Abstract
Recent studies have suggested that renal Nox is important in the progression of diabetic nephropathy. Therefore, we investigated the effect of a novel pan-NOX-inhibitor, APX-115, on diabetic nephropathy in type 2 diabetic mice. Eight- week-old db/m and db/db mice were treated with APX-115 for 12 weeks. APX-115 was administered by oral gavage at a dose of 60 mg/kg per day. To compare the effects of APX-115 with a dual Nox1/Nox4 inhibitor, db/db mice were treated with GKT137831 according to the same protocol. APX-115 significantly improved insulin resistance in diabetic mice, similar to GKT137831. Oxidative stress as measured by plasma 8-isoprostane level was decreased in the APX-115 group compared with diabetic controls. All lipid profiles, both in plasma and tissues improved with Nox inhibition. APX-115 treatment decreased Nox1, Nox2, and Nox4 protein expression in the kidney. APX-115 decreased urinary albumin excretion and preserved creatinine level. In diabetic kidneys, APX-115 significantly improved mesangial expansion, but GKT137831 did not. In addition, F4/80 infiltration in the adipose tissue and kidney decreased with APX-115 treatment. We also found that TGF-β stimulated ROS generation in primary mouse mesangial cells (pMMCs) from wild-type, Nox1 KO, and Duox1 KO mice, but did not induce Nox activity in pMMCs from Nox2 knockout (KO), Nox4 KO, or Duox2 KO mice. These results indicate that activating Nox2, Nox4, or Duox2 in pMMCs is essential for TGF-β-mediated ROS generation. Our findings suggest that APX-115 may be as effective or may provide better protection than the dual Nox1/Nox4 inhibitor, and pan-Nox inhibition with APX-115 might be a promising therapy for diabetic nephropathy.
Similar content being viewed by others
Main
Reactive oxygen species (ROS) are constitutively generated in cells and are required to sustain numerous physiologic processes.1 Generally, ROS mediate cellular signaling; however, when the balance between ROS production and scavenging is disrupted, they cause oxidative stress resulting in tissue injury.2 Excessive ROS production has been implicated in various renal disease processes, including diabetic nephropathy, hypertensive nephropathy, acute kidney injury, and immune-mediated glomerulonephritis.3
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (Noxs) catalyze electron transfer from NADPH to molecular oxygen and are the major sources of ROS in the kidney.4 There are seven Nox isoforms (Nox1–5, Duox, Duox2), and Nox1, Nox2, Nox4, and Nox5 are widely expressed throughout renal tissues.5 Nox4 is the most abundant Nox in the kidney and has been reported to have a central role in oxidative stress induced by type 1 and 2 diabetes.
Several lines of evidence suggest that Nox4 may also have a protective role. Overexpression of Nox4 in endothelial cells promotes angiogenesis and recovery from hypoxia in an eNOS-dependent manner.6 In addition, Nox4-transgenic mice showed cardiac protective effect by Nox4-mediated activation of vascular endothelial growth factor in chronic load-induced stress in mouse hearts.7 Moreover, endogenous Nox4 protects the vasculature during ischemic or inflammatory stress.8 These results suggest that adequate constitutive production of H2O2 by this enzyme could maintain a basal anti-oxidative tone and thus desensitize organs against acute redox challenges.
However, a large body of literature suggest that oxidative stress is increased and Nox4 has been proposed as a major source of ROS in diabetic nephropathy. Accordingly, several studies have focused on Nox4 as a potential therapeutic target for diabetic kidney disease.9, 10, 11, 12 A study by Gorin et al13 showed that administering antisense oligonucleotides for Nox4 to rats with streptozotocin-induced diabetes prevented renal hypertrophy and matrix expansion. Also, systemically knocking out Nox4 in mice with streptozotocin-induced diabetes protected the kidneys from glomerular injury.9
As all components of Nox isoforms are expressed in various parts of the kidney, including renal vessels, glomeruli, and tubulointerstitial tissues, ROS generation can be induced in different renal tissues by many pathologic stimuli such as high glucose, angiotensin II, advanced glycation end products, and aldosterone.4 In addition to Nox4, Nox1 and Nox2 are also involved in the development of diabetic nephropathy.14 Therefore, targeting all the Nox components may be a promising therapeutic strategy to ameliorate renal damage from ROS in diabetic nephropathy. Recently, pharmacologically inhibiting Nox4 and Nox1 with dual inhibitors GKT136901 and GKT137831 in mouse models of type 1 and type 2 diabetic nephropathy effectively reduced systemic oxidative stress, albuminuria, and kidney fibrosis.15, 16 These previous studies suggested the potential protective effect of dual Nox1/Nox4 inhibition in diabetic kidney disease. In this study, we investigated the effect of a recently developed pan-Nox-inhibitor, APX-115, compared with a dual Nox1/Nox4 inhibitor, GKT137831, in a mouse model of type 2 diabetic kidney disease.
