follows: REG4, 5′-CAGATCCTGGTCTGGCAAGT-3′ (forward) and 5′-ATTCGTTGCTGCTCCAAGTT-3′ (reverse); CDX2, 5′-CTCCTCCCCAGCTCTTCTCT-3′ (forward) and 5′-CCTTTCCCTTGAGTCCCTCT-3′ (reverse); 18S rRNA, 5′-GTAACCCGTTGAACCCCATT-3′ (forward) and 5′-CCATCCAATCGGTAGTAGCG-3′ (reverse). One-step qRT-PCR was performed using a QuantiTect SYBR Green RT-PCR kit (Qiagen) with a Chrom4 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) as described previously.28 Each sample was run twice, and any sample showing more than 10% deviation in RT-PCR values was tested a third time. The quantitative range of threshold cycles observed was 20–35 cycles for REG4, CDX2 and 10–30 cycles for 18S rRNA The level of mRNA in each sample was calculated from a standard curve generated with total RNA from the Suit2 human pancreatic cancer cell line. Levels of REG4 and CDX2 mRNA were normalized to that of 18S rRNA.
Statistical Analysis
All calculations were carried out using StatView 5.0J software (Abacus Concepts, Berkeley, CA, USA). Data were analyzed by the Mann–Whitney U–test, if comparisons involved two groups because a normal distribution was not obtained. The positive rates of REG4 and CDX2 expressions with respect to clinicopathological variables were compared using the Fisher's exact test. The relationships between REG4 and CDX2 mRNA levels were assessed by Spearman's correlation test. All statistical analyses were considered to be significant if the P value was <0.05 and the significance of multiple comparisons was evaluated with Bonferroni's correction.
Results
Immunohistochemical Patterns of REG4 Expression in IPMNs According to Morphological Phenotype
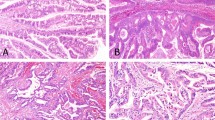
REG4 expression was detected in islet cells of Langerhans as previously reported,14 and these were considered to be internal positive controls. However, REG4 expression was not detected in normal pancreatic ductal epithelium, acini and stromal fibroblasts. In the IPMN samples, 43 (34%) were positive for REG4 (Figure 1). We investigated REG4 expression according to the subtype of IPMN. Among 38 cases of intestinal-type IPMN, 35 (92%) were positive for REG4 at the apical surface and in the cytoplasm of the tumor cells (Figure 1a and b). REG4 was detected in only one case of pancreatobiliary-type IPMN and was not detected in oncocytic-type IPMN. In gastric-type IPMN, only 4 cases (6%) were positive (>2+) for REG4 and its expression was detected focally (1+) in 16 cases (Figure 1c–e). The positive rate of REG4 expression was significantly higher in intestinal-type IPMN compared to that in the other types of IPMN (Table 1; P<0.001).
Microphotographs of REG4 expression in each subtype of IPMN and in IDC without IPMN. H&E stain of intestinal-type IPMN (carcinoma; a) and gastric-type IPMN (adenoma; c). In intestinal-type IPMN, REG4 is detected diffusely at the apical surface and in the cytoplasm of tumor cells (b). In gastric-type IPMN, REG4 expression was focally detected (d) and goblet cell in gastric-type IPMN also showed its expression (e). REG4 is expressed in the cytoplasm of colloid carcinoma (f), but REG4 is not detected in tubular carcinoma (g) or in IDC without IPMN (h).
With regard to the invasive component of IPMN, the positive ratio of REG4 expression in colloid carcinoma, which was always associated with intestinal-type IPMN, (Figure 1f; 5 of 7, 71%) was significantly higher compared to that in tubular ductal adenocarcinoma derived from IPMN (Figure 1g; 1 of 17, 6%; Table 2; P=0.003).
