Abstract
The balance of nitric oxide (NO) versus superoxide generation has a major role in the initiation and progression of endothelial dysfunction. Under conditions of high glucose, endothelial nitric oxide synthase (eNOS) functions as a chief source of superoxide rather than NO. In order to improve NO bioavailability within the vessel wall in type-1 diabetes, we investigated treatment strategies that improve eNOS phosphorylation and NO-dependent vasorelaxation. We evaluated methods to increase the eNOS activity by (1) feeding Ins2Akita spontaneously diabetic (type-1) mice with l-arginine in the presence of sepiapterin, a precursor of tetrahydrobiopterin; (2) preventing eNOS/NO deregulation by the inclusion of inhibitor kappa B kinase beta (IKKβ) inhibitor, salsalate, in the diet regimen in combination with l-arginine and sepiapterin; and (3) independently increasing eNOS expression to improve eNOS activity and associated NO production through generating Ins2Akita diabetic mice that overexpress human eNOS predominantly in vascular endothelial cells. Our results clearly demonstrated that diet supplementation with l-arginine, sepiapterin along with salsalate improved phosphorylation of eNOS and enhanced vasorelaxation of thoracic/abdominal aorta in type-1 diabetic mice. More interestingly, despite the overexpression of eNOS, the in-house generated transgenic eNOS-GFP (TgeNOS-GFP)-Ins2Akita cross mice showed an unanticipated effect of reduced eNOS phosphorylation and enhanced superoxide production. Our results demonstrate that enhancement of endogenous eNOS activity by nutritional modulation is more beneficial than increasing the endogenous expression of eNOS by gene therapy modalities.
Similar content being viewed by others
Main
Endothelial nitric oxide synthase (eNOS) that utilizes l-arginine as a substrate to generate nitric oxide (NO) is a key regulator of vascular function. Even though endothelial cells, a predominant source of eNOS exhibit constitutive enzyme activity, several other regulators are necessary to induce eNOS activity based on local demand for NO. Reduced bioavailability of NO that causes endothelial dysfunction is a hallmark of diabetes. Several mechanisms have been implicated causing ‘endothelial dysfunction’ in diabetes. The reduced availability of the substrate l-arginine and the cofactor tetrahydrobiopterin (BH4) has been implicated due to increased arginase activity and enhanced superoxide generation.1 Uncoupling of eNOS has been shown to be central to robust superoxide generation and the associated vascular dysfunction.2 Increased levels of asymmetric NG, NG-dimethyl arginine (ADMA-a substrate analog of l-arginine) in circulation have also been shown in diabetic patients limiting the availability of l-arginine to eNOS.3
In vitro studies from our laboratory and reports from other investigators have shown that the regulation of eNOS activity is mediated by heat shock protein-90 (Hsp-90).4, 5 The interaction of eNOS with Hsp-90 favors the phosphorylation of eNOS at Ser1177 by Akt kinase. This phosphorylation process is a key event which augments eNOS activity leading to NO production.5
Our earlier studies have shown negative regulation of eNOS–Hsp-90 axis by inhibitor kappa B kinase beta (IKKβ), an upstream mediator of NF-κB signaling pathway in endothelial cells under the influence of high glucose (HG). We have also demonstrated that blocking IKKβ restores eNOS–Hsp-90 interaction and NO production. Earlier in vivo studies have shown supportive evidence demonstrating the positive outcome of nutritional supplementation of l-arginine and BH4 in the diet in augmenting NO production and vasorelaxation in animal models. On the basis of the results from our in vitro studies on IKKβ-mediated NO dysregulation6 and the in vivo nutritional supplementation studies on enhancing eNOS regulation, in this current study, we investigated the effect of upregulation of eNOS activity with the simultaneous suppression of IKKβ activity. We tested the effect of daily oral intake of salsalate, a potent and less toxic FDA-approved IKKβ blocker along with supplementing l-arginine/BH4.
The validity of this eNOS/IKKβ/Hsp-90 regulatory axis in terms of eNOS phosphorylation and restoration of eNOS-driven NO production has been tested both in in vitro endothelial cell cultures and in vivo animal models. The C57BL/6-Ins2Akita+/− mice, a type-1 diabetic mouse model is characterized by chronic hypoinsulinemia and hyperglycemia, and reported to develop spontaneous diabetes by 4 weeks of age. Heterozygous C57BL/6-Ins2Akita+/− mice were used for diet-supplementation experiments. Both age-matched female and male animals were included in this study even though blood glucose levels were noticed to be high in males than females. We also generated TgeNOS-GFP-Ins2Akita+/− cross-transgenic mice by breeding TgeNOS-GFP mice that express human eNOS predominantly in vascular endothelial cells with heterozygous type-1 Ins2-Akita+/− mice.
