Abstract
Inflammation and apoptosis are crucial mechanisms for the development of the acute respiratory distress syndrome (ARDS). Currently, there is no specific pharmacological therapy for ARDS. We have evaluated the ability of a new family of 1,2,3,5-tetrasubstituted pyrrol compounds for attenuating lipopolysaccharide (LPS)-induced inflammation and apoptosis in an in vitro LPS-induced airway epithelial cell injury model based on the first steps of the development of sepsis-induced ARDS. Human alveolar A549 and human bronchial BEAS-2B cells were exposed to LPS, either alone or in combination with the pyrrol derivatives. Rhein and emodin, two representative compounds with proven activity against the effects of LPS, were used as reference compounds. The pyrrol compound that was termed DTA0118 had the strongest inhibitory activity and was selected as the lead compound to further explore its properties. Exposure to LPS caused an intense inflammatory response and apoptosis in both A549 and BEAS-2B cells. DTA0118 treatment downregulated Toll-like receptor-4 expression and upregulated nuclear factor-κB inhibitor-α expression in cells exposed to LPS. These anti-inflammatory effects were accompanied by a significantly lower secretion of interleukin-6 (IL-6), IL-8, and IL-1β. The observed antiapoptotic effect of DTA0118 was associated with the upregulation of antiapoptotic Bcl-2 and downregulation of proapoptotic Bax and active caspase-3 protein levels. Our findings demonstrate the potent anti-inflammatory and antiapoptotic properties of the pyrrol DTA0118 compound and suggest that it could be considered as a potential drug therapy for the acute phase of sepsis and septic ARDS. Further investigations are needed to examine and validate these mechanisms and effects in a clinically relevant animal model of sepsis and sepsis-induced ARDS.
Similar content being viewed by others
Main
The acute respiratory distress syndrome (ARDS) develops as a result of an acute and intense inflammation of the lung.1, 2 Sepsis is the most common predisposing factor and sepsis-associated ARDS has a higher disease severity, poorer recovery from acute lung injury, and higher mortality compared with non-sepsis-related ARDS.3 Bacterial lipopolysaccharide (LPS), also termed endotoxin, is the major component of the outer membrane of Gram-negative bacteria and one of the major pathogen-associated pattern recognition molecules that activate the innate immunity. LPS has a crucial role in the inflammatory response triggered by Gram-negative bacteria in epithelial and other cells of the lung. Toll-like receptor-4 (TLR4) recognizes LPS and initiates a signaling cascade by several downstream adapter proteins that leads to the activation of nuclear factor-κB (NF-κB),4 involving phosphorylation and degradation of the inhibitor of NF-κB (IκBα), and the translocation of NF-κB heterodimers into the nucleus of the cells. Eventually, the NF-κB system exerts transcriptional regulation on the expression of genes related to innate immunity and inflammation, and other gene products.5
LPS can induce apoptosis in lung endothelial and epithelial cells6, 7 and contributes to the pathogenesis of ARDS. Recent studies strongly suggested a role for epithelial apoptosis as a key profibrotic event in lung fibrogenesis.8 Thus, there is a rationale to manipulate apoptosis therapeutically. Currently, there is no specific pharmacological treatment of proven benefit in ARDS. Based on a well-established protocol of the National Cancer Institute of the United States,9 we used a validated screening strategy to identify potential therapeutic molecules by examining the effects of a novel family of tri- and tetrasubstituted pyrrols, which were previously studied by our group.10 In the present study, we report the marked anti-inflammatory and antiapoptotic properties of a compound termed by us as DTA0118 in a well-established in vitro LPS-induced airway epithelial cell injury model,11, 12, 13, 14, 15, 16 based on the initial steps of the development of sepsis and sepsis-induced ARDS, using human alveolar A549 and human bronchial BEAS-2B cells. We used rhein and emodin, two anthracene-derived anthraquinones with proven activity against the effects of LPS,17 as reference compounds. Our results demonstrate that the compound DTA0118 prevents LPS-induced cell death and inhibits TLR4 upregulation and IκBα downregulation.