Quantitative Analysis of REG4 mRNA in IPMN and in Normal Pancreatic Ductal Cells
We isolated IPMN and normal ductal epithelial cells from frozen sections by microdissection. As shown in Figure 2, REG4 mRNA was differentially expressed among normal pancreatic ductal epithelia (n=7), gastric-type (n=7) and intestinal-type (n=5) IPMN cells. There were significant differences in the levels of REG4 mRNA among these cells (P=0.002, normal pancreatic ductal cells vs gastric-type IPMN cells; P=0.004, normal pancreatic ductal cells vs intestinal-type IPMN cells; P=0.005, gastric-type IPMN cells vs intestinal-type IPMN cells).
Scattergram of REG4 mRNA levels in microdissected tissues. We used a microdissection technique to isolate normal pancreatic epithelial cells (normal; n=7), gastric-type (G-type) IPMN (n=7) and intestinal-type (I-type) IPMN (n=5) from frozen sections. 18S rRNA was used as a reference gene. Differential expression of REG4 mRNA is observed among normal cells (median, 0.07), G-type IPMN (median, 2.61) and I-type IPMN (median, 563.9; P=0.002, G-type IPMN cells vs normal cells; P=0.005, G-type IPMN cells vs I-type IPMN cells; P=0.004, I-type IPMN vs normal cells).
Relationship Between REG4 and MUC2 Protein Expressions in IPMN
MUC2 was expressed in 39 (31.2%) of 125 IPMNs, and in almost all cases MUC2 expression was observed in REG4-expressing cells (Figure 3a–c). Interestingly, in some cases of gastric-type IPMN there were some tumor cells that expressed REG4 without MUC2 expression (Figure 3d and e). These findings suggest that REG4 expression is more widespread than the intestinal phenotype that is detected by MUC2 expression.
Microphotographs of MUC2 and CDX2 localization compared with REG4 expression. Double immunofluorescence staining in IPMN: REG4 (a), MUC2 (b) and merged images (c). REG4 and MUC2 are coexpressed. Representative figure of the discrepancy between REG4 expression and MUC2 expression: REG4 (d) and MUC2 (e). CDX2 showed nuclear staining in tumoral cells (f).
Immunohistochemical Patterns of CDX2 and Quantitative Analysis of CDX2 mRNA Levels in IPMN
In our immunohistochemical analysis, REG4 was observed more frequently in CDX2-positive cases (31 of 33, 94%) compared to CDX2-negative cases (12 of 92, 13%; Table 3; P<0.001). Most (32 of 35, 91%) of the intestinal-type IPMN with REG4-positive cells expressed CDX2 in the nuclei of tumor cells (Figure 3f). In gastric-type IPMN, on the other hand, CDX2 expression was barely detected, including in goblet-like cells focally detected in this type of tumor (data not shown).
In the analyses of microdissected samples, CDX2 mRNA levels were also significantly higher in intestinal-type IPMN cells compared to normal pancreatic ductal epithelial cells (P=0.028). Although the median CDX2 mRNA levels were higher in intestinal-type IPMN cells compared to gastric-type IPMN cells, the difference was not statistically significant (Figure 4). There was a significant correlation between REG4 and CDX2 mRNA levels (r=0.735, P=0.001).
Immunohistochemical Patterns of REG4 Expression in IPMNs According to the Histological Grade
The positive rates of REG4 in IPM adenoma (IPMA), IPMB and IPMC were 8/52 (15%), 14/28 (50%) and 21/45 (47%), respectively. The REG4 positive rates were significantly higher in borderline and carcinoma compared to adenoma (P<0.01, P<0.01 respectively). On the other hand, among 40 IDCs without IPMN and 46 PanIN lesions, 5 (13%) were positive for REG4 expression in the cytoplasm of cancer cells (Figure 1h), and REG4 was negative or its intensity was very weak in PanIN lesions (Table 4).