We expected that the overexpression of eNOS will upregulate NO production and cause improved vasodilation in response to carbamylcholine (CCh) induction. Earlier studies have shown that eNOS overexpression protects from endotoxin shock,7 limits neo-intimal lesion formation and medial thickening in a model of vascular remodeling8 and protects against skeletal muscle I/R injury associated with reduced leukocyte-endothelial cell adhesion and leukocyte trafficking into the ischemic tissue. Genetic overexpression of eNOS has also been shown to reduce the severity of congestive heart failure after severe myocardial infarction in mice.9 Cardiac myocyte-specific overexpression of eNOS demonstrated significant cardioprotection in gene-targeted mice.10 Despite these beneficial outcomes, the TgeNOS-GFP overexpression mice did not improve vasorelaxation, eNOS expression or eNOS phosphorylation in Ins2Akita+/− diabetic mice. Although these beneficial effects could be observed in Ins2Akita+/− mice fed with l-arginine, sepiapterin and salsalate indicating that more than overexpressing eNOS, maintaining the local balance between the enzyme, substrate and the cofactor results in favorable outcome, which is further improved by blocking IKKβ function.
MATERIALS AND METHODS
Generation of TgeNOS-GFP-Ins2Akita+/− Heterozygous Type-1 Diabetic Mouse
Heterozygous C57BL/6-Ins2Akita+/−/J mice (Jackson Laboratory, Bar Harbor, ME, USA; Stock # 003548) were used to obtain successive generation of Ins2Akita+/− heterozygotes. DNA obtained from tail snips of backcrossed litters at the time of weaning (about 3 weeks after birth) were used to obtain the PCR products using a forward primer: 5′-TGC TGA TGC CCT GGC CTG CT-3′ and a reverse primer: 5′-TGG TCC CAC ATA TGCA CAT G-3′ sequences. The PCR was performed using conditions recommended by Jackson Laboratories. The PCR products digested with Fnu-1 restriction enzyme were used to confirm Ins2Akita+/− genotype (Table 1).
A representative gel profile of a TgeNOS-GFP-Ins2Akita+/− is presented in Figure 1. Litters from backcrossed hemizygous breeding pair of TgeNOS-GFP overexpressing mice (human eNOS-GFP expressed in vascular endothelial cells) on a C57BL/6 background11 were genotyped using 5′-GAT GGA AGA CTT GGT GGG GAG-3′ as forward primer, and 5′-GTT CTC CCT GGA TTA AGT GAG-3′ as reverse primer that specifically amplifies the human eNOS gene (Figure 1 and Table 1). The results were further confirmed by the presence of GFP expression (Transnetyx, Cordova, TN, USA). Next, Ins2Akita+/− mice that expresses human eNOS-GFP were generated by crossbreeding the hemizygous TgeNOS-GFP expressing mice with hemizygous Ins2Akita+/− mice for two to three generations to obtain genetic homogeneity even though both the strains were already available on a C57Bl/6 background. The litters were genotyped for both Ins2Akita+/− and hemizygous TgeNOS-GFP and mice that are positive for both strains (hemizygous) were referred as TgeNOS-GFP-Ins2Akita+/− cross in this study. In addition to the genotype, the diabetic status of both the Ins2Akita+/− and the TgeNOS-GFP-Ins2Akita+/− cross mice were validated by measuring blood glucose using the One Touch Ultra Test Strips (Table 2). Animals were provided with ad libitum or the recommended breeding diet while breeding. The cages with pregnant females were housed in special breeding rooms. Animals of both the genders between 15 and 20 weeks old were euthanized for collection of aorta. Control groups include wild-type C57BL/6 mice, TgeNOS-GFP mice (hemizygous), Ins2Akita+/− (hemizygous) mice that were generated during the crossbreeding process to obtain TgeNOS-GFP/Ins2Akita+/− cross. Genotypes were determined by resolving the PCR products in 12% polyacrylamide gels after their respective restriction digestions. All procedures were performed in accordance with National Institutes of Health guidelines with approved protocol by the UTHSCSA Institutional Animal Care and use Committee.
Treatment with l-Arginine, Sepiapterin and Salsalate
After weaning, Ins2Akita+/− mice were fed with l-arginine12 in drinking water at 2 mg per gram of body weight. Sepiapterin was administered by oral gavage at 0.1 mg per gram of body weight,13 and salsalate was given at 0.1 g per gram of body weight14 as an additive mixed in ad libitum diet. The treatments were continued for 16 weeks. At the end of the treatment regime, mice were euthanized. Blood samples were collected by cardiac puncture. Aortae were isolated to perform isometric arterial ring assay and immunoblotting.