MATERIALS AND METHODS
Cell Lines, Cell Cultures, and Reagents
We used human lung alveolar type-II A549 cell line and human bronchial epithelial BEAS-2B cells (American Type Culture Collection, Manassas, VA, USA). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% (v/v) inactivated fetal bovine serum (FBS) and in antibiotics (0.1 mg/ml streptomycin, 100 U/ml penicillin) in a humidified incubator at 37 °C with 5% CO2 and 95% humidified air (see Electronic Supplementary Material (ESM) for details).
Survival Assays and Cell Growth
A total of 3 × 104 A549 and BEAS-2B cells per well were seeded in a 96-well plate in a volume of 100 μl of DMEM supplemented with 2% (v/v) inactivated FBS and in antibiotics. After 24 h, cells were exposed to 100 ng/ml LPS either alone or in combination with 10 μM rhein, emodin, or 1,2,3,5-tetrasubstituted pyrrol compounds (Figure 1). After 18 h, cell survival was analyzed by precipitation with 25 μl ice-cold TCA 50% (w/v) and fixed for 60 min at 4 °C. Then, we performed the sulforhodamine B (SRB) assay.18 We then studied the anti-inflammatory and antiapoptotic properties of the most active compound (termed by us as DTA0018). The SRB assay was used to test the effects of various concentrations of DTA0118 (0.1, 1, 10, 100, and 1000 μM) in the presence of A549 and BEAS-2B cells (see ESM for details).
Western Blot Analysis
After 18 h of compound incubation (control-vehicle: 100 ng/ml Escherichia coli LPS alone or in combination with 10 μM rhein, emodin, or DTA0118), A549 and BEAS-2B cells from each experimental group were harvested and centrifuged at 1200 r.p.m. for 7 min using a protocol described previously by our group.5 Protein concentrations in extracts were determined with the Bio-Rad DC protein assay (Bio-Rad DC, Hercules, CA, USA). For immunoblotting, 30 μg of samples were electrophoresed and transferred to polyvinylidene difluoride (PVDF) membranes, blocked for 1 h at room temperature, and probed with primary antibodies to TLR4, IκBα, Bax, Bcl-2, caspase-3, and β-actin in all experimental groups (see ESM for details). Chemiluminiscence detection was performed with the ECL Kit (Thermo Fisher Scientific, Waltham, MA, USA). Densitometry in each band was performed and measured using the Scion Image software package. Western blots were repeated three times in each experimental condition.
Detection of Apoptosis
We used an Annexin V-FITC Apoptosis Detection Kit (BD Biosciences) for measuring early apoptosis and cell death according to the manufacturer’s instructions. A549 and BEAS-2B cells were seeded at 2 × 106 in DMEM supplemented with 2% inactivated FBS and 1% antibiotics for 24 h. Then, cells were treated during 18 h with or without 100 ng/ml E. coli LPS alone or in combination with 10 μM rhein, emodin, or DTA0118. After the treatment, A549 and BEAS-2B cells were analyzed by means of flow cytometry according to 100 000 cells (events). Results were analyzed using the WinMDI 2.8 software (Scripps Institute, La Jolla, CA, USA) (see ESM for details).
Measurement of Cytokines
A total of 3 × 105 A549 and BEAS-2B cells were cultured in 96-well plates. After 24 h, cells were treated with 100 ng/ml LPS alone or in combination with 10 μM rhein, emodin, or DTA0118 for 18 h. Afterwards, the medium of each well was collected and stored at −80 °C. Then, we measured the levels of interleukin-6 (IL-6), IL-8, and IL-1β in A549 and BEAS-2B cell culture supernatants using Cytometric Bead Array Human Inflammatory Cytokines Kit (BD Biosciences) in a FACSCanto II Flow Cytometer (see ESM for details).
Statistical Analysis
Data are expressed as mean±s.d. and analyzed for significant differences among groups using one-way analysis of variance followed by post hoc testing using the Bonferroni corrections for multiple comparisons. Densitometric data were normalized to β-actin as loading controls. Data are from three independent experiments. A two-sided P-value <0.05 was considered statistically significant.
