Abstract
The poor response to drug therapy often seen in hepatocellular carcinoma requires insight into the molecular interplay responsible for intrinsic or acquired drug resistance. We previously demonstrated that the CD133−/EpCAM− subpopulation of the Huh-7 hepatoma cell line features aberrant activation of the hedgehog signaling (Hh) pathway and chemoresistance. The prevailing hypothesis of the present study is that hedgehog signaling may govern expression of ATP-binding cassette (ABC) transporters, which are responsible for drug resistance in the CD133−/EpCAM− subpopulation. Our aim is to reveal the molecular interplay in the mediation of drug resistance with a newly established Huh-7 subpopulation featuring high Hh signaling activity and drug resistance. In this study, chemoresistance was determined in a newly established Huh-7-DN subpopulation featuring the CD133−/EpCAM− surface marker profile, aberrant expression of Hh pathway, and epithelial–mesenchymal transition (EMT). Expression of ABC transporter proteins (ABCB1, ABCC1, and ABCG2) and Hh transcription factor Gli-1/2 was evaluated with and without Hh signaling antagonists LDE225 or itraconazole. We found that hedgehog signaling activity as determined by transfection with a Gli-Lux reporter cassette and gene expression levels tended to increase from Huh-7 CD133+/EpCAM+ to CD133−/EpCAM−, and the highest levels were found in Huh-7-DN cells. The Huh-7-DN subpopulation exhibited characteristics of EMT as evidenced by increased expression of vimentin and loss of E-cadherin. Sorafenib significantly inhibited the viability of all subpopulations except the Huh-7-DN subpopulation. Compared with other sorafenib-sensitive subpopulations, the Huh-7-DN subpopulation showed enhanced expression of Hh transcription factor Gli-2 and ABCC1 transporter protein. Silencing Gli-2 by lentivirus harboring shRNA against Gli-2 or LDE225 significantly suppressed expression of Gli-2 and ABCC1 genes in Huh-7-DN subpopulation. In conclusion, aberrant hedgehog signaling activation is linked to poor differentiation, epithelial–mesenchymal transition, and chemoresistance in the Huh-7-DN subpopulation. Hedgehog signaling transcription factor Gli-2 appears to be the primary regulator for drug sensitivity of hepatoma through the ABCC1 transporter.
Similar content being viewed by others
Main
As the third most common death of malignancy, more than 700 000 new cases of hepatocellular carcinoma (HCC) occur worldwide every year.1 Despite the steadily increasing incidence, only 25% of patients benefit from surgical intervention.2 The advanced stage at the time of diagnosis, aggressive behavior of HCC progression, the high recurrence after resection (60–80% in 5 years), and ablation (40–70%) are major causes of a very low 5-year survival rate.3 Limited adjuvant therapies are available for advanced HCC patients.4, 5 Sorafenib, a Raf-1 kinase inhibitor, was reported to extend patient survival for nearly 3 months.6 However, a recent multicenter, double-blinded trial concluded that sorafenib did not extend recurrence-free survival, but increased adverse effects of the treatment,3 and the findings are consistent with a growing list of negative reports from primary or adjuvant use after resection, ablation, or transarterial chemoembolization.7
Primary or acquired multidrug resistance (MDR) is one of the reasons leading to HCC treatment failure and progression.8 It is known that MDR increases drug efflux by adenosine triphosphate binding cassette (ABC) transporters, resulting in a decrease in intracellular concentrations of chemotherapeutic agents. ABCB1 (MDR-1, P-glycoprotein), ABCC1 (multidrug resistance-associated protein 1, MRP1), and ABCG2 (breast cancer-resistance protein, BCRP) are the best-characterized ABC transporters that contribute to chemoresistance.9 However, it is not yet understood how ABC transporter proteins affect the development of intrinsic and acquired resistance to sorafenib in HCC.
We previously demonstrated that hepatoma cell lines could be separated into subpopulations according to the expression profile of cell surface markers CD133 and EpCAM. The CD133−/EpCAM− subpopulation is more resistant to cisplatin, doxorubicin, and sorafenib in comparison with the CD133+/EpCAM+ subpopulation.10 Moreover, our data suggest that the chemoresistance is correlated with aberrant activation of the hedgehog (Hh) signaling pathway and occurrence of epithelial–mesenchymal transition (EMT).10 Hedgehog signaling is quiescent in adult tissues. Overexpression of Hh pathway molecules, such as sonic Hh, PTCH1, SMO, and Gli-1/2, has been reported in 60–70% of HCC specimens,11, 12 and dysregulated expression of Hh molecules is one of disordered signaling mechanisms crucial for hepatic carcinogenesis13 and highly metastatic behavior of HCC.14 Hh signaling inhibitors, such as vismodegib (GDC-0449) and sonidegib (LDE225), are approved for the treatment of advanced basal cell carcinoma;15 and sonidegib is in phase I–II clinical trials for the treatment of a variety of malignancies, including esophageal, pancreatic, ovarian cancers, and hepatocellular carcinoma.16 However, whether abnormal hedgehog signaling has a critical role in modulating drug resistance through ABC transporter molecules has not been thoroughly investigated. Therefore, we tested the hypothesis that hedgehog signaling may govern expression of ABC transporter proteins, which are responsible for chemoresistance in hepatoma cells.
In the present study we established a unique Huh-7 subpopulation, which features higher hedgehog signaling activity with EMT characteristics and drug resistance. We investigated how drug resistance is developed and the underlying controlling mechanisms within this specific subpopulation.