Cell Culture
Human aortic endothelial cells (HAECs, Invitrogen, CA, USA) and bovine aortic endothelial cells (Clonetics, San Diego, CA, USA) were cultured as reported earlier.6 In brief, cells were revived in MCDB-131 medium (Sigma, St Louis, MO, USA) containing 10% fetal bovine serum (FBS, Hyclone, Kansas City, KS, USA) and enriched with 250 ng/ml fibroblast growth factor (PeproTech, Rocky Hill, NJ, USA), 20 ng/ml of epidermal growth factor (PeproTech), 1 μg/ml of hydrocortisone (Sigma), 100 U/ml of penicillin, and 100 mg/ml streptomycin (Media-Tech, Herndon, VA, USA). For routine maintenance, only 5% FBS was used. Before treatment conditions, the cells were incubated in reduced serum concentration (2% FBS) at least for 16 h. Cells from passages 4–7 were used for all the experiments.
NO Measurement in Endothelial Cells by DAF-FM Using FACS
HAECs were cultured in 25 mM α d(+) glucose (HG) medium containing 2 mM of l-arginine, 50 μM of salsalate, 100 μM of sepiapterin or as a combined supplement for >48 h. Cells grown in basal medium containing 5 mM α d(+) glucose or 25 mM l-glucose were used as controls. Cells were washed three times with Krebs–Ringer Bicarbonate Buffer (KREBS, Sigma-Aldrich) and further incubated with 0.5 μM of DAF-FM (DA) (Life Technologies) for 30 min at 37°C. Cells were then trypsinized, and re-suspended in KREBS buffer containing 2% FBS. Cells were analyzed on a FACSCanto II (BD Biosciences, San Jose, CA, USA) analyzer set at 100 000 events per sample. The data was represented as differences in mean fluorescence intensity (excitation wavelength of 488 nm and emission wavelength of 530 nm) at single-cell level. In each sample, the mean fluorescence intensity of the analyzed cells was determined after gating the cell population by forward and side-light scatter signals as recorded on a dot plot. Data were analyzed using BD Diva software (BD Biosciences). To exclude any intracellular background fluorescence signal, cells incubated with DAF-FM (DA) alone in parallel with glucose-treated cells were used as basal level fluorescence. The fluorescence data calculated by subtracting unstained cells from stained cells were expressed as the percentage of mean fluorescence and the increment of NO generation obtained between experimental groups were shown in terms of fold increase.
NO Measurement in Aortic Tissue by Arginine–Citrulline Conversion Method
Aortic homogenates from frozen aortic tissue samples were prepared using 50 mM of Tris–HCl buffer (pH 7.4) containing 0.1 mM EDTA. Supernatants were collected after centrifugation at 100 000 rpm using an Optima Max TL centrifuge and subjected to the measurement of NO production using the arginine–citrulline conversion assay.15 In brief, 35 μl of cell lysates were incubated with 15 μM 14C-l-arginine, 15 μM l-arginine, 10 μM calmodulin, 25 μM BH4, and 1 mM NADPH in 50 mM Tris–HCl, pH 7.4, 100 mM NaCl, and 200 μM CaCl2 in a 50-μl reaction volume. Samples were incubated at 37 °C for 30 min, then reactions were terminated with 400 μl ice-cold stop buffer (50 mM HEPES, pH 5.5, 5 mM EDTA, 5 mM EGTA) and stored on ice. Dowex 50Wx8 beads, 500 μl, equilibrated in H2O, were added to each sample and mixed. Mixtures were loaded into spin cups (Bio-Rad, Hercules, CA, USA) and spun for 1 min. Eluates were collected and radioactivity was measured in a scintillation counter. High (total counts, no Dowex beads) and low (background, with Dowex beads) controls were also measured.
Measurement of Superoxide in In Vitro Cell Cultures and In Vivo Frozen Aortae from Mice
Aortae excised from Ins2Akita+/−, TgeNOS-GFP, TgeNOS-GFP-Ins2Akita+/− cross- and wild-type C57BL/6 mice were placed in optimal cutting temperature compound (Tissue-Tek O.C.T Compound Sakura, The Netherlands) and frozen at −80 °C. These frozen tissues were sectioned at a thickness of 8 μm and incubated with dihydroethidium (DHE; 5 μM; Life Technologies) for 30 min at 37 °C.2 Some sections were pretreated with superoxide dismutase-polyethylene glycol (PEG-SOD; 300 U/ml)/catalase-polyethylene glycol (PEG-CAT; 200 U/ml) or Apocynin (250 μM) or l-NG-Nitroarginine Methyl Ester (l-NAME; 1 mM) before incubation with DHE for 10 min at 37 °C. The aortic specimen were visualized by Olympus IX81 confocal fluorescence microscopy using an excitation at 546±25 nm band-pass filter, emission at 590 nm long-pass filter followed by a 640±25 nm band-pass filter. Aortic elastic laminae were recorded as an auto fluorescence using an excitation at 465±25 nm and emission at 525 nm long-pass filter. Identical laser settings were used for acquisition of images from different groups of aortae. Fluorescent images were captured by Flowview1000 software and fluorescence quantitation was performed using FIJI/ImageJ2 analysis software. DHE signals from each aortic specimen were calculated by subtracting the auto fluorescence green signal and the ratios were compared by each region on an aorta.