RESULTS
Effects of Pyrrol Compounds on Cell Survival and Growth
Mean percentage of cell survival (PS) with rhein was −64.2±20.1% in A549 cells and −51.0±15.2% in BEAS-2B cells, and with emodin it was 26.5±12.6% in A549 cells and 32.2±18.9% in BEAS-2B cells. Cells treated with pyrrol compounds showed higher PS values. The best results were obtained for the derivative DTA0118 with a PS of 53.3±25.8% in A549 cells and 67.1±15.4% in BEAS-2B cells (Figure 2a and Table 1). No A549 and BEAS-2B cell death occurred at any concentration of DTA0118 (Figure 2b).
Effects of pyrrol compounds on A549 and BEAS-2B cells survival. (a) Percentage of survival of 100 ng/ml lipopolysaccharide (LPS)-stimulated A549 and BEAS-2B cells in combination with 10 μM rhein (R), emodin (E) and 15 1,2,3,5-tetrasubstituted pyrrol synthetic compounds. (b) Percentage of A549 and BEAS-2B cell growth under the effects of 0.1, 1, 10, 100, and 1000 μM of compound DTA0118. In (a and b), A549 and BEAS-2B cells were stimulated with 100 ng/ml LPS alone or in combination with 10 μM rhein (R), emodin (E) and 15 1,1,3,5-tetrasubstituted pyrrol synthetic compounds (A) or with 0.1, 1, 10, 100, and 1000 μM of lead compound DTA0118 for 18 h (B). (c) Morphological features of human A549 and BEAS-2B cells following the stimulation with 100 ng/ml of LPS (LPS) plus R (LPS+rhein), E (LPS+emodin), and DTA0118(LPS+DTA0118) for 18 h. Panels correspond to x200 objective. C=untreated control-vehicle cells. *P<0.05 vs R; **P<0.01 vs R; ***P<0.001 vs R. Note the marked growth of A549 and BEAS-2B cells treated with 1000 μM of lead compound DTA0118.
DTA0118 Prevented LPS-Induced Cellular Morphological Changes
Control-vehicle cells showed an intact appearance, whereas the LPS-stimulated cells displayed morphological changes, such as stretching. Additionally, LPS- plus rhein- and emodin-treated cells displayed a round morphology as well as floating round shapes. Of note, LPS-induced morphological changes were prevented after treatment with DTA0118 (Figure 2).
DTA0118 Decreased Cytokine Levels
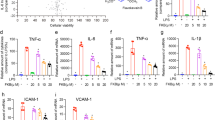
IL-6, IL-8, and IL-1β levels increased in LPS-treated cells compared with untreated control-vehicle cells (P<0.001 in all cases; P<0.01 for IL-8 in A549 cells). This effect was reduced after cotreatment with 10 μM rhein or emodin (P<0.001 for all cases; P<0.01 for IL-8) in cotreatment with 10 μM rhein and A549 cells (P<0.05 for IL-1β in cotreatment with 10 μM rhein and A549 cells). Inhibition of LPS-induced effects was marked after cotreatment with 10 μM DTA0118 (P<0.01 for IL-6, P<0.05 for IL-8, and P<0.001 for IL-1β in A549 cells; P<0.001 for all cytokines in BEAS-2B cells) (Figure 3).
Cytometric bead array analyses to determine interleukin-6 (IL-6), IL-8 and IL-1β. C: untreated control-vehicle cells, C+: cells treated with lipopolysaccharide (LPS), R: LPS+rhein, E: LPS+emodin, DTA0118: LPS+DTA0118 for 18 h. **P<0.01 vs C; ***P<0.001 vs C; ‡P<0.05 vs LPS; #P<0.01 vs LPS; ¶P<0.001 vs LPS.