Materials and methods
Cell Culture and Reagents
Hepatoma cell lines, Huh-7 and Hep3B, were obtained from the American Type Culture Collection (Manassas, VA, USA). The Hep3B cells were incubated in minimum essential medium (MEM, Gibco, Grand Island, NY, USA), and Huh-7 cells were incubated in Dulbecco’s modified essential medium (DMEM, Gibco), supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco) and 1% (v/v) penicillin–streptomycin.
Fluorescence Activation Cell Sorting Enrichment and Transwell Matrigel Invasion Assay for Subpopulation Selection
CD133+/EpCAM+ and CD133−/EpCAM− subpopulations were enriched from Huh-7 cells by fluorescence activation cell sorting (FACS) using allophycocyanin (APC)-conjugated monoclonal antibodies against human CD133 and fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies against human EpCAM as we previously described.10 Briefly, the Huh-7 cells were detached by 0.05% Trypsin-EDTA (Gibco) and incubated with corresponding antibodies (CD133/EpCAM) for 45 min. After washing, the cells were suspended in DMEM containing 10% FBS until sorting. All the cells were sorted in a high-speed MoFlo XDP Cell Sorter (Beckman, Indianapolis, IN, USA). The Huh-7-DN subpopulation, which stably retained negative CD133/EpCAM expression profile was isolated by FACS enrichment plus culture selection from CD133−/EpCAM− subpopulation. The Huh-7-trans subpopulation was isolated using Matrigel invasion transwell selection as we previously described.14
Immunofluorescent Staining
CD133+/EpCAM+, CD133−/EpCAM−, Huh-7-DN, and Huh-7-trans subpopulations were seeded on coverslips and fixed in 4% buffered paraformaldehyde. Then cells on coverslips were incubated with primary antibodies against Gli-1, E-cadherin, vimentin, Gli-2, and ABCC1 overnight at 4 °C, and were stained with secondary antibodies (Alexa Fluor 488-conjugated donkey anti-mouse IgG or Alexa Fluor 594/488-conjugated donkey anti-rabbit IgG) as we previously described.17 All the cells were counter-stained with 4', 6-diamidino-2-phenylindole (DAPI) for nuclear visualization. All electronic images were captured under a Leica TCS SP8 Confocal laser scanning microscope. The sources of all antibodies were listed in the Supplementary Table 1.
Hh Signaling Activity by a Gli-Lux Reporter System
The Gli-Lux reporter system, in which the firefly luciferase gene is driven by the Gli promoter, was used to determine Hh signaling activity in different subpopulations. pRL-thymidine kinase (TK) renilla luciferase control reporter (Promega, Madison, WI, USA) was used for transfection normalization. The Gli-Lux reporter system was kindly provided by Dr Hiroshi Sasaki from the RIKEN Center for Developmental Biology, Kobe, Hyogo, Japan.18 FACS-enriched Huh-7 CD133+/EpCAM+ or CD133−/EpCAM− subpopulations were transfected with both Gli-Lux and pRL-TK reporter gene plasmids by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). To determine the inhibitory effect of Hh antagonists, culture medium was replaced with medium containing itraconazole or LDE225 24 h after transfection. Firefly and renilla luciferase activity in transfected cells was determined by a dual-luciferase reporter system (Promega).19
Assays of Cell Viability
CD133+/EpCAM+, CD133−/EpCAM−, Huh-7-DN, and Huh-7-trans subpopulations were seeded at 104/well in 96-well plates and allowed to adhere overnight. The cells were treated with Sorafenib (Selleck, TX, USA), Itraconazole (Biovision, Milpitas, CA, USA) and LDE225 (Cellagen Technology, San Diego, CA, USA) at various concentrations for 24 h. The cell viability was assayed with thiazolyl blue tetrazolium bromide (MTT) assay.20 The MTT assay was also used for the determination of IC50 in various cell types in exposure to sorafenib as shown in the Supplementary Information.
RNA Isolation and Quantitative RT-PCR
Total RNA was extracted from various cells using RNAprep Pure Cell kit (TIANGEN, Beijing, China) according to the manufacturer’s instruction. cDNA was synthesized using PrimeScript RT reagent Kit (TAKARA, Dalian, China). Quantitative RT-PCR was performed with Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) in the Eppendorf AG 22331 RT-PCR system (Eppendorf, Hamburg, German). Primer pair sequences for Gli-1, Gli-2, ABCB1, ABCC1, ABCG2, Twist, Snail, Bcl-2, and Bax are listed in Supplementary Table 2. Relative gene expression was normalized to the housekeeping gene, human glyceraldehyde phosphate dehydrogenase (GAPDH) and expressed as 2(−ΔΔCT) as previously described.21, 22 cDNA generated from human primary hepatocytes was provided by Dr Ping Zhou, Stem Cell Program, UC Davis Medical Center, Sacramento, CA, USA, and used as a control for human gene expression level. Human primary hepatocytes were obtained from the Liver Tissue Procurement and Distribution System (LTPDS), Pittsburgh, PA, USA.