Materials and methods
Animal Studies
Six-week-old male diabetic db/db mice (C57BLKS/J-leprdb/leprdb) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The mice had free access to food and tap water and were caged individually under controlled temperature (23±2 °C) and humidity (55±5%) with an artificial light cycle. The mice were fed standard chow (Cargill Agri Purina Korea, Korea). APX-115 and GKT137831 were designed and manufactured as described previously.17 APX-115 was originally named as Ewha-18278 before it was transferred to Aptabio Therapeutics, and these two drugs were same compounds. To investigate the effect of APX-115 compared with GKT137831, 8-week-old mice were divided into five groups: (1) nondiabetic control db/m mice (n=7), (2) db/m mice treated with APX-115 (n=10) (3) control db/db mice treated with vehicle (n=8), (4) db/db mice treated with GKT137831 (n=9), and (5) db/db mice treated with APX-115 (n=10). APX-115 and GKT137831 were administered by oral gavage at a dose of 60 mg/kg per day for 12 weeks. As the IC50s for GKT137831 was 110–140 nM, and that for APX-115 was 100–150 nM, we used the same dosage of 60 mg/kg per day in this study. Food and water intake, urine volume, body weight, fasting blood glucose concentration, and HbA1c level were measured every month. Plasma glucose level was measured with glucose oxidase method, and HbA1c level was calculated with the IN2IT system (Bio-Rad Laboratories, Hercules, CA, USA). Plasma levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were determined using the ELISA kit (BioVision, Milpitas, CA, USA), following the manufacturers’ instructions. The homeostasis model assessment index of insulin resistance (HOMA-IR) was calculated as fasting glucose (mmol/l) × fasting insulin (mU/l)/22.5. Plasma triglyceride and cholesterol analyses were performed with a GPO-Trinder kit (Sigma-Aldrich, St Louis, MO, USA). Plasma lipoprotein profiles were measured with a fast protein HPLC system. Insulin tolerance testing was conducted in db/db mice after an 8 h fast, and the blood samples were collected from the tail vein. The mice received 0.75 U/kg regular insulin by i.p. injection, and blood glucose was subsequently measured at 0, 30, 60, 90, and 120 min. Individual mice were separated in a metabolic cage where urine was collected and measured for a 24 h period every month. Urinary albumin excretion was determined with a competitive ELISA (ALPCO, Westlake, OH, USA). Plasma and urinary levels of 8-isoprostane were measured with an ELISA kit (Cayman Chemical, Ann Arbor, MI, USA), and urinary levels were corrected according to urine creatinine concentrations. Lipids from hepatic, adipose, and renal cortex tissues were extracted with the Bligh and Dyer method.18 Total cholesterol and triglyceride content were measured with a commercial kit (Wako Chemicals, Richmond, VA, USA). The extent of peroxidative reaction in hepatic tissue, adipose tissue, and kidney tissue was determined by directly measuring lipid hydroperoxide (LPO) with an LPO assay kit (Cayman Chemical) as described previously.19 At the end of the study period, systolic blood pressure (BP) was measured by tail-cuff plethysmography (LE 5001-Pressure Meter, Letica SA, Barcelona, Spain). All ELISA analyses were performed in triplicate, and the results were averaged. The mice were killed under anesthesia with i.p. injections of tribromoethanol (Avertin; 50 mg/kg) and epididymal fat, liver, and kidney tissues were weighed and snap frozen in liquid nitrogen. All the experiments were conducted in accordance with NIH guidelines and with the approval of the Korea University Institutional Animal Care and Use Committee.
Histological and Immunohistochemical Analysis
Kidney, hepatic, and adipose tissues were fixed for 48 h with 10% paraformaldehyde at 4 °C, dehydrated, embedded in paraffin, cut into 4 μm thick slices, and stained with periodic acid-Schiff (PAS), Masson’s trichrome (MT), and hematoxylin and eosin. For immunohistochemical staining, the sections were microwaved for 10–20 min to retrieve antigens for TGF-β1, nephrin, α-SMA, nitrotyrosine, WT-1, and F4/80 staining. Alternatively, the sections were transferred to Biogenex Retrievit buffer (pH 8.0; InnoGenex, San Ramon, CA, USA) and microwaved for 10–20 min to retrieve antigens for plasminogen activator inhibitor (PAI)-1 staining, or treated with trypsin (Sigma-Aldrich) for 30 min at 37 °C to detect type IV collagen. To block endogenous peroxidase activity, 3.0% H2O2 in methanol was applied to the tissue sections for 20 min. The samples were then incubated at room temperature for 60 min in 3% BSA/3% normal goat serum (type IV collagen, α-SMA nitrotyrosine, WT-1, and F4/80), 30 min with 5% normal goat serum (nephrin), 15 min with 10% powerblock (PAI-1), or 30 min with 20% normal sheep serum (TGF-β1). The slides were incubated overnight at 4 °C with rabbit polyclonal anti-TGF-β1 antibodies (1:200; Santa Cruz Biotechnology, CA, USA), mouse monoclonal anti-F4/80 antibodies (1:100; Serotec, Raleigh, NC, USA), rabbit polyclonal anti-type IV collagen antibodies (1:150; BioDesign International, Sarco, ME, USA), guinea pig polyclonal anti-nephrin antibodies (1:50; PROGEN Biotechnik GmbH, Heidelberg, Germany), rabbit polyclonal anti-PAI-1 antibodies (1:60; American Diagnostica, Stamford, CT, USA), rabbit polyclonal anti-WT-1 antibodies (1:400, Santa Cruz Biotechnology), mouse monoclonal anti-nitrotyrosine antibodies (1:400; Santa Cruz Biotechnology), or rabbit polyclonal anti-α-SMA antibodies (1:100; Santa Cruz Biotechnology). After incubating overnight, the slides were incubated with secondary antibodies for 30 min. The immunoreactive areas were detected via incubation at room temperature in 0.05% 3,3-diaminobenzidine containing 0.01% H2O2 followed by counterstaining with Mayer’s hematoxylin. Negative control sections were stained under identical conditions but with a buffer solution instead of primary antibody. Glomerular mesangial expansion was scored semiquantitatively, and the percentage of mesangial matrix occupying each glomerulus was rated from 0 to 4 as follows: 0, 0%; 1, <25%; 2, 25–50%; 3, 50–75%; and 4, >75%. To evaluate immunohistochemical staining for type IV collagen, TGF-β1, nitrotyrosine, WT-1, and PAI-1, glomerular fields were graded semiquantitatively under a high-power field containing 50–60 glomeruli, and an average score was calculated.19 All histologic examinations were conducted by a trained pathologist in a blinded manner.
Analysis of Gene Expression by Real-Time Quantitative PCR
Total RNA was extracted from renal cortex tissues and experimental cells with Trizol reagent. The primers were designed with Primer 3 software, while the secondary structures of the templates were examined and excluded using mfold software. The nucleotide sequences of all primers used in this study are shown in Supplementary Table 1. Quantitative gene expression was performed on a LightCycler 1.5 system (Roche Diagnostics Corporation, Indianapolis, IN, USA) using SYBR Green technology. Thermocycling conditions consisted of 22–30 cycles of denaturation for 10 s at 95 °C followed by annealing and extension for 30 s at 60 °C. The expression of each gene relative to β-actin (relative gene expression) was calculated by subtracting the threshold cycle number (Ct) of the target gene from that of β-actin and taking 2 to the power of that number. The specificity of each PCR product was evaluated by melting curve analysis, followed by agarose gel electrophoresis.