In addition, HAECs cultured in α d(+) glucose (25 mM) for 48 h were incubated with l-NAME (1 mM), PEG-SOD (300 U/ml)/PEG-CAT (200 U/ml) or Apocynin (250 μM) for 30 min prior to adding DHE. Cells were also incubated with 2 mM l-arginine, 100 μM sepiapterin and 50 μM of salsalate for 48 h along with HG. The cells were harvested by trypsinization and further incubated with 5 μM of DHE for 10 min at 37 °C. The cells were washed three times with Krebs buffer and re-suspended at a density of 1 × 106 cells/ml in Krebs buffer contains 2% FBS. Cells were analyzed on a FACSCanto II (BD Biosciences) analyzer set at 100 000 events per sample at an excitation and emission wavelengths of 520 and 610 nm, respectively. The observed mean fluorescence intensities calculated by subtracting unstained from stained cells were analyzed using BD Diva software (BD Biosciences). The cells stained with DHE were used to exclude the intracellular background fluorescence signal. Mean fluorescence of test samples are expressed as the percentage of control mean fluorescence intensity.
Measurement of HG-Induced TNF-α Generation in HAEC
The culture supernatants from HAECs treated with or without HG in the presence of l-arginine, sepiapterin and salsalate were collected. The level of secreted TNF-α in the culture supernatants was determined by a bead-based immunoassay complemented with FCAP Array v3.0 software (BD Biosciences). The levels of secreted TNF-α in the supernatant from activated normal human monocytes (Mono Mac 6; MM6) by stimulating with 1 ng/ml of LPS and harvested at 24 h were used as assay control. Recombinant TNF-α at known concentrations was used to derive standard curve as recommended by the manufacturer’s protocol.
Western Blotting
After clearing remnant blood components, the vessels were homogenized on ice using tissue protein extraction buffer (1 × HEPES-based buffer, pH 7.5). Clear supernatants were obtained after centrifugation of the tissue homogenates at 100 000 × g at 4 °C using Optima Max-TL-ultracentrifuge (Beckman Coulter). Protein from whole cell lysates and aortic extracts were separated by electrophoresis on a 10% SDS–polyacrylamide gel and immunoblotted as described earlier.6 Primary antibodies used were rabbit anti-human eNOS (1:1000 dilution) and rabbit anti-human phospho eNOS. Beta actin or GAPDH expression was used as internal loading control. Blots were quantitated using NIH ImageJ software.
Isometric Assay
Thoracic aorta was carefully removed and immediately rinsed in warm, oxygenated Kreb’s solution (35±2 °C). After clearing fatty tissue, the aorta was cut into rings of 2 mm in width with care being taken not to damage the endothelial cells and transferred to the apparatus for equilibration for 30 min. The tissues were mounted in double-jacketed vertical organ baths filled with oxygenated (95% O2, 5% CO2) Kreb’s solution maintained at physiological temperature. Maximum contraction was observed at both 0.8 and 1.0 g tension. Therefore, 1.0 g was employed as the baseline tension in all experiments. The solution was continuously oxygenated and changed every 15 min. The experiment was initiated after 30 min of equilibration, and progressed as stated in the table below. Recording was done using Axoscope software 10.2.0.14.
The concentrations of the contracting and relaxing agents applied to the aortic rings (60 mM KCl, and CCh 0.3, 1.0, 3.0, and 10 μM) were selected based on literature.16, 17, 18, 19 The optimal concentration of phenylephrine to be used for pre-contracting tissues was determined to be 1.0 μM and this concentration was used consistently throughout the experiments.

Continuous recording of the contraction/relaxation was initiated starting 5 min before the end of the equilibration period and was terminated at the end of the last experimental step.
In the table above, steps 1 through 3 served to acclimate the rings by conditioning them to contraction with phenylephrine. Rings not meeting the following criteria were considered damaged:
(a) vascular rings developed at least 0.1 g tension in the presence of 60 μM KCl; (b) when stimulated with 1 μM phenylephrine, vascular rings developed 40% of ‘maximum’ tension (as determined with 60 μM KCl) (individual contraction/relaxation cycles were eliminated from the data set based on this criteria rather than entire tissues); (c) vascular rings in the presence of 10 μM CCh relaxed by 25% of the tension developed following 1 μM phenylephrine exposure; and (d) vascular rings in the presence of 3.0 μM CCh relaxed by 10% of the tension developed following 1 μM phenylephrine exposure. All tissues that were adequately large in diameter were mounted in the baths and the experimental procedure was recorded for further analysis (tissues which did not meet the functional criteria outlined above were only eliminated after analysis of the raw data). Additional validation steps as shown below were performed following step 11 in the table above to validate the assay in three wild-type mice.