Western Blot Analysis
Total protein was extracted from different cell subpopulations using RIPA buffer (Ruian BioTechnologies, Shanghai, China). The protein concentration was determined using a bicinchoninic acid (BCA) protein assay (Pierce Biotech, Rockford, USA). Protein lysate (40 μg) was loaded and separated by 4–12% gradient Tris-glycine gels and was transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, MA, USA) at 80 V for 1.5 h. Then, membrane of protein extracts was blocked with 5% skim milk and incubated with monoclonal anti-Gli-1, ABCG2, ABCC1, and β-actin or polyclonal anti-Gli-2 in Tris-buffered saline containing 0.1% bovine serum albumin (BSA) overnight at 4 °C. Blotted membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA, USA) for 1 h at room temperature. Specific protein bands were visualized by the enhanced chemiluminescent reagent (Tanon, Shanghai, China) as previously reported.14
Statistical Analysis
All the experiments were performed three times with a minimum of triplicates. Statistical analysis was performed with SPSS 20.0. The data were expressed as means±standard deviation (s.d.), and analyzed by independent-samples T-test between two groups or one-way ANOVA for difference between more than two groups, and further by Newman–Keuls or Bonferroni tests for multiple comparisons between given two groups. P<0.05 was considered as statistically significant.
Results
Stable CD133−/EpCAM− Expression Profile of Huh-7-DN and Huh-7-Trans Subpopulations
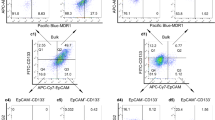
Well-differentiated hepatoma Huh-7 cells were separated into CD133+/EpCAM+ and CD133−/EpCAM− subpopulations by FACS enrichment. As shown in Figure 1a, CD133+/EpCAM+ and CD133−/EpCAM− subpopulations constituted 48.7 and 21.0% of unsorted Huh-7 cells. Flow cytometric analysis verified that more than 80% of freshly sorted CD133+/EpCAM+ and CD133−/EpCAM- cells possessed their own surface marker profile (Figures 1b and c). However, freshly sorted CD133+/EpCAM+ and CD133−/EpCAM− subpopulations gradually lost characteristics of their surface marker profile, and tended to return to an unsorted state after a few passages. To obtain a subpopulation, which stably retained CD133−/EpCAM− expression profile, Huh-7-DN and Huh-7-trans subpopulations were established by FACS enrichment plus culture selection or Matrigel invasion transwell selection. Both Huh-7-DN and Huh-7-trans subpopulations remained CD133−/EpCAM− profile (>98%) after more than 10 passages (Figures 1d and e).
Huh-7-DN and Huh-7-trans subpopulations retained CD133−/EpCAM− cell surface marker profile over multiple passages. (a) Unsorted Huh-7; (b) Freshly sorted CD133+/EpCAM+ subpopulation; (c) Freshly sorted CD133−/EpCAM− subpopulation; (d) Huh-7-DN subpopulation at passage 16; (e) Huh-7-trans subpopulation.
Huh-7-DN Cells were Positive for Hh Transcription Factors Gli-1/2 and EMT Markers
As shown in Figure 2, Gli-1 and Gli-2 were positive in CD133−/EpCAM− subpopulations, especially in Huh-7-DN and Huh-7-trans subpopulations. Staining of EMT markers demonstrated that E-cadherin was only observed in CD133+/EpCAM+ subpopulation, whereas other three subpopulations with a negative CD133/EpCAM expression profile exhibited obvious vimentin positivity and loss of E-cadherin expression, indicating that these subpopulations with CD133−/EpCAM− expression acquired an EMT status.
Immunofluorescent staining of Hh signaling transcription factors Gli-1/2 and EMT markers in various Huh-7 subpopulations. CD133+/EpCAM+, CD133−/EpCAM−, Huh-7-DN, and Huh-7-trans cells were seeded on coverslips and stained with primary and secondary antibodies as described in the 'Materials and Methods' section. All subpopulations were counter-stained with DAPI for nuclear imaging. Magnification: × 400.
Enhanced Hh Signaling Activity in CD133−/EpCAM− Subpopulation
To evaluate the Hh signaling activity, freshly sorted CD133+/EpCAM+ and CD133−/EpCAM− subpopulations were transfected with the Gli-Lux firefly reporter cassette, and co-transfected with pRL-TK renilla luciferase reporter control vector for the normalization of transfection efficiency. Two days after transfection, normalized luciferase activity of CD133−/EpCAM− subpopulation was 1.8-fold higher than CD133+/EpCAM+ subpopulation (P<0.01). These two subpopulations did not show any significant difference in Hh signaling activity when transfected with a mutated Gli-Lux reporter cassette (Figure 3a). Moreover, both itraconazole and LDE225, two Hh signaling SMO antagonists, significantly suppressed luciferase activity of CD133−/EpCAM− subpopulation (Figures 3b and c). Notably, LDE225 was effective at 100 nM in suppressing Hh signaling activity in this subpopulation. This data demonstrated that CD133−/EpCAM− subpopulation displayed higher Hh signaling activity than CD133+/EpCAM+ subpopulation, and that specific antagonists targeting the SMO molecule were effective in suppressing Hh signaling activity in this subpopulation.
Hedgehog signaling activity as reflected by Gli-Lux reporter transfection. Normalized luciferase activity of CD133+/EpCAM+ and CD133−/EpCAM− subpopulations was evaluated by co-transfecting with the Gli-Lux in which firefly luciferase is under the control of the Gli promoter and pRL-TK luciferase reporter system in which renilla luciferase is under the control of the TK promoter. (a) Relative luciferase activity in CD133−/EpCAM− subpopulation was determined after transfection with Gli-Lux and a mutated plasmid (Gli-Lux-M). **P<0.01 compared with CD133+/EpCAM+ subpopulation. Treatment with Hh signaling SMO antagonists, itraconazole (b), or LDE225 (c) resulted in a decrease in firefly luciferase activity in CD133−/EpCAM− subpopulation. **P<0.01 compared with CD133−/EpCAM− subpopulation without treatment.