To investigate involvement of REG4 in the ‘intestinal’ pathway of carcinogenesis, we analyzed the relationship between REG4 expression and histological grading, focusing on gastric- and intestinal-type IPMN. REG4 expression showed a significantly increasing trend from adenoma to carcinoma (Figure 5; P<0.001, IPMC vs IPMA; P=0.002, IPMB vs IPMA).
Clinical Significance of REG4 Expression in the IPMN Limited to the Branch Ducts
Of 51 IPMNs limited to the branch ducts, 36 cases (71%) were gastric-type IPMN. In branch duct IPMN, the positive rates of REG4 in IPMB and IPMC were higher than those in IPMA (Table 5a; P=0.01), and these findings are also detected in main duct and mixed-type IPMN (Table 5b; P=0.04).
Relationship Between REG4 Expression and Ki-67 Labeling Index
In IPMB, REG4-positive cases showed a significantly higher Ki-67 LI compared to REG4-negative cases (Figure 6b; P=0.013). In IPMA and IPMC, the median Ki-67 LI was higher in REG4-positive cases compared to REG4-negative cases, but this difference was not statistically significant (Figure 6a and c).
Transition Area Between Gastric- and Intestinal-Type IPMN
We found gastric-type IPMA lesions directly connected to IPMB lesions, and also observed intestinal-type IPMC around these lesions (Figure 7a). This IPMB area seemed to be neither completely gastric-type nor intestinal-type epithelium. Immunohistochemically, MUC2 and CDX2 expressions were detected in this IPMB lesion and in intestinal-type IPMC lesion (Figure 7b and c). This lesion is a possible transition area between gastric- and intestinal-type IPMN and corresponds to IPMB. We observed that the positive area of the Ki-67 staining was similar to that of REG4 (Figure 7d and e), and we suggest a close relationship of these proteins.
Immunohistochemical staining in the transition lesion (IPMB) between gastric-type IPMA and intestinal-type IPMC: HE (a), MUC2 (b), CDX2 (c), REG4 (d) and Ki-67-I (e). All stainings are detected in the transition area and in intestinal-type IPMC. Arrow, intestinal-type IPMC; black arrowhead, gastric-type IPMA; white arrowhead and inset, transition area.
Discussion
This is the first report regarding the involvement of REG4 and CDX2 in IPMN using both immunohistochemistry and microdissection-based quantitative analyses of mRNA. We demonstrated that REG4 is frequently detected in intestinal-type IPMN, which also expressed MUC2 protein, and is frequently coexpressed with CDX2. We also observed a proliferative function in REG4-positive IPMN cells.
IPMNs are reported to be precursors of invasive cancer, that is, colloid (mucinous noncystic) carcinoma and tubular ductal adenocarcinoma. Colloid carcinoma is more frequently associated with intestinal-type IPMN compared to the other types of IPMN,5, 10 which is consistent with our present findings. REG4 expression was more frequently observed in intestinal-type IPMN and colloid carcinoma than in gastric-type IPMN and tubular ductal adenocarcinoma with IPMN. These findings were confirmed by microdissection-based analysis of REG4 mRNA. We also showed that REG4 expression was significantly correlated with CDX2 and MUC2 expressions at both protein and mRNA levels. CDX2 is a transcriptional factor that is important for the maintenance of intestinal identity.29 MUC2, on the other hand, is a major mucin detected in intestinal epithelium. Considering these findings, REG4 is significantly related with the intestinal phenotype and its expression may be regulated by CDX2.
Intestine-specific gene REG4 is detected in some goblet cells of gastric-type IPMN unlike goblet cells of small intestine and colon.14 Thus, goblet cells in gastric-type IPMN may have different biological characteristics from those in the small intestine and colon.
Focusing on intestinal- and gastric-type IPMN, REG4 expression showed a significantly increasing trend from adenoma to carcinoma. Previous reports revealed that REG4 was detected in colorectal adenomas and cancers13, 15, 30, 31 and that it might be involved in early carcinogenesis of colorectal cancer.31 Therefore, we considered the association of REG4 with carcinogenesis of IPMN.