Effects of DTA0118 on TLR4 and IκBα Protein Levels
The expression of TLR4 increased in A549 and BEAS-2B cells after LPS stimulation (P<0.001) (Figure 4) (see Supplementary Table E3 in ESM for mean±s.d. values of densitometric analysis in each experimental group). Downregulation of TLR4 protein levels was observed when both cell types were cotreated with LPS plus 10 μM emodin or rhein, but these effects were maximum with cotreatment with 10 μM DTA0118 (P<0.001). IκBα protein levels decreased after LPS stimulation compared with control-vehicle cells (P<0.001) and increased after cotreatment with LPS plus 10 μM rhein or emodin (P<0.01 for rhein in A549 cells; P<0.001 for the rest of experimental conditions). These changes were inhibited after treatment with DTA0118 (P<0.001) (Figure 4) (see Table in the ESM for mean±s.d. values of densitometric analysis in each experimental group). These results were replicated with TLR4 and IκBα immunocytochemical staining (see ESM for further details). We found a high intensity of IκBα staining in the nuclei and low-intensity staining in the cytoplasm of A549 and BEAS-2B cells stimulated with LPS, consistent with the low transcript level of NF-κB (see ESM for details).
Effects of DTA0118 on lipopolysaccharide (LPS)-induced changes of Toll-like receptor-4 (TLR4) and IκBα. Representative blots from each experimental condition and histograms exhibiting the changes in TLR4 and IκBα protein levels. C: untreated control-vehicle cells; C+: cells treated with LPS; R: LPS+rhein; E: LPS+emodin; DTA0118: LPS+DTA0118 for 18 h. ***P<0.001 vs C; #P<0.01 vs LPS; ¶P<0.001 vs LPS.
LPS-Mediated Apoptosis was Prevented by DTA0118
After LPS incubation, the percentage of apoptosis and cell death increased when compared with untreated cells (P<0.001 in both cell types) (Figure 5). This effect was prevented after treatment with DTA0118 (P<0.001) (Figures 5a and b).
Lipopolysaccharide (LPS)-induced apoptosis is prevented by DTA0118. (a) Representative dot-plot diagrams demonstrating typical apoptotic and death in A549 and BEAS-2B cells populations detected by Annnexin V-FITC and propidium iodide (PI) staining. (b) Percentages of early apoptosis and death of A549 and BEAS-2B under all experimental conditions. C: untreated control-vehicle cells; C+: cells treated with LPS; DTA0118: LPS+DTA0118 for 18 h. ***P<0.001 vs C; ¶P<0.001 vs LPS.
DTA0118 Inhibited LPS-Induced Changes of Bax, Bcl-2, and Caspase-3 Proteins
We performed additional western blot assays (Figure 6) for evaluating the mechanisms induced by DTA0118. Stimulation with LPS induced upregulation of proapoptotic Bax protein when compared with control-vehicle cells (P<0.001). This effect was prevented in cells cotreated with emodin and DTA0118 compared with LPS-stimulated cells (P<0.001). Furthermore, a significant downregulation of the antiapoptotic Bcl-2 protein was observed in LPS-stimulated cells (P<0.001). Emodin and DT0118 prevented the LPS-induced downregulation of Bcl-2 protein (P<0.001). Stimulation with LPS induced an upregulation of procaspase-3 and downregulation of caspase-3 when compared with control-vehicle cells (P<0.001 for A549 cells and P<0.01 for BEAS-2B cells). This effect was maximum with DTA0118 (P<0.001 for both cell types) (Figure 6). See Supplementary Table E3 in the ESM for mean±s.d. values of densitometric analysis in each experimental group.
Immunobloting analyses for Bax, Bcl-2, and procaspase-3/caspase-3 in A549 and BEAS-2B cells. Immunobloting and β-actin-normalized densitometry of Bax, Bcl-2, procaspase-3 (32 kDa)/caspase-3 (11 kDa) western blots from protein extracts of A549 and BEAS-2B cells treated or not for 18 h with 100 ng/ml lipopolysaccharide (LPS) (C+) in combination or not with rhein (R), emodin (E), or DTA0118. C: untreated control-vehicle cells. **P<0.01 vs C; ***P<0.001 vs C; #P<0.01 vs LPS; ¶P<0.001 vs LPS.