Protein Extraction and Western Blot Analysis
Nuclear and cytoplasmic proteins from renal cortex tissues or cells were extracted with a commercial nuclear extraction kit according to the manufacturer’s instructions (Active Motif, Carlsbad, CA, USA). Protein concentration was determined with the bicinchoninic acid method (Pierce Pharmaceuticals, Rockford, IL, USA). For western blotting, 40 μg of protein was electrophoresed on a 10% SDS-PAGE minigel. The proteins were transferred onto polyvinylidene difluoride membrane, which was hybridized in blocking buffer overnight at 4 °C with goat polyclonal anti-TLR4 (1:500, Santa Cruz Biotechnology), rabbit polyclonal anti-NF-kB p65 antibody (1:1000, Cell Signaling Technology, USA), rabbit monoclonal anti-phospho IκBα antibody (1:1000, Cell Signaling Technology), rabbit polyclonal anti-Nox1 (1:1000, Abcam Plc, Cambridge, MA, USA), rabbit polyclonal anti-Nox2 (1:1000, Bioworld Technology, St Louis Park, MN, USA), rabbit polyclonal anti-Nox4 (1:1000, Bioworld Technology), mouse monoclonal anti-β actin antibody (1:5000, Sigma-Aldrich) or TATA binding protein (1:2000, Abcam Plc). The membrane was subsequently incubated with horseradish peroxidase-conjugated secondary antibody diluted 1:1000 for 60 min at room temperature. The signals were detected with the enhanced chemiluminescence method (Amersham, Buckinghamshire, UK).
Analysis of Antibody Against Nox Isozyme
We generated rabbit polyclonal antibodies against Nox1, Nox2, and Nox4, respectively. Nox1 antibody (Nox1 Ab) detected 63 kDa Nox1 in smooth muscle cells from wild-type mice, whereas the antibody failed to recognize the protein in Nox1 KO mice (Supplementary Figure 1A). Nox2 antibody (Nox2 Ab) detected 65 kDa Nox2 in macrophages from wild-type mice, whereas the antibody failed to recognize the protein in Nox2 KO mice (Supplementary Figure 1B). Nox4 antibody (Nox4 Ab) detected 62 kDa protein in kidney from wild-type mice. However, the antibody did not recognize the protein in Nox4 KO mice (Supplementary Figure 1C).
Nox1, Nox2, Nox4, and Duox2 Knockout Mice
Nox1 KO mice (B6.129X1-Nox1tm1Kkr/J, stock number 018787), Nox2 KO mice (B6.129S-Cybbtm1Din/J, stock number 002365), and Duox2 KO mice (B6.129-Duox2thyd/J, stock number 005543) were purchased from Jackson Laboratory. Generation of Nox4 KO mice was described in a previous report.20
Generation of Duox1 Knockout Mouse using CRISPR-Cas9
C57BL/6J female mouse was superovulated with intraperitoneal (IP) injection of 5 I.U. pregnant mare serum gonadotropin (Merck KGaA, Darmstadt, Germany) followed by human chorionic gonadotropin (Merck KGaA) 48 h later, then bred to C57BL/6J male mice. Next morning, one-cell embryos were obtained from the oviduct and cultured in the microdrop of KSOM media (Merck KGaA) under the mineral oil (Merck KGaA) until microinjection as described elsewhere. Cas9 mRNA and Duox1 sgRNA were purchased from ToolGen (Seoul, South Korea). The target sequence for Duox1 sgRNA is 5′-CTTAGTAACAGGGTCATGAGAGG-3′ (underline indicates PAM sequence). Cas9 mRNA (10 ng/μl) and sgRNA (16 ng/μl) dissolved in 10 mM Tris-HCl, pH 7.4, 0.25 mM EDTA were microinjected into the cytoplasm of one-cell embryos and the embryos that survived microinjection were further cultured overnight as described above. Two-cell embryos were transferred into the oviducts of pseudopregnant ICR females anesthetized with IP injection of 2.5% Avertin (Merck KGaA). Initial screening of Indel mutation was performed with PCR using DNA extract from F0 mice and agarose gel electrophoresis. Only the mutant mice were bred to C57BL/6J mice and the nature of Indel mutation was further characterized with PCR using DNA extract from F1 heterozygotes mice. The PCR product was subcloned into TA cloning vector as per the manufacturer’s protocol (Promega, Fitchburg, WI, USA) and analyzed by sequencing. The primer sequences for PCR are 5′-CTCTCCTATGCCAGGCTCTC-3′ (sense), 5′-TGTACGGTTCCGAAGAGATG-3′ (antisense). All mice experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Ewha Womans University (Permit number: 2015-01-072). We have obtained two F1 heterozygotes mice having 20 bp deletion. A 20 bp deletion (276–295 bp) provided premature stop codon generation. The heterozygotes mice with 11 bp deletion (Duox1em1Bys) were maintained by backcrossing with C57BL/6J mice two to three more generations before obtaining the homozygous mutant mice. The primer sequences for PCR genotyping are as follows. Duox-S: 5′-CTCTCCTATGCCAGGCTCTC-3′ (sense), Duox-AS: 5′-TGTACGGTTCCGAAGAGATG-3′ (antisense). Genotyping with Duox-S/Duox-AS resulted in 160 bp and 140 bp for wild-type allele and mutant-type allele, respectively (Supplementary Figure 2).
Preparation of Primary Mouse Mesangial Cells
To further define the role of various Nox isoforms in glomerular sclerosis, the experiments were performed with mouse mesangial cells. A part of the renal cortex was obtained immediately after surgical nephrectomy from wild-type mice and mice with various Nox isoforms knocked out. Glomeruli were isolated by the differential sieving method with some modifications.21 Isolated glomeruli were plated on a 100 mm culture dish and maintained at 37 °C under 5% CO2 condition in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 20% (v/v) fetal bovine serum (FBS; PAN BIOTECH, Aidenbach, Germany,) and 1% antibiotic-antimycotic solution (Welgene, Daegu, Korea LS 203-01). Migrated mesangial cells from isolated glomeruli transferred onto collagen-coated culture dish. Purity of primary mesangial cells was examined with antibody against α-SMA as a marker of mesangial cell. Immunohistochemistry of primary mesangial cells with antibody against α-SMA resulted in a positive signal. However, the primary cells failed to react with antibody to WT-1. Therefore, purified cells from mice turned out to be mesangial cells (Supplementary Figure 3).
Podocyte Culture Experiments
The mouse podocyte cell line was obtained from Peter Mundel at the Albert Einstein College of Medicine, NY, USA. The cells were cultured in RPMI medium supplemented with 10% FCS. To evaluate the effect of high glucose on NF-κB, NOX2, NOX4, MCP-1, and fibrotic molecules such as TGF-β1, PAI-1, and type IV collagen, subconfluent cells were serum starved for 24 h. Then the medium was replaced with fresh medium containing 30 mM d-glucose and cultured for 72 h, and harvested for measurement of each molecules. To test the effects of APX-115, APX-115 (5 μ M) was added to cells 60 min before treatment with high glucose. All the experiments were performed with three technical replicates.