The relationship of REG4 with proliferative activity in IPMB suggests that REG4 not only reflects the morphological difference of IPMN, but also is involved in an early step of ‘intestinal’ carcinogenesis of IPMN through its growth-promoting action. In IPMA, the median Ki-67 LI was higher in REG4-positive cases than in REG4-negative cases, but this difference was not statistically significant. This is because the number of REG4-positive cases was small and its expression pattern was restricted. We also found no statistical differences in Ki-67 LI between REG4-positive and -negative IPMC and 27 of 45 (60%) IPMCs were not of the intestinal-type. Therefore, REG4-negative IPMC cells are affected by other proliferative mechanisms and a subset of gastric-type IPMNs may progress to pancreatobiliary-, gastric- and oncocytic-type-IPMC by a nonintestinal pathway of carcinogenesis. Furthermore, we also detected a MUC2, CDX2, REG4 and Ki-67 positive transition area between gastric- and intestinal-type IPMN. This observation directly demonstrated that a subset of gastric-type IPMA may obtain the intestinal phenotype and proceed to IPMB and intestinal-type IPMC. Thus, REG4 may be involved in the ‘intestinal’ pathway of carcinogenesis in IPMN.
In a clinical setting, main duct IPMN requires adequate resection as soon as possible depending on its malignant potential, whereas asymptomatic branch duct IPMN without mural nodules may not require immediate surgery. Nonetheless, IPMN without mural nodules sometimes has in situ carcinoma or minimally invasive carcinoma.1, 20 Our results revealed that branch duct IPMNs with REG4 expression were frequently borderline malignant or carcinoma. Recently, REG4 has been reported as a novel serum marker in several malignancies.16, 17, 30 Therefore, REG4 may be a promising marker to indicate the need for surgery for IPMN limited to the branch ducts.
In conclusion, our data suggest that REG4 plays an important role in differentiation of the ‘intestinal’ pathway of IPMN and it may be regulated by CDX2.
References
Tanaka M, Kobayashi K, Mizumoto K, et al. Clinical aspects of intraductal papillary mucinous neoplasm of the pancreas. J Gastroenterol 2005;40:669–675.
Nagai E, Ueki T, Chijiiwa K, et al. Intraductal papillary mucinous neoplasms of the pancreas associated with so-called ‘mucinous ductal ectasia’. Histochemical and immunohistochemical analysis of 29 cases. Am J Surg Pathol 1995;19:576–589.
Sessa F, Solcia E, Capella C, et al. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch 1994;425:357–367.
Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol 2001;25:26–42.
Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol 2002;15:1087–1095.
Adsay NV, Longnecker DS, Klimstra DS . Pancreatic tumors with cystic dilatation of the ducts: intraductal papillary mucinous neoplasms and intraductal oncocytic papillary neoplasms. Semin Diagn Pathol 2000;17:16–30.
Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology 2002;123:1500–1507.
Furukawa T, Kloppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch 2005;447:794–799.
Ban S, Naitoh Y, Mino-Kenudson M, et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am J Surg Pathol 2006;30:1561–1569.
Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an ‘intestinal’ pathway of carcinogenesis in the pancreas. Am J Surg Pathol 2004;28:839–848.
Seidel G, Zahurak M, Iacobuzio-Donahue C, et al. Almost all infiltrating colloid carcinomas of the pancreas and periampullary region arise from in situ papillary neoplasms: a study of 39 cases. Am J Surg Pathol 2002;26:56–63.
Luttges J, Zamboni G, Longnecker D, et al. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol 2001;25:942–948.
Hartupee JC, Zhang H, Bonaldo MF, et al. Isolation and characterization of a cDNA encoding a novel member of the human regenerating protein family: Reg IV. Biochim Biophys Acta 2001;1518:287–293.