DISCUSSION
This is the first report showing that 1,2,3,5-tetrasubstituted pyrrol compounds are able to prevent lung epithelial cell injury induced by bacterial LPS. The main findings of our study are: (i) our screening method identified a pyrrol compound that inhibited LPS-induced cell death in two well-validated in vitro models of endotoxin-induced airway epithelial cell injury; (ii) DTA0118, the candidate drug, inhibited the secretion of proinflammatory cytokines; (iii) this inhibition was associated with downregulation of TLR4 and upregulation of IκBα; (iv) DTA0118 prevented apoptosis by modulating Bax, Bcl-2, and caspase-3 protein levels; and (v) inhibition of LPS effects was seen while human cell integrity and viability was preserved during DTA0118 treatment.
LPS initiates an inflammatory and immune defense response in the lung through recognition by TLR4.4 Our results demonstrate that pyrrol compound DTA0118 inhibited changes in TLR4 and IκBα expression levels in human A549 and BEAS-2B cells stimulated by LPS, and this inhibition was much more effective than the effects produced by the reference compounds. Furthermore, DTA0118 significantly inhibited the synthesis of cytokines. IL-1β was generated presumably by activation of the inflammasome,19 which has been linked to the pathogenesis of ARDS.20 This inhibition of proinflammatory cytokines has also been reported by other compounds, such as inosine,14 bupleurum polysaccharides,21 proxylin A,22 and quercetin,23 and by other pyrrol compounds, such as T-686, an inhibitor of plasminogen activator inhibitor-1 that was associated with a mortality reduction in mice exposed to LPS.24 However, none of those compounds had described any antiapoptotic activity in sepsis-induced lung injury.
We chose A549 and BEAS-2B cell lines as representative human alveolar and bronchial epithelial cells because they have been implicated in the pathogenesis of sepsis-induced ARDS.11, 12, 13, 14, 15, 16 One of the most important determinants of lung severity is the magnitude of injury to the epithelial barrier.2 Epithelial cells generate cytokines, chemokines, and anti-microbial peptides in response to inflammatory stimuli,25, 26 and airway epithelium controls lung inflammation and injury through NF-κB.27 Numerous experimental studies have used human A549 alveolar and BEAS-2B bronchial epithelial cell lines to examine the acute lung inflammatory response induced by LPS, as an acceptable, validated, and suitable in vitro airway epithelial injury model based on the initial steps of the development of sepsis and ARDS11, 12, 13, 14, 15, 16, 25, 28, 29, 30 and continue being extensively used.31, 32, 33, 34, 35 We selected E. coli LPS because it has been used in most endotoxin-induced lung injury models36, 37 and LPS is a key pathogen recognition molecule for sepsis27 that induces apoptosis in lung cells.38 Previous reports examining the efficacy of molecules on the LPS-induced activation of inflammatory markers in the lung, or before evaluating the potential in vivo anti-inflammatory properties, have used a similar in vitro epithelial cell injury model to the one we used.14, 39
The activation of apoptosis following LPS-induced lung inflammation have a key role in the development of sepsis-induced ARDS.38 The induction of apoptosis-mediated DNA damage is an important characteristic in the development of acute lung injury during sepsis and ARDS.40, 41 Apoptosis occurred in airway epithelial cells after LPS administration in an in vivo rat model.42 While proliferation of type-II alveolar cells occurs during the early phase of ARDS as a reparative phenomenon, extensive apoptosis is responsible for the loss of these cells in the resolution phase. Two main pathways are involved in the induction of apoptosis:42 (i) the mitochondrial pathway, which involves cytochrome c release from the mitochondria and the activation of caspase-9, which then cleaves and activates caspase-3, caspase-6, and caspase-7; and (ii) the death domain receptor pathway that involves activation of Fas or tumor necrosis factor receptors followed by the activation of caspase-8 and subsequent activation of downstream caspases. Cytochrome c release is determined by the ratio of antiapoptotic (Bcl-2 and Bcl-xL) and proapoptotic (Bax, Bad, Bik, and Bid) members of the Bcl-2 family proteins.43 In our study, LPS stimulation induced apoptosis that was associated with changes in protein levels of antiapoptotic Bcl-2 and Bax and caspase-3. Previous studies have tested the hypothesis that an increased duration of sepsis stimulates apoptosis in lung tissue,44, 45 whereas others have suggested a biphasic response where changes in Bax/Bcl-2 protein levels were found after sepsis onset.40 Although NF-κB could have a role in apoptosis by regulating the expression of genes involved in cell death, a specific role of NF-κB on the apoptotic process cannot be ascertained from our data. As reported by several investigators, apoptosis is a major pathway responsible for the resolution of alveolar cells in ARDS.6 In our study, LPS induced a significant apoptotic effect in both lung epithelial cell lines and this effect was prevented by the cotreatment with 10 μM of the pyrrol compound DTA0118. Further investigations are needed to determine NF-κB-dependent and/or -independent apoptosis mechanisms.