Intrinsic Chemoresistance of Huh-7-DN Subpopulation
To investigate the influence of Hh signaling activity on drug sensitivity in Huh-7 subpopulations with varying Hh signaling activity, CD133+/EpCAM+, CD133−/EpCAM−, Huh-7-DN, and Huh-7-trans subpopulations were treated with itraconazole, LDE225 and a Raf kinase inhibitor, Sorafenib for 24 h. The cell viability was determined by an MTT reagent. As shown in Figures 4a and b, treatment with itraconazole at 5–10 μM resulted in a decrease in cell viability by 13.15 and 14.57% in CD133+/EpCAM+ subpopulation, but no difference was observed in the other three subpopulations. Treatment with sorafenib at 10 μM significantly compromised cell viability in CD133+/EpCAM+, CD133−/EpCAM−, and Huh-7-trans subpopulations, but did not affect Huh-7-DN subpopulation (Figures 4c–f). Neither itraconazole, LDE225, nor sorafenib (10 μM) suppressed cell viability of Huh-7-DN subpopulation. Moreover, Huh-7-DN and Huh-7-trans subpopulations were treated with various concentrations of sorafenib to determine the effects of hedgehog signaling activity on drug sensitivity. Hep3B cells that are well differentiated in hepatic gene expression profile and exhibit positive CD133/EpCAM expression profile, were used as a control.10 The IC50 was calculated to reflect resistance to sorafenib in Hep3B, Huh-7-DN, and Huh-7-trans cell types, and was higher in Huh-7-DN (21.58 μM) than 11.6 μM, and 17.87 μM in Hep3B and Huh-7-trans cells (Supplementary Figures 2A–D). Taken together, the cell viability assay documented that Huh-7-DN possessed intrinsic chemoresistance to sorafenib.
Cell viability of various subpopulations in exposure to chemotherapeutic agents. CD133+/EpCAM+, CD133−/EpCAM−, Huh-7-DN, and Huh-7-trans subpopulations were treated with chemotherapeutic drugs as indicated concentration for 24 h. Cell viability was determined by MTT and expressed as percentage. CD133+/EpCAM+, CD133−/EpCAM−, Huh-7-DN, and Huh-7-trans subpopulations were treated with itraconazole (0–10 μM) only (a and b); or with LDE225 (0–10 μM) plus/minus sorafenib at 10 μM (c–f). *P<0.05, **P<0.01 compared with LDE225 only at corresponding concentrations.
Increased Expression of Hh Signaling Molecules Contributed to Chemoresistance of Huh-7-DN Subpopulation
In order to reveal the controlling mechanisms of chemoresistance of Huh-7-DN subpopulation, expression of Hh signaling transcription factors Gli-1/2 and MDR-related ABC transporters (ABCB1, ABCC1, and ABCG2) was determined in various hepatoma subpopulations. Abnormal activation of Hh signaling in Huh-7-DN and Huh-7-trans subpopulations resulted in an increase in mRNA levels of Gli-1/2 and Bcl-2 genes (Figures 5a–c). Compared with relatively sensitive CD133+/EpCAM+ subpopulation, Gli-1 mRNA levels of Huh-7-DN and Huh-7-trans subpopulations were increased more than 12- and 22-fold. Gli-2 mRNA levels of Huh-7-DN and Huh-7-trans subpopulations were increased more than 31- and 35-fold. Of note, ABCC1 expression levels were higher in Huh-7-DN and Huh-7-trans cells than the other cell types tested (Figure 5d). Therefore, higher expression of Gli-1 and Gli-2 was accompanied with upregulation of ABCC1 expression in Huh-DN and Huh-7-trans subpopulations (2- and 3.5-fold). In contrast, expression levels of ABCB1 and ABCG2 in Huh-7-DN and Huh-7-trans subpopulations were much lower than unsorted Huh-7 and CD133+/EpCAM+ subpopulations (Figures 5e and f). Although Gli-1 and Gli-2 mRNA levels were much higher in Huh-7-DN and Huh-7-trans subpopulations than other hepatoma cells, only Gli-2 protein level was strikingly increased in both Huh-7-DN and Huh-7-trans subpopulations (Figure 6a). Consistent with real-time PCR results, immunofluorescent staining exhibited a similar increase in ABCC1 protein level in Huh-7-DN subpopulation (Figure 6b). Moreover, the ABCC1 protein level of Huh-7-DN was confirmed to be significantly higher than Huh-7-trans subpopulation (Figures 6a and f). These findings indicate that the Hh signaling has a crucial role in the development of chemoresistance in Huh-7-DN subpopulation through the ABCC1 transporter.
Expression of Hh signaling pathway and multidrug-resistant ABC transporters in various subpopulations. Expression of Hh signaling transcription factors, Gli-1 and Gli-2; ABC transporters ABCC1, ABCB1, ABCG2 was determined in Huh-7, CD133+/EpCAM+, CD133−/EpCAM−, Huh-7-DN, and Huh-7-trans subpopulations by quantitative RT-PCR using Huh-7 as a control. (a and b) Relative Gli-1 and Gli-2 mRNA expression over primary human hepatocyte (PH). (c) Relative BCL-2 mRNA expression levels (fold) over Huh-7 cells. (d–f) Relative ABCC1, ABCB1, and ABCG2 mRNA expression levels (fold) over primary human hepatocyte (PH). *P<0.05 compared with Huh-7.