Oue N, Mitani Y, Aung PP, et al. Expression and localization of Reg IV in human neoplastic and non-neoplastic tissues: Reg IV expression is associated with intestinal and neuroendocrine differentiation in gastric adenocarcinoma. J Pathol 2005;207:185–198.
Nanakin A, Fukui H, Fujii S, et al. Expression of the REG IV gene in ulcerative colitis. Lab Invest 2007;87:304–314.
Takehara A, Eguchi H, Ohigashi H, et al. Novel tumor marker REG4 detected in serum of patients with resectable pancreatic cancer and feasibility for antibody therapy targeting REG4. Cancer Sci 2006;97:1191–1197.
Mitani Y, Oue N, Matsumura S, et al. Reg IV is a serum biomarker for gastric cancer patients and predicts response to 5-fluorouracil-based chemotherapy. Oncogene 2007;26:4383–4393.
Violette S, Festor E, Pandrea-Vasile I, et al. Reg IV, a new member of the regenerating gene family, is overexpressed in colorectal carcinomas. Int J Cancer 2003;103:185–193.
Wong WM, Stamp GW, Elia G, et al. Proliferative populations in intestinal metaplasia: evidence of deregulation in Paneth and goblet cells, but not endocrine cells. J Pathol 2000;190:107–113.
Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006;6:17–32.
Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology 2007;133:72–79; quiz 309–310.
Sugiyama M, Izumisato Y, Abe N, et al. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg 2003;90:1244–1249.
Hamilton SR . Targeted therapy of cancer: new roles for pathologists in colorectal cancer. Mod Pathol 2008;21 (Suppl 2):S23–S30.
Moskaluk CA, Zhang H, Powell SM, et al. Cdx2 protein expression in normal and malignant human tissues: an immunohistochemical survey using tissue microarrays. Mod Pathol 2003;16:913–919.
Terada T, Ohta T, Kitamura Y, et al. Cell proliferative activity in intraductal papillary-mucinous neoplasms and invasive ductal adenocarcinomas of the pancreas: an immunohistochemical study. Arch Pathol Lab Med 1998;122:42–46.
Ohuchida K, Mizumoto K, Ishikawa N, et al. The role of S100A6 in pancreatic cancer development and its clinical implication as a diagnostic marker and therapeutic target. Clin Cancer Res 2005;11:7785–7793.
Tachikawa T, Irie T . A new molecular biology approach in morphology: basic method and application of laser microdissection. Med Electron Microsc 2004;37:82–88.
Ohuchida K, Mizumoto K, Ohhashi S, et al. Twist, a novel oncogene, is upregulated in pancreatic cancer: clinical implication of Twist expression in pancreatic juice. Int J Cancer 2007;120:1634–1640.
Beck F, Chawengsaksophak K, Waring P, et al. Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice. Proc Natl Acad Sci USA 1999;96:7318–7323.
Oue N, Kuniyasu H, Noguchi T, et al. Serum concentration of Reg IV in patients with colorectal cancer: overexpression and high serum levels of Reg IV are associated with liver metastasis. Oncology 2007;72:371–380.
Zhang Y, Lai M, Lv B, et al. Overexpression of Reg IV in colorectal adenoma. Cancer Lett 2003;200:69–76.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakata, K., Nagai, E., Ohuchida, K. et al. REG4 is associated with carcinogenesis in the ‘intestinal’ pathway of intraductal papillary mucinous neoplasms. Mod Pathol 22, 460–468 (2009). https://doi.org/10.1038/modpathol.2008.205
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2008.205
Keywords
This article is cited by
-
Different subtypes of intraductal papillary mucinous neoplasm in the pancreas have distinct pathways to pancreatic cancer progression
Journal of Gastroenterology (2012)
-
Expression of claudin-4 (CLDN4) mRNA in intraductal papillary mucinous neoplasms of the pancreas
Modern Pathology (2011)
-
Malignant potential of intraductal papillary mucinous neoplasms of the pancreas
Surgery Today (2010)