Although our data may imply a role for pyrrol compounds in the therapy of the acute phase of sepsis and septic ARDS, we acknowledge some limitations to this study. First, we describe in vitro studies using micromolar concentrations; further studies are necessary to prove the relevance of these findings using nanomolar concentrations and in vivo models. Second, we did not use primary alveolar epithelial cells; however, there is a plethora of published studies validating the use of human BEAS-2B and A549 cells as an acceptable model of epithelial cell injury and for studying the LPS-induced effects in the airway epithelium.11, 12, 13, 14, 15, 16, 25, 28, 29, 30, 31, 32, 33, 34, 35 Third, further studies are needed to elucidate whether there are other mechanisms by which the pyrrol compound DTA0118 elicits its anti-inflammatory and antiapoptotic activities. Fourth, TLR4, Ikβα, and β-actin were not adjusted for the cell number. Furthermore, although we did not test whether these compounds modulate pulmonary vascular function by impacting endothelial cell function, Wang et al32 have recently reported that the presence of A549 cells or primary human alveolar epithelial cells in a coculture model with endothelial cells attenuated septic pulmonary endothelial cell permeability by 40–100% using an in vitro model of LPS-induced ARDS. Based on these results, it is plausible that our pyrrol compound would have an additive effect by modulating vascular permeability during sepsis and sepsis (LPS)-induced ARDS. More studies are needed to explore the specific role of pyrrol compound DTA0118 in the context of sepsis and septic ARDS.
In conclusion, this study showed E. coli LPS stimulated the production of proinflammatory mediators in an in vitro human airway epithelial cell injury model, and provided evidence that DTA0118, a novel pyrrol compound, inhibited the cytokine overproduction through the modulation of TLR4/NF-κB pathway. LPS induced an apoptotic response that was prevented by DTA0118 through the modulation of antiapoptotic proteins. Our study suggests that DTA0118 can be considered as a potential therapy in the acute phase of sepsis and septic ARDS. Further investigations are needed to examine and validate these new effects and mechanisms in a clinically, relevant animal model of sepsis and sepsis-induced ARDS.
References
Villar J . What is the acute respiratory distress syndrome? Respir Care 2011;56:1539–1545.
Ware LB, Matthay MA . The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349.
Sheu CC, Gong MN, Zhai R et al. Clinical characteristics and outcomes of sepsis-related vs non-sepsis-related ARDS. Chest 2010;138:559–567.
Baumgarten G, Knuefermann P, Wrigge H et al. Role of Toll-like receptor-4 for the pathogenesis of acute lung injury in Gram-negative sepsis. Eur J Anaesthesiol 2006;23:1041–1048.
Villar J, Cabrera N, Casula M et al. Mechanical ventilation modulates Toll-like receptor signaling pathway in a sepsis-induced lung injury model. Intens Care Med 2010;36:1049–1057.
Bardales RH, Xie SS, Schaefer RF et al. Apoptosis is a major pathway responsible for the resolution of type II pneumocytes in acute lung injury. Am J Pathol 1996;149:845–852.
Fujita M, Kuwano K, Kunitake R et al. Endothelial cell apoptosis in lipopolysaccharide-induced lung injury in mice. Int Arch Allergy Immunol 1998;117:202–208.
Janssen-Heininger YM, Aesif SW, van der Velden J et al. Regulation of apoptosis through cysteine oxidation: implications for fibrotic lung disease. Ann NY Acad Sci 2010;1203:23–28.
Shoemaker RH . The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer 2006;6:813–823.