Measurement of Intracellular ROS by DCF-DA
Primary mouse mesangial cells (pMMCs) from wild-type and Nox knockout mice were serum starved for 6 h and then stimulated with recombinant human TGF-β1 (10 ng/ml) for 10 min. The cells were washed with Hank’s balanced salt solution (HBSS) and incubated for 10 min in the dark at 37 °C in HBSS containing 10 μM DCF-DA (2',7'-dichlorofluorescindiacetate, Molecular Probes). Fluorescence from oxidized DCF was detected by a Zeiss LSM510 confocal microscope (version 2.3 Minneapolis, MN, USA) at excitation and emission wavelengths of 488 nm and 515–540 nm, respectively. Five fields were randomly selected for fluorescence measurements and the mean relative fluorescence intensity was used. All the experiments were repeated at least three times.
Statistical Analysis
Nonparametric analyses were used due to the relatively small number of samples. The results were expressed as mean±s.e.m. Comparisons were performed using Wilcoxon rank-sum tests and Bonferroni correction. A Kruskal–Wallis test was used to compare more than two groups, followed by a Mann–Whitney U-test. The values of P<0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS for Windows, version 20.0 (IBM Corporation, New York, NY, USA).
Results
Physical and Biochemical Parameters in Experimental Animals
Table 1 shows the physical and biochemical results of each group. The db/db mice had significantly higher body weights, blood glucose levels, and HbA1c levels than did db/m controls, as expected. These parameters did not differ among the treated groups and vehicle group in diabetic mice. Food and water intake at 12 weeks in the APX-115 group and water intake during the treatment period in the GKT137831 group were significantly increased. However, these differences did not affect the body weight or urine volume. Systolic blood pressure was significantly lower in diabetic mice than in db/m controls, and the diabetic groups did not differ. Kidney, liver, and fat tissue mass were significantly increased in db/db mice, and did not change with Nox inhibitor treatments (Table 2).
Effect of APX-115 on Lipid Peroxidation and Oxidative Stress in Experimental Animals
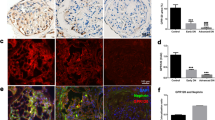
APX-115 and GKT137831 target Noxs and are expected to reduce oxidative stress. Therefore, the plasma and urine 8-isoprostane levels as an oxidative stress marker in mice were examined. The plasma and urine 8-isoprostane levels increased significantly in db/db mice. APX-115 significantly reduced plasma but not urine 8-isoprostane levels. GKT137831 did not affect plasma or urine 8-isoprostane levels (Figure 1a and b). Tissue lipid peroxidase levels in the kidney, liver, and adipose tissue did not differ significantly among the groups (Figure 1c–e). We next measured ROS generation in the kidney tissues of db/m and db/db mice with dihydroethidium (DHE). Kidney tissues in control db/db mice showed a significant increased DHE staining, compared with that in db/m mice. Treatment of db/db mice with APX-115 or GKT137831 resulted in inhibited DHE production in kidney tissues (Figure 1f and g). Furthermore, nitrotyrosine expression was markedly increased in the diabetic kidney, treatment with APX-115 showed significant reduction in nirotyrosine levels in the diabetic kidney (Supplementary Figure 4). Taken together, these results indicate that urine isoprotane production was mediated by DHE generation and nitrotyrosine production in db/db mice (Figure 1f and g, Supplementary Figure 4).
Effect of APX-115 on oxidative markers. (a) Plasma 8-isoprostane level. (b) Urine 8-isoprostane level. (c) Kidney tissue lipid peroxidase (LPO) level. (d) Fat tissue LPO level. (e) Liver tissue LPO level among groups. (f) Kidney tissues from untreated or APX-115 group of db/m or db/db mice were embedded and freshly cut at 10 μm thickness. Tissue slides were stained with dihydroethidium (DHE, 5 μ M, Molecular Probes) for 10 min at 37 °C to evaluate superoxide production with red fluorescence (585 nm) by Carl Zeiss vision system (LSM510 meta). (g) Quantification of DHE level in (f). *P<0.05, **P<0.01, ***P<0.001 vs db/m groups. ##P<0.01, ###P<0.001 vs db/db control.
Effect of APX-115 on Insulin Resistance in Experimental Animals
We examined whether reduced oxidative stress changed the metabolic profiles. Blood glucose and HbA1c levels did not differ among diabetic groups, but insulin resistance improved significantly after 12 weeks of Nox inhibition. Insulin resistance was determined by the insulin tolerance test. Both the APX-115 and GKT137831 groups had a larger decrease in blood glucose concentration at 60 min after insulin injection than did control mice (Figure 2a). This result was consistent with plasma insulin and HOMA-IR, another index of insulin resistance (Figure 2b and c).
Effect of APX-115 on insulin resistance and lipid profiles. (a) Insulin tolerance test after 12-week treatment with Nox inhibitors. (b) Plasma insulin level. (c) Homeostasis model assessment of insulin resistance (HOMA-IR) level. (d) Plasma lipid concentrations. (e) Tissue cholesterol concentrations. (f) Tissue triglyceride concentrations among groups. *P<0.05, **P<0.01, ***P<0.001 vs db/m groups, #P<0.05, ##P<0.01, ###P<0.001 vs db/db control.
Effect of APX-115 on Lipid Profile in Experimental Animals
Considering the remarkable improvement in insulin resistance, we examined the systemic lipid profile and tissue lipid profile. Lipid abnormalities improved significantly with APX-115 treatment. Plasma lipid concentrations improved significantly with both APX-115 and GKT137831 treatment (Figure 2d). Cholesterol content in the kidney, fat, and liver tissues was significantly reduced with APX-115 or GKT137831 treatment (Figure 2e). Triglyceride content in the kidney decreased significantly with APX-115, but not GKT137831, whereas triglyceride content in the liver decreased significantly with GKT137831 (Figure 2f). Supplementary Figure 5 shows the representative liver pathology in the experimental groups at the end of the study period. In accordance with improved tissue lipid abnormalities, both GKT137831 and APX-115 treatment improved the liver function and hepatic steatosis (Supplementary Figure 5).