Protein levels of Hh signaling pathway molecules and ABC transporters in various hepatoma cells. (a) Gli-1/2, ABCC1, and ABCG2 protein levels as determined by western blot analysis, and (c–f) arbitrary value of densitometrical analysis of three independent experiments is shown, using β-actin as a loading control. *P<0.05, **P<0.01 compared with Huh-7. (b) Immunofluorescent staining of ABCC1 in CD133+/EpCAM+ (A), CD133-/EpCAM- (B), Huh-7-DN (C), and Huh-7-trans (D). Magnification: × 400.
Pharmacological Suppression of the Hh Signaling Activity Decreased ABCC1 Expression
To further investigate whether Hh signaling is involved in the regulation of ABCC1 transporter gene expression, Huh-7-DN and Huh-7-trans subpopulations were treated with Hh signaling SMO antagonists, LDE225 or itraconazole. As shown in Figures 7 and 8, neither itraconazole nor LDE225-affected Gli-1 mRNA levels in all concentrations tested in both Huh-7-DN and Huh-7-trans subpopulations, whereas Gli-2 expression was suppressed by a high concentration of LDE225 (10 μM) in both Huh-7-DN (P=0.016) and Huh-7-trans subpopulations (P=0.017; Figures 7b and 8b). A significant inhibitory effect on Gli-2 expression was observed by itraconazole at 1 μM, and a dose-dependent decrease of Gli-2 was seen at high concentrations (5–10 μM) in Huh-7-trans subpopulation (Figure 8e). Both LDE225 and itraconazole suppressed expression of ABCC1 in Huh-7-DN subpopulation starting at 1 μM in a dose-dependent manner, and the most obvious decrease of ABCC1 mRNA expression was observed at 10 μM (Figures 7c and f). Consistent with mRNA levels, ABCC1 protein levels were significantly reduced in Huh-7-DN by LDE225 or itraconazole at 10 μM, whereas the reduction of Gli-2 protein levels occurred when the concentration of LDE225 and itraconazole was increased to 5 μM (Figures 7g and h). Both LDE225 and itraconazole (1–10 μM) significantly reduced ABCC1 mRNA expression in Huh-7-trans subpopulation, and subsequently western blot analysis confirmed that ABCC1 protein levels decrease from 1.0 μM (Figures 8g and h). These data further verified that Hh signaling transcription factor Gli-2 appeared to be the primary regulator for drug sensitivity of Huh-7-DN and Huh-7-trans subpopulations through ABCC1 transporter.
The effects of LDE225 and itraconazole on expression of Gli-1, Gli-2, and ABCC1 in Huh-7-DN subpopulation. Huh-7-DN cells were exposed to LDE225 or itraconazole at various concentrations as indicated for 24 h. mRNA levels of Gli-1, Gli-2, and ABCC1 in Huh-7-DN subpopulation were determined by quantitative RT-PCR, using untreated Huh-7 cells as a control. Protein levels of Gli-2 and ABCC1 were determined by western blot analysis, using β-actin as a loading control. Neither LDE225 nor itraconazole effectively suppressed Gli-1 mRNA expression (a and d), whereas LDE225 (10 μM) significantly reduced Gli-2 mRNA expression (b and e). Both LDE225 and itraconazole treatment inhibited ABCC1 mRNA expression in a dose-dependent manner, and a maximum inhibitory effect was observed at 10 μM (c and f). Gli-2 protein levels started to decrease from 5 to 10 μM and ABCC1 protein levels were significantly reduced at 10 μM when exposure to LDE225 or itraconazole in Huh-7-DN subpopulation (g and h). *P<0.05, **P<0.01 compared with untreated Huh-7-DN subpopulation.
The effects of LDE225 and itraconazole on expression of Gli-1, Gli-2, and ABCC1 in Huh-7-trans subpopulation. Huh-7-trans cells were exposed to LDE225 or itraconazole at various concentrations as indicated for 24 h. mRNA levels of Gli-1, Gli-2, and ABCC1 in Huh-7-trans subpopulation were determined by quantitative RT-PCR, using untreated Huh-7 as a control. Protein levels of Gli-2 and ABCC1 were determined by western blot analysis, using β-actin as a loading control. Similar to Huh-7-DN subpopulation, LDE225 or itraconazole did not reduce Gli-1 mRNA expression (a and d). LDE225 or itraconazole could not only significantly inhibit Gli-2 mRNA, but also ABCC1 mRNA expression (b, c, e and f). ABCC1 protein levels started to decrease when LDE225 or itraconazole concentration reached to 1.0 μM (g and h). *P<0.05, **P<0.01 compared to untreated Huh-7-trans subpopulation.