Padron JM, Tejedor D, Santos-Exposito A et al. Antiproliferative activity in HL60 cells by tetrasubstituted pyrroles: a structure–activity relationship study. Bioorg Med Chem Lett 2005;15:2487–2490.
Boots AW, Gerloff K, Bartholome R et al. Neutrophils augment LPS-mediated pro-inflammatory signaling in human lung epithelial cells. Biochim Biophys Acta 2012;1823:1151–1162.
Koyama S, Sato E, Nomura H et al. The potential of various lipopolysaccharides to release monocyte chemotactic activity from lung epithelial cells and fibroblasts. Eur Respir J 1999;14:545–552.
MacRedmond R, Singhera GK, Dorscheid DR . Erythropoietin inhibits respiratory epithelial cell apoptosis in a model of acute lung injury. Eur Respir J 2009;33:1403–1414.
Liaudet L, Mabley JG, Pacher P et al. Inosine exerts a broad range of antiinflammatory effects in a murine model of acute lung injury. Ann Surg 2002;235:568–578.
Muroya M, Chang K, Uchida K et al. Analysis of cytotoxicity induced by proinflammatory cytokines in the human alveolar epithelial cell line A549. Biosci Trends 2012;6:70–80.
Rodriguez-Gonzalez R, Ramos-Nuez A, Martin-Barrasa JL et al. Endotoxin-induced lung alveolar cell injury causes brain cell damage. Exp Biol Med (Maywood) 2015;240:135–142.
Liu X, Cheng J, Zheng X et al. Targeting CpG DNA to screen and isolate anti-sepsis fraction and monomers from traditional Chinese herbs using affinity biosensor technology. Int Immunopharmacol 2009;9:1021–1031.
Skehan P, Storeng R, Scudiero D et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990;82:1107–1112.
Jin C, Flavell RA . Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol 2010;30:628–631.
Dolinay T, Kim YS, Howrylak J et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 2012;185:1225–1234.
Wu J, Zhang YY, Guo L et al. Bupleurum polysaccharides attenuates lipopolysaccharide-induced inflammation via modulating Toll-like receptor 4 signaling. PLoS One 2013;8:e78051.
Tseng TL, Chen MF, Tsai MJ et al. Oroxylin-A rescues LPS-induced acute lung injury via regulation of NF-kappaB signaling pathway in rodents. PLoS One 2012;7:e47403.
Wang L, Chen J, Wang B et al. Protective effect of quercetin on lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammatory cell influx. Exp Biol Med (Maywood) 2014;239:1653–1662.
Murakami J, Ohtani A, Murata S . Protective effect of T-686, an inhibitor of plasminogen activator inhibitor-1 production, against the lethal effect of lipopolysaccharide in mice. Jpn J Pharmacol 1997;75:291–294.
Larsson BM, Larsson K, Malmberg P et al. Gram positive bacteria induce IL-6 and IL-8 production in human alveolar macrophages and epithelial cells. Inflammation 1999;23:217–230.
Strieter RM, Belperio JA, Keane MP . Cytokines in innate host defense in the lung. J Clin Invest 2002;109:699–705.
Cheng DS, Han W, Chen SM et al. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol 2007;178:6504–6513.
Yeh CH, Cho W, So EC et al. Propofol inhibits lipopolysaccharide-induced lung epithelial cell injury by reducing hypoxia-inducible factor-1alpha expression. Br J Anaesth 2011;106:590–599.
Wang YL, Malik AB, Sun Y et al. Innate immune function of the adherens junction protein p120-catenin in endothelial response to endotoxin. J Immunol 2011;186:3180–3187.
Cabrera-Benitez NE, Perez-Roth E, Casula M et al. Anti-inflammatory activity of a novel family of aryl ureas compounds in an endotoxin-induced airway epithelial cell injury model. PLoS One 2012;7:e48468.
Terasaki Y, Terasaki M, Urushiyama H et al. Role of survivin in acute lung injury: epithelial cells of mice and humans. Lab Invest 2013;93:1147–1163.
Wang L, Taneja R, Wang W et al. Human alveolar epithelial cells attenuate pulmonary microvascular endothelial cell permeability under septic conditions. PLoS One 2013;8:e55311.