Validation Steps

Analysis of Experimental Data
The experimental trace was analyzed in Clampfit 10.4.0.36. The contraction and relaxation amplitude of each contraction–relaxation cycle was extracted using digital cursors. Tension levels for each tissue were analyzed for each experimental condition; data were also extracted with the digital cursors in an empty bath at the same time points as maximum contraction and maximum relaxation for each cycle, to allow for correction of changes due to noise in the digital signal rather than tissue contraction/relaxation.
The data was transferred into an experiment-specific MS Excel 2007 template sheet; to correct for signal anomalies, the changes in the baseline signal extracted from an empty bath was subtracted from changes in tension measured in the tissues; any negative relaxation values (contraction) following addition of 0.3 or 1.0 μM CCh were included in the data set, but set to zero relaxation. Exclusion criteria were applied to the extracted data to yield a data set for each group containing sufficiently robust, endothelium intact tissues.
Tension data was plotted as ‘% Relaxation versus Log (CCh concentration in M)’ for each group, where ‘% Relaxation’ represents the amount of relaxation achieved (in grams of tension) as a percent of the amount of contraction (in grams of tension) caused by 1 μM phenylephrine or 60 mM KCl in the same tissue. Validation data were treated in the same way (% Relaxation as percentage of phenylephrine or KCl contraction). Repeat unpaired Student’s t-tests were performed on all experimental conditions and parameters, comparing the post-exposure responses to phenylephrine and CCh in treatment groups to these responses elicited in the control groups. The threshold for statistical significance was set at P≤0.05.
RESULTS
Development of the TgeNOS-Ins2Akita+/− Cross Mice
A representative genotypic profile (Table 1) of a wild type, Ins2Akita+/− TgeNOS-GFP and TgeNOS-GFP-Ins2Akita+/− cross mice is presented in Figure 1 as gel picture. The genotyping was performed by PCR and restriction digestion as described in the Materials and Methods section. Both Ins2Akita+/− females and males were used for breeding with TgeNOS-GFP males and females. No differences were observed in the probability of obtaining the hemizygous TgeNOS-GFP-Ins2 Akita+/−cross mice. In general, from this crossbreeding, only a 15–20% success rate of obtaining TgeNOS-GFP-Ins2Akita+/− cross mice was noticed. Also, as shown in Table 2, the body weight of the Ins2Akita+/− mice (19.21±2.07 g) and the TgeNOS-GFP-Ins2Akita+/− mice (20.26±1.09 g) were slightly lower than the wild type and the TgeNOS-GFP mice (23.27±2.24 g and 25.86±0.3 g, respectively). The blood-glucose levels determined at the time of euthanasia were elevated for both Ins2Akita+/− and TgeNOS-GFP-Ins2Akita+/− mice with 431.41±30.0 and 484.5±77.2 mg/dl, respectively. Although the males showed very high blood-glucose levels, the females demonstrated only moderately elevated blood-glucose levels compared with the wild-type mice (Table 2).
Endothelial NOS Is a Chief Source of Superoxide Generated in Endothelial Cells Cultured in Medium Containing HG
HAECs cultured in HG medium for 48 h exhibited increased DHE fluorescence (75.40±4.11% compared with 10.66±0.93% of control or 15.6±0.87% of 25 mM l-glucose). This was a significant (85.78±1.96%; P<0.001) increment over the control (Figure 2). When cells were pre-incubated with 1 mM l-NAME, a specific inhibitor of eNOS in the presence of HG for 30 min, the DHE fluorescence decreased (19.5±0.95%, P<0.001) showing a 74.07±2.28% reduction in HG-mediated superoxide generation. Although PEG-SOD (300 U/ml)/PEG-CAT (200 U/ml) incubated cells showed a decrement of 45.3±0.72%, addition of l-NAME further (additional 55.53±0.53%) reduced the DHE fluorescence indicating the eNOS-specific generation of superoxide. Similarly, the NADPH oxidase inhibitor apocynin (250 μM) inhibited 33.7±1.8% of HG-induced superoxide generation. However, in the presence of l-NAME, there was 56.9±0.96% enhanced inhibition compared with the inhibition obtained by apocynin alone. Altogether, the results indicated that l-NAME, independently and in combination, robustly suppressed superoxide generated by HG in HAEC suggesting that eNOS is the predominant source of superoxide under these conditions.