Effect of APX-115 on Renal Function in Experimental Animals
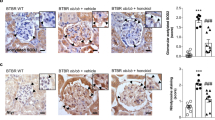
Twenty-four hour urinary albumin excretion was significantly higher in db/db mice than in db/m controls throughout the study period, indicating overt kidney injury in db/db mice. APX-115 markedly decreased urinary albumin excretion at the end of the study compared with diabetic controls and the GKT137831 group (Figure 3a). Serum creatinine levels were significantly lower in the Nox inhibitor groups (Figure 3b). Renal histology findings were consistent with improved urinary albumin excretion and creatinine levels (Figure 4). APX-115 treatment significantly attenuated mesangial expansion in diabetic mice, but GKT137831 did not (Figure 3c). Immune reactivity to profibrotic and proinflammatory molecules improved significantly with both APX-115 and GKT137831 in diabetic mice. As expected, nephrin and WT-1 expression was markedly decreased in diabetic kidneys, and APX-115 attenuated the reduction (Figure 4,Supplementary Figure 6C).
Effect of APX-115 on renal function. (a) Twenty-four hour urine albumin excretion after 12 weeks of treatment. (b) Plasma creatinine level. (c) Semi-quantitative scoring of the kidney as described in the ‘Materials and Methods’. *P<0.05, **P<0.01, ***P<0.001 vs db/m groups, #P<0.05, ##P<0.01, ###P<0.001 vs db/db control.
Immunohistochemistry findings of the kidney. (a1–a5) PAS, periodic acid-Schiff stain; (b1–b5) α-SMA, alpha-smooth muscle actin stain; (c1–c5) PAI-1, plasminogen activator inhibitor-1 stain; (d1–d5) TGF-β, transforming growth factor beta-1 stain; (e1–e5) type IV collagen stain; (f1–f5) nephrin stain. 1, db/m control; 2, db/m+APX-115; 3, db/db control; 4, db/db+GKT; 5, db/db+APX-115. Original magnification × 1000.
Effect of APX-115 on Inflammatory and Profibrotic Processes in Experimental Animals
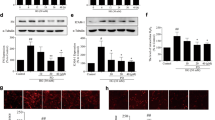
As shown in Figure 5a, macrophage infiltration, as measured by F4/80 staining, was remarkably increased in the glomeruli of db/db mice. APX-115 and GKT137831 treatment ameliorated macrophage infiltration of diabetic glomeruli. Fat tissue of diabetic mice had significantly increased F4/80 staining with adipocyte enlargement. APX-115-treated mice had reduced macrophage infiltration. Because the renal effects of APX-115 were remarkable, we analyzed Nox protein expression in the kidney. Expression of Nox2 and Nox4 protein was significantly increased in db/db controls (Figure 5b). TLR4 and NF-κB p65 expression also increased, which was consistent with Nox protein expression. In addition, APX-115 treatment significantly suppressed the expression of inflammatory molecules including MCP-1, IL-6, and TNFα in the diabetic kidney (Figure 5c). APX-115 significantly attenuated Nox1, Nox2, and Nox4 protein expression, whereas GKT137831 attenuated only Nox1 and Nox4 expression. Both TLR4 and NF-κB p65 expression was reduced in both APX-115- and GKT137831-treated kidneys. The improved lipid profile and insulin resistance and reduced F4/80-positive cells in fat tissue indicate that APX-115 exerts certain effects on fat tissue. Therefore, we examined gene expression in fat tissue. Moreover, mRNA expression of the inflammatory chemokines MCP-1 and PAI-1 decreased significantly with both Nox inhibitors in diabetic mice (Figure 5d).
Effect of APX-115 on inflammatory and profibrotic processes in experimental animals. (a) F4/80 staining in the kidney and fat tissues. (b) Protein expression of Noxs in kidney tissue. TLR4, Toll-like receptor 4, TBP, TATA binding protein. (c) mRNA expression of inflammatory cytokines in kidney tissue. (d) mRNA expression of proinflammatory and profibrotic gene expressions in fat tissue. *P<0.05, **P<0.01 vs db/m groups, #P<0.05, ###P<0.001 vs db/db control.
Effects of APX-115 on Proinflammatory and Profibrotic Molecule Synthesis in Cultured Podocytes
Finally, we performed an in vitro experiment to further evaluate the direct effect of APX-115 treatment in terms of anti-inflammatory and anti-fibrotic effects in cultured podocytes. As shown in Supplementary Figure 6A, B and D, stimulation with high glucose-induced significant increases in the expression of NF-κB p65, NOX2, NOX4, MCP-1, and profibrotic molecules such as TGF-β1, PAI-1, and collagen IV. Prior treatment with APX-115 almost completely suppressed this high glucose-induced proinflammatory and profibrotic molecule expression. Taken together, these results suggested the direct renoprotective effects of APX-115 in diabetic condition.
Effect of Knocking out Nox Isoforms on TGF-β-Induced ROS Generation in Primary Cultured Mesangial Cells
It has been well known that diabetic nephropathy (DN) can be induced by NADPH oxidase (Nox)-dependent reactive oxygen species (ROS). To investigate whether TGF-β, as a fibrogenic factor in renal disease, stimulates Nox isozymes in glomeruli, we prepared primary mouse mesangial cells (pMMCs) from wild-type and Nox knockout mice (Nox1 KO, Nox2 KO, Nox4 KO, Duox1 KO, and Duox2 KO). TGF-β stimulated ROS generation in pMMCs from WT, Nox1 KO, and Duox1 KO mice, but did not induce Nox activity in pMMCs from Nox2 KO, Nox4 KO, or Duox2 KO mice (Figure 6). These results indicate that activating Nox2, Nox4, or Duox2 in pMMCs is essential for TGF-β-mediated ROS generation. Therefore, these Nox isozymes serve as potential therapeutic targets for treating diabetic nephropathy. Thus, a Nox2/Nox4/Duox2 inhibitor is likely to be a more effective agent for treating diabetic nephropathy. Recently, we reported that APX-115 had Ki values for Nox2, Nox4, and Duox2 of 0.86, 2.04, 1.66 μ M, respectively. These results indicate that APX-115 is a good inhibitor of Nox2/Nox4/Duox2 isozymes.17 We validated the inhibitory function of APX-115 on HG-mediated ROS and fibronectin production. Treatment of mesangial cells with APX-115 as a pan-Nox inhibitor resulted in suppressed ROS and fibronectin (FN) production (Supplementary Figure 7).