Discussion
In the present study, we compared the phenotypic features of a newly established subpopulation of Huh-7-DN with the previously reported Huh-7-trans subpopulation,14 and other subpopulations or hepatoma cells, and characterized their sensitivity to sorafenib, LDE225 and another Hh inhibitor, itraconazole. The latter is an antifungal agent, and was defined as a potent Hh inhibitor that targets SMO and is distinct from other Hh inhibitors, such as LDE225 or cyclopamine.23 In contrast to the well-differentiated CD133+/EpCAM+ subpopulation that possessed epithelial morphology and α-fetoprotein expression,10 Huh-7-DN and Huh-7-trans cells derived from the CD133−/EpCAM− subpopulation are unique subpopulations characterized by the occurrence of EMT (Figure 2) and enhanced hedgehog signaling activity (Figure 3). These CD133−/EpCAM− subpopulations have been previously demonstrated to be poorly differentiated in terms of their hepatocyte-specific gene expression profile and function.10 Not only did both Huh-7-DN and Huh-7-trans subpopulations retain CD133−/EpCAM− expression profile, but also they appeared to have striking karyotypic alterations and different transcriptome by RNA-Seq (data not shown). Huh-7-trans subpopulation displayed metastatic capability with upregulation of matrix metalloproteinase (MMP)-1/2/9 as we reported recently.14 Huh-7-DN subpopulation exhibited much more resistance to sorafenib than Hun-7-trans and other subpopulations. The viability of Huh-7-DN was not compromised by structurally unrelated chemotherapeutic agents in single use or combination (sorafenib, LDE225, and itraconazole; Figure 4); however, both LDE225 and itraconazole suppressed Hh signaling activity in Huh-7 CD133−/EpCAM− subpopulation in a dose-dependent manner (Figure 3). These data verify that Huh-7-DN cells possess an intrinsic resistance to the chemotherapeutic agents tested, and could be a valuable tool in exploring how Hh signaling modulates drug resistance in HCC.
To explore the underlying mechanisms of chemoresistance in Huh-7-DN cells, we first examined levels of the ABC transporter molecules, and found that ABCC1 expression was significantly increased in both mRNA and protein levels in Huh-7-DN compared with other subpopulations. However, the other two ABC transporters (ABCB1 and ABCG2) were decreased in both Huh-7-DN and Huh-7-trans cells compared with other subpopulations. At the same time, Gli-2 expression levels in both Huh-7-DN and Huh-7-trans cells were much higher (61- and 68-fold compared with Huh-7 cells) than other subpopulations, though there was a moderate increase in Gli-1 level in Huh-7 DN (6.8-fold), and a larger increase in Huh-7-trans cells (12.2-fold) compared with Huh-7 cells. The aberrant Gli-1 expression, especially the occurrence of truncated Gli-1, was thought to be responsible for the highly metastatic property in Huh-7-trans subpopulation as we previously reported.14 Based on these results, it is reasonable to believe that Gli-2 has a more dominant role than Gli-1 in the mediation of chemoresistance in Huh-7-DN subpopulation. Our findings are consistent with a notion that ABCC1 is not expressed in mature hepatocytes (Figure 5d), and its expression levels are negatively correlated with differentiation grade in untreated HCC.24 Moreover, constitutive activation of the Hh pathway maintains chemoresistance through increasing drug efflux of ABC transporters (ABCB1 and ABCG2),25 and Gli-1 maintained multidrug-resistant phenotype of myeloid leukemia by ABCB1.26 These findings are further supported by the observation that ABCB1 and ABCG2 promoter regions have the consensus sequence of Gli-binding site, and Gli-1 directly bound to the consensus GACCACCCA-like motif located in the promoter regions of ABCB1 and ABCG2 to regulate their transcription in B-cell lymphoma and ovarian cancer cells.27, 28 However, our results demonstrate that ABCC1 was highly expressed in Huh-7-DN and Huh-7-trans cells; in contrast, ABCB1 and ABCG2 were downregulated in these two subpopulations. Accordingly, the protein levels of Gli-2 were remarkably increased in Huh-7-DN and Huh-7-trans subpopulations. Given the fact that both Gli-1 and Gli-2 may bind to the same motif in Gli-binding site and initiate transcription of target genes, we speculate that Gli-2, rather than Gli-1, is the major transcription factor controlling the resistance of Huh-7-DN subpopulation to sorafenib through the ABCC1 transporter, which is obviously different from what was observed in myeloid leukemia.
To further confirm the regulation of Gli-2 on ABCC1 expression, itraconazole and LDE225 were separately used to treat various subpopulations. As shown in Figures 7a and d and 8a and d, both LDE225 and itraconazole did not display any inhibition on Gli-1 gene expression in either Huh-7-trans or Huh-7-DN subpopulations. The results demonstrated that inhibition of Gli-2 expression by either itraconazole or LDE225 simultaneously suppressed ABCC1 expression in Huh-7-DN or Huh-7-trans subpopulations (Figures 7 and 8), although the inhibition on Gli-2 expression took place when the concentration of LDE225 was reached to 10 μM. The discrepancy in suppressing Gli-1 and Gli-2 gene expression by SMO antagonists, LDE225 or itraconazole, may be partially attributed to the fact that hedgehog signaling activation is under the control of canonical and noncanonical pathway, and in noncanonical pathway, hedgehog activation is operated in SMO-independent manner.29 This is intriguing, and more experimentation is needed to confirm our speculation. It is also possible that LDE225 was less sensitive in indirectly suppressing Gli expression when compared with its direct inhibition on Hh signaling activity in CD133−/EpCAM− Huh-7 cells. Drug resistance has apparently developed in these cells because LDE225 has been shown to be effective at nM levels.14, 30
To further verify the effect of Gli-2 on ABCC1 expression, we used RNA interference (RNAi) and suppressed Gli-2 expression with transduction of a lentiviral-particle-harboring shRNA against Gli-2 gene in both Huh-7-DN and Huh-7-trans cells, and used cells transduced with lentiviral particles harboring scrambled shRNA as a control (Supplementary Figure 3). Our data demonstrate that shRNA against Gli-2 reduced Gli-2 gene expression by 50–70% at mRNA levels in two clones, and nearly 25–30% reduction at protein levels in both Huh-7-trans and Huh-7-DN cells (P<0.05–0.01). Subsequently, a 10–20% decrease (P<0.05) in ABCC1 mRNA and protein levels was seen in cells transduced with a lentiviral particles harboring shRNA against Gli-2 compared with those transduced with a lentiviral vector harboring scrambled shRNA. Therefore, these data partially support that ABCC1 is the target gene of the Hh signaling in both Huh-7-DN and Huh-7-trans cells, and Gli-2 has a major role in the development of drug resistance through modulating ABCC1 gene expression, although other factors, such as EMT transcription factors, may also participate in this process as discussed below.