Elevated α d(+) glucose (25 mM) enhances ROS production. HAECs incubated with HG for 48 h in the presence or absence of 1 mM l-NAME, 250 μM apocynin or 300 U/ml SOD plus 200 U/ml CAT were subjected for DHE staining. FACS analysis of DHE-stained cells demonstrated production of superoxide to be 85.78±1.96% elevated in HG. Although SOD plus CAT and apocynin inhibited 45.31±0.72% and 33.7±1.8%, respectively, l-NAME inhibited significant (74.06±2.28%) amount of ROS indicating a major portion of ROS released to be eNOS dependent under conditions of HG.
Endothelial NOS Is a Chief Source of Superoxide Generated in Diabetic Vessels
To establish the significance and the physiological relevance of enhanced superoxide generation in vivo, frozen sections of thoracic and abdominal aortae of wild type, TgeNOS-GFP, Ins2Akita+/− type-1 diabetic mice and TgeNOS-GFP-Ins2Akita+/− cross mice were subjected for DHE staining (Figure 3a). Although, some background fluorescence was present in wild-type mice, the TgeNOS-GFP mice, Ins2Akita+/− and the TgeNOS-GFP-Ins2Akita+/− cross mice exhibited increased DHE fluorescence (3.79±0.5-, 5.01±0.4-, 5.65±0.48-fold, respectively) compared with the wild-type mice. Incubation of aorta from TgeNOS-GFP mice with PEG-SOD (300 U/ml) and PEG-CAT (200 U/ml) showed 78.66±2.33% blockade of superoxide generation. Apocynin (250 μM) inhibited 71.91±2.26% of DHE fluorescence intensity. However, incubation with l-NAME (1 mM) showed 82.14±2.19% decrement in DHE fluorescence in TgeNOS-GFP mice. The 5.01±0.4-fold increase of DHE staining shown in Ins2Akita+/− mice reduced to 66.48±0.67% by apocynin, 69±1.22% by PEG-SOD/PEG-CAT and 75.57±1.63 by l-NAME. Similar to Ins2Akita+/− mice, TgeNOS-GFP/Ins2Akita+/− cross mice demonstrated 64.61±2.61% blocking by apocynin, 69.59±0.53% by PEG-SOD/PEG-CAT and 76.08±0.32% by l-NAME. Overall, l-NAME demonstrated enhanced blocking of DHE fluorescence in TgeNOS-GFP mice, Ins2Akita+/− and the TgeNOS-GFP/Ins2Akita+/− cross mice relative to apocynin and PEG-SOD/CAT indicating the chief source of superoxide to be eNOS in Ins2Akita+/− type-1 diabetic vessels. Quantitation of the DHE staining performed by FIJI/ImageJ2 analysis software as shown in Figure 3b demonstrated enhanced superoxide generation not only in Ins2Akita+/− type-1 diabetic mice and TgeNOS-GFP/Ins2Akita+/− cross mice, but also in TgeNOS-GFP mice, which was not reported earlier.
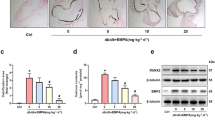
(a) Increased dihydroethidium (DHE) fluorescence in TgeNOS-GFP-Ins2Akita+/− arteries. Frozen aortae from Ins2,Akita+/− TgeNOS-GFP, TgeNOS-GFP-Ins2Akita+/− cross- and wild-type (C57BL/6) mice were processed and then incubated with 5 μM DHE for 30 min at 37 °C and subsequently visualized by fluorescence microscopy using fluorescein isothiocyanate filter to visualize the endogenous elastic laminar fluorescence of blood vessels and rhodamine red to visualize DHE-positive fluorophore. Although background fluorescence was present in wild-type mice, TgeNOS-GFP, Ins2Akita+/−, and the TgeNOS-GFP-Ins2Akita+/− cross mice exhibited increased DHE fluorescence (3.79±0.5-, 5.01±0.4-, 5.65±0.4-fold) relative to wild-type mice. Incubation of tissue with PEG-SOD/CAT and apocynin reduced DHE staining but l-NAME even more robustly reduced the high DHE fluorescence in TgeNOS-GFP, Ins2Akita+/− and TgeNOS-GFP-Ins2Akita+/− cross mice indicating eNOS as a predominant source of superoxide generated in diabetic vessels. (b) Quantitation of DHE fluorescent images: images were captured by Flowview1000 software and quantitation was performed using FIJI/ImageJ2 analysis software. Contribution of l-NAME in blocking DHE fluorescence is shown in fold increase compared with untreated control. Significant blocking of DHE fluorescence indicates eNOS as a key source of superoxide generated in the vessel wall.