Effect of knocking out Nox isoforms on TGF-β-induced ROS generation in primary cultured mesangial cells. Primary mouse mesangial cells (pMMCs) from wild-type (WT), Nox1 knockout (KO), Nox2 KO, Nox4 KO, Duox1 KO, and Duox2 KO mice were serum starved for 6 h, stimulated with TGF-β1 (10 ng/ml) for 10 min, and then incubated within 2', 7'-dichlorofluorescindiacetate (DCF-DA) for 10 min. ROS generation from each sample was monitored by confocal microscopy (N=3, data shown as mean±s.d.).
Discussion
Our study provides evidence that a novel compound, APX-115 exerts a renoprotective effect in type 2 diabetic mice. In diabetic mice, APX-115 treatment significantly improved systemic oxidative stress, insulin sensitivity, and lipid profile. In the kidney, APX-115 attenuated Nox gene upregulation and protein expression while improving inflammatory and fibrotic processes. Broad inhibition of Nox isoforms by APX-115 was similar or superior to selective inhibition by GKT137831 in reducing albuminuria and preserving renal function.
Several lines of evidence indicate that reactive oxygen species (ROS) seem to be essential mediators of normal cell physiology.22, 23, 24, 25 Intracellular ROS produced by Nox regulate transcriptional factors either by forming disulfide bonds with DNA-binding domains or by modulating redox signaling pathways. Excessive ROS activate mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK1/2), c-Jun N-terminal protein kinases (JNKs), and NF-κB, thereby inducing proinflammatory gene expression leading to an inflammatory cascade.1, 2 Moreover, under pathological conditions, uncontrolled ROS formation is associated with the upregulation of different Nox subtypes.24, 25, 26, 27 Inhibition of Nox isozymes by treatment of APX-115 reduced ROS generation and then attenuated Nox isozymes expression. The result suggests that Nox inhibitor regulated vicious cycle of oxidative stress leading to downregulation of Nox isozymes expression (Supplementary Figure 2). Pharmaceutical Nox inhibition is expected to reduce ROS production, thereby reducing tissue injury induced by oxidative stress. Recent studies in different animal models of diabetes have shown that selective Nox1/Nox4 inhibitors protect against glomerulosclerosis and progressive renal injury.15, 16
Of the seven Nox isoforms, Nox1, Nox2, Nox4, and recently Nox5 have been found in glomerular cells (mesangial and podocytes), glomerular endothelial cells, vascular smooth muscle cells, and tubulointerstitial cells.28 Although Nox5 has been recently identified in human diabetic kidneys, Nox5 is absent from the mouse and rat genome, not that it has not been identified there.29 Therefore, we did not address Nox5 in our study. Growing bodies of evidence indicated that various Nox isozymes including Nox1, Nox2, and Nox4 are involved in pathogenesis of diabetic nephropathy.5, 9, 30, 31 Nox4 is the most abundant isoform in the kidney and has attracted the most interest as a potential therapeutic target in diabetic kidney disease. Although the role of Nox4 in the progression of renal diseases, such as unilateral ureteral ligation (UUO) and the 5/6 nephrectomy model, has been contested,32 a huge body of evidence suggests a pathogenic role of Nox4 in diabetic nephropathy. Recent studies demonstrate a major pathological role of Nox1 in the progression of diabetic atherosclerosis.33, 34 In our results, Nox2, Nox4, and Duox2 were required for ROS generation in response to TGF-β. It is likely that activity of three different Nox isozymes contributes to TGF-β-mediated ROS generation in mesangial cells. Regulation mechanisms of each Nox isozyme, Nox2, Nox4, and Duox2 are different, whereas coordination of Nox isozymes in the same cell is far from clear. Furthermore, physiological signals emanating from Nox-dependent ROS generation are integrated into a pathogenesis of inflammation, and fibrosis in kidney remains to be elucidated. Function of Nox4 and Duox2 in Nox2 KO mice, Nox2 and Duox2 in Nox4 KO mice, and Nox2 and Nox4 in Duox2 KO mice await further studies. Therefore, these results imply that various Nox isoforms have different roles in the pathogenesis of diabetic vascular complications, and, more generally, that inhibiting ROS generation may be a promising strategy to improve diabetic vasculopathy.
In the present study, we found that APX-115 treatment markedly improved insulin resistance, including reducing insulin levels and HOMA-IR index scores. Improved insulin resistance was further confirmed by an insulin tolerance test. APX-115-treated animals exhibited much better lipid profiles, including decreased total cholesterol and triglyceride levels associated with improved lipid contents in various organs such as kidney, adipose tissue, and liver. However, we did not observe a significant decrease in tissue LPO levels, possible due to the overtly diabetic, severely insulin-resistant state of db/db mice. In addition, we also observed that APX-115 treatment markedly improved systemic oxidative stress as reflected by plasma 8-isoprostane levels similar to a previous report.16, 34 However, APX-115 had a similar or larger effect on oxidative stress than did a dual Nox1/Nox4 inhibitor, GKT137831. The effect of APX-115 might have been mediated by broadly inhibiting various Nox isoforms, since different isoforms have potential ROS generation activity.
The most important finding of this study was that APX-115 treatment decreased the urinary excretion of albumin and plasma creatinine levels. In addition, APX-115 decreased mesangial expansion in renal tissues, accompanied by suppressed synthesis of profibrotic and proinflammatory molecules. Nox2 and Nox4 expression increased significantly in db/db mice, associated with increased TLR4 and NF-κB p65 expression and macrophage infiltration. APX-115 significantly attenuated Nox1, Nox2, and Nox4 protein expression, whereas GKT137831 attenuated only Nox1 and Nox4 expression. Metabolic endotoxemia is associated with release of endotoxin such as lipopolysaccharide (LPS) into blood stream and causes a chronic low-grade inflammation in chronic kidney injury and cardiovascular disease.35, 36 HFD in db/db mice was known to trigger chronically elevated circulating gut-generated LPS contributing to the development of hepatic insulin resistance and diabetes.37 Previously, we reported that interaction of TLR4 with Nox4 is involved in LPS-mediated ROS generation and NF-κB activation. The result suggested that Nox4-dependent ROS generation mediates TLR4-induced inflammation processes including activation of NF-κB and proinflammatory cytokines. Recently, we showed that APX-115 suppressed phosphorylation of IκBα in response to RANKL resulting in attenuated NF-κB activation.17 Taken together, these results suggest that APX-115 in DN inhibited TLR4/NFkB cascade through reduced Nox activity and ROS generation.