Hh signaling has a critical role during embryonic development, and is often silent in adult tissue. It is activated to participate in tissue repair in chronic damage.31 With Hh pathway activation, hedgehog ligands (Sonic, Indian or Desert) bind to the PTCH1 receptor to release the G-protein-coupled receptor (GPCR)-family protein smoothened (SMO), and the latter leads to stabilization and nuclear translocation of GLI family members. Gli-1/2 then transact target genes, such as B-cell lymphoma 2 (Bcl-2), twist, snail, Gli-1 and PTCH-1.32 SMO, one of critical molecules in the Hh signaling pathway, has been a major target for pharmaceutical intervention in cancer therapy, and has been clinically proved to be effective in basal cell cancer and other malignancies although drug resistance has been reported in basal cell cancer against vismodegib.15, 33 As one of Gli-1/2 target genes, BCL-2 exerts its anti-apoptosis effects by binding to pro-apoptotic proteins and preventing the release of cytochrome c from mitochondria.34 Consistent with the expression trend of ABCC1 protein, Huh-7-DN subpopulation had the highest BCL-2 expression level, which was followed by Huh-7-trans subpopulation. Thus, Hh signaling maintains chemoresistance of hepatoma cells through increasing drug efflux mediated by ABCC1 transporter and promoting cell survival by overexpression of Bcl-2.
Huh-7-DN and Huh-7-trans cells exhibited EMT features by overexpression of relevant transcription factors, twist and snail. The occurrence of EMT has been implicated as another mechanism of hedgehog signaling in the mediation of MDR (Supplementary Figure 1). Hh pathway activation directly contributes to the expression of twist and snail.35 The expression levels of twist in Huh-7-DN and Huh-7-trans cells were 300–400-fold higher than in Huh-7 cells, which indicates that this transcription factor may participate in modulating drug resistance through regulating the EMT status. Hence, it was postulated that aberrant hedgehog signaling activation promotes the transacting activity of twist and snail, which may in turn increase the activity of ABC transporters in the downstream. It remains to be seen whether Gli-2 directly transacts ABCC1 or indirectly transacts it through activation of twist and/or snail at a transcriptional level in Huh-7-DN cells.
In conclusion, the present study established a poorly differentiated and chemoresistant CD133−/EpCAM− Huh-7-DN subpopulation exhibiting EMT and upregulation of the ABCC1 transporter. These characteristics are attributed to increased expression of Hh transcription factor Gli-2, which may govern expression of the ABCC1 transporter and contribute to sorafenib resistance of hepatoma cells. Our results reveal the molecular mechanism underlying HCC chemoresistance, and may aid in improving the outcome of chemotherapy in HCC.
References
Lozano R, Naghavi M, Foreman K et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–2128.
Alves RC, Alves D, Guz B et al. Advanced hepatocellular carcinoma. Review of targeted molecular drugs. Ann Hepatol 2011;10:21–27.
Bruix J, Takayama T, Mazzaferro V et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344–1354.
Samuel M, Chow PK, Chan Shih-Yen E et al. Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma. Cochrane Database Syst Rev 2009;1:CD001199.
Ge S, Huang D . Systemic therapies for hepatocellular carcinoma. Drug Discov Ther 2015;9:352–362.
Llovet JM, Ricci S, Mazzaferro V et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390.
Kelley RK . Adjuvant sorafenib for liver cancer: wrong stage, wrong dose. Lancet Oncol 2015;16:1279–1281.
Tovar V, Cornella H, Moeini A et al. Tumour initiating cells and IGF/FGF signalling contribute to sorafenib resistance in hepatocellular carcinoma. Gut 2017;66:530–540.
Sharom FJ . ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics 2008;9:105–127.
Chen X, Lingala S, Khoobyari S et al. Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. J Hepatol 2011;55:838–845.
Chen XL, Cheng QY, She MR et al. Expression of sonic hedgehog signaling components in hepatocellular carcinoma and cyclopamine-induced apoptosis through Bcl-2 downregulation in vitro. Arch Med Res 2010;41:315–323.
Huang S, He J, Zhang X et al. Activation of the hedgehog pathway in human hepatocellular carcinomas. Carcinogenesis 2006;27:1334–1340.
Sicklick JK, Li YX, Jayaraman A et al. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis 2006;27:748–757.
Fan YH, Ding J, Nguyen S et al. Aberrant hedgehog signaling is responsible for the highly invasive behavior of a subpopulation of hepatoma cells. Oncogene 2016;35:116–124.
Jacobsen AA, Aldahan AS, Hughes OB et al. Hedgehog pathway inhibitor therapy for locally advanced and metastatic basal cell carcinoma: a systematic review and pooled analysis of interventional studies. JAMA Dermatol 2016;152:816–824.
Burness CB . Sonidegib: first global approval. Drugs 2015;75:1559–1566.
Lingala S, Cui YY, Chen X et al. Immunohistochemical staining of cancer stem cell markers in hepatocellular carcinoma. Exp Mol Pathol 2010;89:27–35.