Expression of Total versus Phospho eNOS and Endothelial NOS Activity Is Significantly Reduced in Homogenates of Aortae of Ins2Akita+/− Type-1 Diabetic Mice and TgeNOS-GFP-Ins2Akita+/− Cross Mice
Phosphorylation of eNOS at Ser1177 (bovine Ser 1179) has an important role in the regulation of eNOS activity.4 Recent evidence also demonstrated that Ser1179 phosphorylation exclusively exists in eNOS dimer at both resting and stimulated condition.5 As shown in Figure 4a, our results demonstrated a significant downregulation of both total and phospho eNOS expression in Ins2Akita+/− mice and the TgeNOS-GFP/Ins2Akita+/− cross mice compared with age-matched wild type and TgeNOS-GFP mice. However, it is interesting to note here that the overexpression of eNOS as shown in TgeNOS-GFP mice did not rescue the Ins2Akita+/− mice both in total eNOS or phospho eNOS expression. In support of the phosphorylation results, the wild type and the TgeNOS-GFP mice showed the presence of the dimer form of eNOS heavily expressed than the Ins2Akita+/− mice (data not shown).
(a) Reduced phospho eNOS expression in TgeNOS-GFP Ins2Akita+/− cross mice. Representative immunoblot showing total and phospho eNOS expression in aortae of wild type, TgeNOS-GFP, TgeNOS-GFP-Ins2Akita+/− cross and Ins2Akita+/− mice. Although the TgeNOS mice expressed significantly elevated total eNOS and phospho eNOS, the TgeNOS-GFP-Ins2Akita+/− cross mice expressed significantly low total and phospho eNOS proteins. An insignificant difference was noticed between the expression levels of total and phospho eNOS in Ins2Akita+/− and TgeNOS-GFP-Ins2Akita+/− cross mice. (b) Reduced eNOS activity in TgeNOS-GFP-Ins2Akita+/− cross mice. Endothelial NOS activity was determined by the conversion of l-arginine to l-citrulline in aortae. Protein lysates from isolated aortae of wild type, TgeNOS-GFP mice, TgeNOS-GFP-Ins2Akita+/− cross and Ins2Akita+/− mice were examined for eNOS activity. Although, TgeNOS-GFP mice showed significantly enhanced NO production (7.51±1.45 pmol/min/mg), both Ins2Akita+/− and TgeNOS-GFP-Ins2Akita+/− cross mice demonstrated significantly reduced NO production.
In support of the results obtained from phospho eNOS expression, endothelial NOS activity as shown in Figure 4b measured in homogenates of aorta by the l-arginine to l-citrulline conversion assay demonstrated a significant increase in eNOS activity in TgeNOS-GFP mice (7.51±1.45 pmol/min/mg) compared with wild-type mice (5.47±0.94 pmol/min/mg, P-value<0.05). However, the eNOS activity was significantly reduced in Ins2Akita+/− type-1 diabetic mice. Although the control mice exhibited 5.47±0.94 pmol/min/mg of activity, Ins2Akita+/− type-1 diabetic mice showed 1.71±0.045 pmol/min/mg of activity (a 68% reduction from the wild-type mice with a P-value≤0.019). In addition, these results were interesting in those TgeNOS-GFP/Ins2Akita+/− cross mice, which had the eNOS-GFP overexpression in their vessels, did not show enhanced eNOS activity. The activity levels were 2.06±0.31 pmol/min/mg compared with the TgeNOS-GFP mice, which exhibited 7.51±1.45 pmol/min/mg of activity. The difference in eNOS activity between Ins2Akita+/− type-1 diabetic mice and the TgeNOS-GFP/Ins2Akita+/− cross mice was not significant. These results clearly demonstrated the influence of hyperglycemia over eNOS activity.
Supplementing l-Arginine, Sepiapterin and Salsalate Improves eNOS Phosphorylation and NO Production in ECs Grown in HG Medium
In our attempts to investigate the effect of supplementing l-arginine (2 mM), sepiapterin (100 μM) and salsalate (50 μM) to improve NO production under conditions of HG, first we evaluated the influence of these treatment conditions on phospho eNOS expression, which is vital for the subsequent NO production. As shown in Figures 5a and b, HAECs treated with HG for >48 h showed significantly reduced phospho eNOS expression (0.77±0.15-fold, P≤0.04) compared with both control (cells grown in basal medium; 5 mM α d(+)glucose) or 25 mM l-glucose medium. When cells were treated with l-arginine in combination with salsalate, the phospho eNOS expression increased (1.26±0.08-fold, P<0.02), which was statistically significant compared with HG-treated cells. However, a consistently increased expression was not noticed in all the experiments (n=9) in cells treated with sepiapterin in combination with l-arginine. The specificity of detection of phospho eNOS has been demonstrated by de-phosphorylating the phospho group by incubating the cell lysates with calf intestinal alkaline phosphatase, after inducing with VEGF, which is used as a positive control (Figure 5a insert).