Although Nox4 is the most widely characterized Nox isoform in diabetic nephropathy, recent evidence implies that Nox2 may also have a role in diabetic nephropathy. Fukuda et al38 demonstrated that Nox2 expression is upregulated in the kidneys of diabetic mice. Treatment with candesartan and pioglitazone inhibited Nox2 expression in the kidney, associated with decreased oxidative stress and reduced renal fibrosis.38 In addition, Oudit et al39 showed increased Nox2 gene expression in diabetic kidneys. Treatment with human recombinant angiotensin converting enzyme 2 decreased Nox2 expression accompanied by reduced renal oxidative stress, urinary albumin, and renal fibrosis in type I diabetic Akita mice.39 In further support of the possible role of Nox2 in diabetic nephropathy, a high dose of valsartan or probucol treatment slows the progression of diabetic nephropathy in db/db mice by reducing renal Nox2 expression associated with decreased renal oxidative stress, inflammation, and fibrosis.40, 41 Furthermore, Nagasu et al14 recently demonstrated that transgenic Akita overexpressing Nox2 in the endothelium exhibited high levels of Nox activity in glomeruli, developed glomerular endothelial perturbations, increased glomerular permeability, and exacerbated diabetic nephropathy.14 Taken together, these findings suggest a possible role of Nox2 in the development of renal oxidative stress and diabetic nephropathy. APX-115 significantly attenuated Nox1, Nox2, and Nox4 protein expression, whereas GKT137831 attenuated only Nox1 and Nox4 expression. These findings suggest that APX-115 may be as effective or may provide better renoprotection than GKT137831.
One of the interesting findings in this study was that broad Nox inhibition with APX-115 significantly changed adipose tissue. Recent studies showing the results of dual Nox1/Nox4 inhibitor in a type 2 diabetic nephropathy model have primarily demonstrated the potential therapeutic effects in the kidney but not in adipose tissue.15, 16 Chronic insult from a diabetic milieu triggers and accelerates renal injury, but also directly influences adipose tissue.42, 43 Adipose tissue in obesity has been proposed to be a pivotal organ in insulin resistance, where inflammatory processes such as macrophage infiltration and increased cytokine synthesis occur, inducing systemic insulin resistance.44 In the adipocytes, Nox4 is the main source of ROS, and Nox4 expression in adipose tissue is thought to be linked to insulin resistance and adipocyte differentiation.45 However, recent studies have identified that Nox2 is also present in adipose tissue. Pepping et al46 demonstrated that visceral adipocyte hypertrophy and macrophage infiltration were attenuated in Nox2-deficient mice in a diet-induced obesity model. Our study demonstrated that APX-115 suppresses Nox1 and Nox2 mRNA expression in adipose tissue, in concordance with reduced expression of inflammatory chemokines MCP-1 and PAI-1. Moreover, APX-115 significantly reduced adipocyte hypertrophy and inflammatory cell accumulation in adipose tissue. All of these anti-inflammatory effects on adipose tissue may explain why APX-115 exerts similar or superior effects to GKT137831 in diabetic renal injury.
The above-mentioned APX-115-induced improvements in renal function are further supported by the in vitro experiments that demonstrated the role of Nox2, Nox4, or Duox2 in TGF-β-mediated ROS generation in primary mouse mesangial cells (pMMCs) from wild-type and Nox knockout mice (Nox1 KO, Nox2 KO, Nox4 KO, Duox1 KO, and Duox2 KO). TGF-β stimulated ROS generation in pMMCs from WT, Nox1 KO, or Duox1 KO mice, but failed to induce Nox activity in pMMCs from Nox2 KO, Nox4 KO, or Duox2 KO mice. These results indicated that activating Nox2, Nox4, or Duox2 is essential for TGF-β-mediated ROS generation in pMMCs, and these Nox isozymes serve as potential therapeutic targets for reducing ROS. Thus, pan-NOX inhibition, including Nox2/Nox4/Duox2, is likely to be similar or more effective than dual Nox1/Nox4 inhibition for treating diabetic nephropathy.
As expression of functional Nox2 in monocytes/macrophages protects against bacterial infection, infection due to Nox2 inhibition is a serious concern.47 In our experimental model, APX-115 did not increase the susceptibility to infections and had no lethality. Our experiments demonstrated that broad inhibition of Nox isoforms may be a potential therapeutic approach for type 2 diabetic nephropathy by targeting adipose tissue as well as the kidney. In conclusion, our findings provide evidence that pan-Nox inhibition by APX-115 may be as effective or may provide better renoprotection than GKT137831 in diabetic nephropathy. These findings suggest that APX-115 may be a useful new therapeutic agent in treating type 2 diabetes and diabetic nephropathy. An agent that simultaneously inhibits various Noxs therefore holds a considerable promise as a new antidiabetic drug.
References
Bae YS, Oh H, Rhee SG et al, Regulation of reactive oxygen species: generation in cell signaling. Mol Cells 2011; 32: 491–509.
Ogura S, Shimosawa T . Oxidative stress and organ damages. Curr Hypertens Rep 2014; 16: 452.
Forbes JM, Coughlan MT, Cooper ME . Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 2008; 57: 1446–1454.
Gill PS, Wilcox CS . Forum review NADPH oxidases in the kidney. Antioxid Redox Signal 2006; 8: 1597–1607.
Gorin Y, Block K . Nox as a target for diabetic complications. Clin Sci 2013; 125: 361–382.
Craige SM, Chen K, Pei Y et al, NADPH oxidase4 promotes endothelial angiogenesis through endothelial nitricoxide synthase activation. Circulation 2011; 124: 731–740.
Zhang M, Brewer AC, Schroder K et al, NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA 2010; 107: 18121–18126.
Schroder K, Zhang M, Benkhoff S et al, Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 2012; 110: 1217–1225.
Jha JC, Gray SP, Barit D et al, Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol 2014; 25: 1237–1254.
Sedeek M, Callera G, Montezano A et al, Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 2010; 299: F1348–F1358.
Shah A, Xia L, Goldberg H et al, Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J Biol Chem 2013; 288: 6835–6848.
Etoh T, Inoguchi T, Kakimoto M et al, Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia 2003; 46: 1428–1437.
Gorin Y, Block K, Hernandez J et al, Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 2005; 280: 39616–39626.
Nagasu H, Satoh M, Kiyokage E et al, Activation of endothelial NAD(P)H oxidase accelerates early glomerular injury in diabetic mice. Lab Invest 2016; 96: 25–36.
Gorin Y, Cavaglieri RC, Khazim K et al, Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am J Physiol Renal Physiol 2015; 308: F1276–F1287.
Sedeek M, Gutsol A, Montezano AC et al, Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of Type 2 diabetes. Clin Sci 2013; 124: 191–202.