Maloney JP, Gao L . Proinflammatory cytokines increase vascular endothelial growth factor expression in alveolar epithelial cells. Mediators Inflamm 2015;2015:387842.
Cox R Jr, Phillips O, Fukumoto J et al. Resolvins decrease oxidative stress mediated macrophage and epithelial cell interaction through decreased cytokine secretion. PLoS One 2015;10:e0136755.
Cui J, Zhao H, Yi B et al. Dexmedetomidine attenuates bilirubin-induced lung alveolar epithelial cell death in vitro and in vivo. Crit Care Med 2015;43:e356–e368.
Fortis S, Spieth PM, Lu WY et al. Effects of anesthetic regimes on inflammatory responses in a rat model of acute lung injury. Intens Care Med 2012;38:1548–1555.
Mittal N, Sanyal SN . In vivo effect of surfactant on inflammatory cytokines during endotoxin-induced lung injury in rodents. J Immunotoxicol 2011;8:274–283.
Tang PS, Mura M, Seth R et al. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol 2008;294:L632–L641.
Magalhaes CB, Riva DR, DePaula LJ et al. In vivo anti-inflammatory action of eugenol on lipopolysaccharide-induced lung injury. J Appl Physiol (1985) 2010;108:845–851.
Chopra M, Reuben JS, Sharma AC . Acute lung injury:apoptosis and signaling mechanisms. Exp Biol Med (Maywood) 2009;234:361–371.
Matute-Bello G, Liles WC, Radella F II et al. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med 1997;156:1969–1977.
Rudkowski JC, Barreiro E, Harfouche R et al. Roles of iNOS and nNOS in sepsis-induced pulmonary apoptosis. Am J Physiol Lung Cell Mol Physiol 2004;286:L793–L800.
Ackermann EJ, Taylor JK, Narayana R et al. The role of antiapoptotic Bcl-2 family members in endothelial apoptosis elucidated with antisense oligonucleotides. J Biol Chem 1999;274:11245–11252.
Chopra M, Sharma AC . Distinct cardiodynamic and molecular characteristics during early and late stages of sepsis-induced myocardial dysfunction. Life Sci 2007;81:306–316.
Gupta A, Sharma AC . Metalloendopeptidase inhibition regulates phosphorylation of p38-mitogen-activated protein kinase and nitric oxide synthase in heart after endotoxemia. Shock 2003;20:375–381.
Acknowledgements
This study was supported by grants from the Instituto de Salud Carlos III Madrid, Spain (CB06/06/1088, PI10/0393, PI11/00840). JMP acknowledges financial support from the EU Research Potential (FP7-REGPOT-2012-CT2012-31637-IMBRAIN), and the European Regional Development Fund (FEDER).
Author Contributions
All authors participated in the design, interpretation of the studies and analysis of data, and review of the manuscript. JV and JMP obtained funding for the study. NECB, ARN, EPR, IS, and CRG performed the experiments and the statistical analysis. JV, NECB, EPR, and ASS conceived the study and drafted the first manuscript. JV coordinated the study. All authors participated in the writing process of the manuscript and read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website
There is no specific therapy for acute respiratory distress syndrome (ARDS). This study evaluated the ability of a pyrrol compound, DTA0118, to attenuate lipopolysaccharide-induced injury in an airway epithelial cell injury model. The potent anti-inflammatory and anti-apoptotic properties of DTA0118 suggest that it is a potential drug therapy for the acute phase of sepsis and septic ARDS.
Supplementary information
Rights and permissions
About this article
Cite this article
Cabrera-Benítez, N., Pérez-Roth, E., Ramos-Nuez, Á. et al. Inhibition of endotoxin-induced airway epithelial cell injury by a novel family of pyrrol derivates. Lab Invest 96, 632–640 (2016). https://doi.org/10.1038/labinvest.2016.46
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2016.46
This article is cited by
-
Prolyl oligopeptidase inhibition ameliorates experimental pulmonary fibrosis both in vivo and in vitro
Respiratory Research (2023)
-
Dermal fibroblast cells interactions with single and triple bacterial-species biofilms
Molecular Biology Reports (2021)