Sasaki K, Matsuda M, Ohkura Y et al. In hepatocellular carcinomas, any proportion of poorly differentiated components is associated with poor prognosis after hepatectomy. World J Surg 2014;38:1147–1153.
Liu L, Zern MA, Lizarzaburu ME et al. Poly(cationic lipid)-mediated in vivo gene delivery to mouse liver. Gene Ther 2003;10:180–187.
Zhang Y, Gu J, Zhao L et al. Complete elimination of colorectal tumor xenograft by combined manganese superoxide dismutase with tumor necrosis factor-related apoptosis-inducing ligand gene virotherapy. Cancer Res 2006;66:4291–4298.
Wege H, Le HT, Chui MS et al. Telomerase reconstitution immortalizes human fetal hepatocytes without disrupting their differentiation potential. Gastroenterology 2003;124:432–444.
Wu J, Liu L, Yen RD et al. Liposome-mediated extracellular superoxide dismutase gene delivery protects against acute liver injury in mice. Hepatology 2004;40:195–204.
Kim J, Tang JY, Gong R et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell 2010;17:388–399.
Vander Borght S, Komuta M, Libbrecht L et al. Expression of multidrug resistance-associated protein 1 in hepatocellular carcinoma is associated with a more aggressive tumour phenotype and may reflect a progenitor cell origin. Liver Int 2008;28:1370–1380.
Sims-Mourtada J, Izzo JG, Ajani J et al. Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene 2007;26:5674–5679.
Queiroz KC, Ruela-de-Sousa RR, Fuhler GM et al. Hedgehog signaling maintains chemoresistance in myeloid leukemic cells. Oncogene 2010;29:6314–6322.
Singh RR, Kunkalla K, Qu C et al. ABCG2 is a direct transcriptional target of hedgehog signaling and involved in stroma-induced drug tolerance in diffuse large B-cell lymphoma. Oncogene 2011;30:4874–4886.
Chen Y, Bieber MM, Teng NN . Hedgehog signaling regulates drug sensitivity by targeting ABC transporters ABCB1 and ABCG2 in epithelial ovarian cancer. Mol Carcinog 2014;53:625–634.
Blotta S, Jakubikova J, Calimeri T et al. Canonical and noncanonical Hedgehog pathway in the pathogenesis of multiple myeloma. Blood 2012;120:5002–5013.
Fendrich V, Wiese D, Waldmann J et al. Hedgehog inhibition with the orally bioavailable Smo antagonist LDE225 represses tumor growth and prolongs survival in a transgenic mouse model of islet cell neoplasms. Ann Surg 2011;254:818–823.
Omenetti A, Choi S, Michelotti G et al. Hedgehog signaling in the liver. J Hepatol 2011;54:366–373.
Scales SJ, de Sauvage FJ . Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci 2009;30:303–312.
Gonnissen A, Isebaert S, Haustermans K . Targeting the Hedgehog signaling pathway in cancer: beyond Smoothened. Oncotarget 2015;6:13899–13913.
Kim R, Emi M, Tanabe K . Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol 2006;57:545–553.
Kong Y, Peng Y, Liu Y et al. Twist1 and Snail link Hedgehog signaling to tumor-initiating cell-like properties and acquired chemoresistance independently of ABC transporters. Stem Cells 2015;33:1063–1074.
Acknowledgements
The authors are grateful to Dr Ping Zhou, Stem Cell Program, UC Davis Medical Center, Sacramento, CA, USA for providing cDNA of human primary hepatocytes; and to Mrs Ke Qiao, Shuhui Sun, and Jing Qian in the Departments of Medical Microbiology and Immunology, School of Basic Medical Sciences, Fudan University, for their technical support in the use of confocal microscope and FACS enrichment. The authors thank Professor Zhaoyuan Hou and Dr Yimin Chu at the Faculty of Basic Medicine, Shanghai Jiaotong University School of Medicine, for providing Gli-2 shRNA lentiviral plasmid and their helpful suggestions for Gli-2 knockdown experiments. This work is supported by the National Natural Science Foundation (NSFC #81272436 and 81572356) and the Ministry of Science and Technology (#2016YFE0107400), China, to JW as well as the Young Investigator Program of Jing’an District Municipal Commission of Health and Family Planning (JWRC2014Q01) to JD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
The abstract of this work was presented in the 66th Annual Meeting of the American Association for the Study of Liver Disease (AASLD), 11–15 November 2016, Boston, MA, USA.
Supplementary Information accompanies the paper on the Laboratory Investigation website
This study employed a newly characterized CD133−/EpCAM− subpopulation (Huh-7-DN) that features high hedgehog signaling activity and epithelial-mesenchymal transition to investigate how hedgehog signaling controls chemosensitivity by an ATP-binding complex molecule, ABCC1. The findings demonstrate that transcription factor Gli-2 governs the target gene ABCC1 that modulates drug resistance in this subpopulation.
Rights and permissions
About this article
Cite this article
Ding, J., Zhou, XT., Zou, HY. et al. Hedgehog signaling pathway affects the sensitivity of hepatoma cells to drug therapy through the ABCC1 transporter. Lab Invest 97, 819–832 (2017). https://doi.org/10.1038/labinvest.2017.34
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2017.34
This article is cited by
-
Understanding the versatile roles and applications of EpCAM in cancers: from bench to bedside
Experimental Hematology & Oncology (2022)
-
Drug resistance and Cancer stem cells
Cell Communication and Signaling (2021)
-
miR-1268a regulates ABCC1 expression to mediate temozolomide resistance in glioblastoma
Journal of Neuro-Oncology (2018)