Joo JH, Huh JE, Lee JH et al, A novel pyrazole derivative protects from ovariectomy-induced osteoporosis through the inhibition of NADPH oxidase. Sci Rep 2016; 6: 22389.
Bligh EG, Dyer WJ . A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959; 37: 911–917.
Kim JE, Lee MH, Nam DH et al, Celastrol, an NF-κB inhibitor, improves insulin resistance and attenuates renal injury in db/db mice. PLoS One 2013; 8: e62068.
Lee JH, Joo JH, Kim J et al, Interaction of NADPH oxidase 1 with Toll-like receptor 2 induces migration of smoothmuscle cells. Cardiovasc Res 2013; 99: 483–493.
Harper PA, Robinson JM, Hoover RL et al, Improved methods for culturing rat glomerular cells. Kidney Int 1984; 26: 875–880.
Ratliff BB, Abdulmahdi W, Pawar R et al, Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal 2016; 25: 119–146.
Marco ED, Jha JC, Sharma A et al, Are reactive oxygen species still the basis for diabetic complications? Clin Sci 2015; 129: 199–216.
Holterman CE, Read NC, Kennedy CRJ . Nox and renal disease. Clin Sci 2015; 128: 465–481.
Choi J, Corder NLB, Koduru B et al, Oxidative stress and hepatic Nox proteins in chronic hepatitis C and hepatocellular carcinoma. Free Radic Biol Med 2014; 72: 267–284.
Manea SA, Constantin A, Manda G et al, Regulation of Nox enzymes expression in vascular pathophysiology: focusing on transcription factors and epigenetic mechanisms. Redox Biol 2015; 5: 358–366.
Claudia Goettsch C, Babelova A, Trummer O et al, NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J Clin Invest 2013; 123: 4731–4738.
Holterman CE, Thibodeau JF, Kennedy CR . NADPH oxidase 5 and renal disease. Curr Opin Nephrol Hypertens 2015; 24: 81–87.
Holterman CE, Thibodeau JF, Towaij C et al, Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J Am Soc Nephrol 2014; 25: 784–797.
Zhu K, Kakehi T, Matsumoto M et al, NADPH oxidase NOX1 is involved in activation of protein kinase C and premature senescence in early stage diabetic kidney. Free Rad Biol Med 2015; 83: 21–30.
You YH, Okada S, Ly S et al, Role of Nox2 in diabetic kidney disease. Am J Physiol Renal Physiol 2013; 304: F840–F848.
Babelova A, Avaniadi D, Jung O et al, Role of Nox4 in murine models of kidney disease. Free Radic Biol Med 2012; 53: 842–853.
Gray SP, Di Marco E, Okabe J et al, NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013; 27: 1888–1902.
Di Marco E, Gray SP, Chew P et al, Pharmacological inhibition of NOX reduces atherosclerotic lesions, vascular ROS and immune-inflammatory responses in diabetic Apoe(−/−) mice. Diabetologia 2014; 57: 633–642.
Wong J, Vilar E, Farrington K . Endotoxemia in end-stage kidney disease. Semin Dial 2015; 28: 59–67.
Brown JM, Hazen SL . The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med 2015; 66: 343–359.
Uchimura K, Hayata M, Mizumoto T et al, The serine protease prostasin regulates hepatic insulin sensitivity by modulating TLR4 signalling. Nat Commun 2014; 5: 3428.
Fukuda M, Nakamura T, Kataoka K et al, Potentiation by candesartan of protective effects of pioglitazone against type 2 diabetic cardiovascular and renal complications in obese mice. J Hypertens 2010; 28: 340–352.
Oudit GY, Liu GC, Zhong J et al, Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes 2010; 59: 529–538.
Zhou G, Cheung AK, Liu X et al, Valsartan slows the progression of diabetic nephropathy in db/db mice via a reduction in podocyte injury, and renal oxidative stress and inflammation. Clin Sci 2014; 126: 707–720.
Zhou G, Wang Y, He P et al, Probucol inhibited Nox2 expression and attenuated podocyte injury in type 2 diabetic nephropathy of db/db mice. Biol Pharm Bull 2013; 36: 1883–1890.
Cha JJ, Hyun YY, Lee MH et al, Renal protective effects of toll-like receptor 4 signaling blockade in type 2 diabetic mice. Endocrinology 2013; 154: 2144–2155.
Min HS, Kim JE, Lee MH et al, Effects of Toll-like receptor antagonist 4,5-dihydro-3-phenyl-5-isoxasole acetic acid on the progression of kidney disease in mice on a high-fat diet. Kidney Res Clin Pract 2013; 33: 33–44.
Ferrante AW Jr . Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med 2007; 262: 408–414.
Schröder K, Wandzioch K, Helmcke I et al, Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol 2009; 29: 239–245.
Pepping JK, Freeman LR, Gupta S et al, NOX2 deficiency attenuates markers of adiposopathy and brain injury induced by high-fat diet. Am J Physiol Endocrinol Metab 2013; 304: E392–E404.
Pizzolla A, Hultqvist M, Nilson B et al, Reactive oxygen species produced by the NADPH oxidase 2 complex in monocytes protect mice from bacterial infections. J Immunol 2012; 188: 5003–5011.
Acknowledgements
This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C0223).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SHM and SCL work at Aptabio Therapeutics Inc, Korea, which develops APX-115. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website
In this study, the authors aimed to ameliorate renal damage from reactive oxygen species in diabetic nephropathy. They found that a recently developed pan-Nox-inhibitor, APX-115, may provide better renoprotection than the dual Nox1/Nox4 inhibitor. Pan-Nox inhibition with APX-115 might be a promising therapy for diabetic nephropathy and targeting all Nox components may be a new therapeutic strategy
Rights and permissions
About this article
Cite this article
Cha, J., Min, H., Kim, K. et al. APX-115, a first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db mice from renal injury. Lab Invest 97, 419–431 (2017). https://doi.org/10.1038/labinvest.2017.2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2017.2
This article is cited by
-
NADPH oxidase family proteins: signaling dynamics to disease management
Cellular & Molecular Immunology (2022)
-
NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets
Nature Reviews Cardiology (2020)
-
Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches
Signal Transduction and Targeted Therapy (2020)
-
Targeting oxidative stress and anti-oxidant defence in diabetic kidney disease
Journal of Nephrology (2020)
-
Diabetic Nephropathy: a Tangled Web to Unweave
Cardiovascular Drugs and Therapy (2017)