(a) Expression of phospho eNOS in HAECs treated with l-arginine (2 mM) in combination with salsalate (50 μM) and sepiapterin (100 μM) in the presence of HG. Cell lysates prepared from HAECs treated with l-arginine (2 mM) salsalate (50 μM) and sepiapterin (100 μM) and further cultured for >48 h in HG were subjected to immunoblotting using anti-rabbit phospho eNOS and total eNOS antibodies. Membranes were re-blotted for house-keeping protein GAPDH for normalizing equal protein loading. Insert shows validation of phospho-specific antibody. Cell lysates treated with 20–40 units of calf intestinal alkaline phosphatase abolished the phospho protein expression, but the heat-inactivated enzyme did not dephosphorylate the protein. (b) Densitometric quantitation was performed using NIH ImageJ software and the ratio of phospho versus total eNOS expressions are indicated as fold increase. (c) Elevated α d(+)glucose concentration mitigates NO generation. Supplementation of l-arginine, in combination with salsalate and sepiapterin significantly increases NO production in vascular endothelial cells. HAECs were grown in 25 mM α d(+)glucose for >48 h. VEGF induction (25 ng/ml for 15 min) is shown as positive control (insert). Blocking NO production by l-NAME (1 mM) validates the specificity of NO measurement by DAF-FM (DA) staining. HG significantly downregulated NO production compared with 5 mM glucose incubation (*P<0.05). Incubation of salsalate independently upregulated NO production compared with l-arginine alone (#P≤0.005) or in combination with l-arginine (##P≤0.05). Combination of l-arginine with salsalate and sepiapterin achieved maximum NO production compared with l-arginine alone or in combination with sepiapterin (**P≤0.01). (d) HAECs treated with l-arginine (2 mM), sepiapterin (100 μM) and salsalate (50 μM) reduce HG-induced superoxide generation. HAECs incubated with HG for 48 h in the presence or absence of l-arginine, in combination with salsalate and sepiapterin were subjected for DHE staining. FACS analysis of DHE-stained cells demonstrated a significant generation of superoxide (81.12±3.23%) in HG-treated cells. Incubation of HAECs with l-arginine, in combination with salsalate and sepiapterin inhibited 32.99± 6.78% of HG-induced ROS production. (e) HAECs treated with l-arginine (2 mM), sepiapterin (100 μM) and salsalate (50 μM) demonstrated no alteration of HG-induced TNF-α production: HAECs treated with l-arginine (2 mM), sepiapterin (100 μM) and salsalate (50 μM) in the presence or absence of HG were used for BD beads TNF-α Flex assay to determine TNF-α production. HG incubation significantly enhanced TNF-α generation compared with cells grown in basal medium containing 5 mM α-d glucose (118±8 pg/ml in HG versus 27±16 pg/ml in control, P≤0.003). This increase in HG-induced TNF-α level did not change in HAECs treated with l-arginine, sepiapterin and salsalate (104±10 pg/ml in treated cells P=0.13). (f) Daily oral intake of l-arginine, in combination with salsalate restores phospho eNOS/total eNOS expression in Ins2Akita+/− mice. Ins2Akita+/− mice were fed with l-arginine (2 mg per gram of body weight in drinking water), sepiapterin (0.1 mg per gram of body weight by oral gavage), and salsalate (0.1 g per gram of body weight mixed in ad libitum diet) for 16 weeks. At the end of the diet regimen mice were killed and the aortae were dissected. Proteins extracted from residual aortae left behind in mice at the time of arterial ring assay were subjected to electrophoresis. Immunoblots were performed to determine phospho eNOS and total eNOS expression. Results show the improved phospho eNOS/total eNOS expression after 16 weeks of oral supplementation of l-arginine, in combination with sepiapterin and salsalate.
To correlate the phospho eNOS expression status described above with subsequent NO production, we used HAECs pre-treated for 48 h with 2 mM l-arginine, or 50 μM of salsalate, or 100 μM of sepiapterin either individually or in combination and further incubated with HG for >48 h. These cells demonstrated improved NO production. As shown in Figure 5c, the HG-treated cells generated 0.87±0.052-fold of NO with a P-value≤0.05 compared with untreated control. However, pre-treatment of cells with l-arginine, or sepiapterin, a precursor of BH4, or salsalate either alone or in combination followed by 25 mM α d(+)glucose incubation demonstrated significantly enhanced NO production as follows: 2.05±0.19-fold for l-arginine, 3.0±0.19-fold for salsalate (P≤0.005) and 3.28±0.22-fold for l-arginine and salsalate in combination (P≤0.05). The ability of sepiapterin in inducing NO production was almost equivalent to salsalate-mediated NO production in combination with l-arginine (3.28±0.3-fold, 3.28±0.2-fold, respectively). However, of all the treatment conditions, combination of both salsalate and sepiapterin with l-arginine demonstrated highest NO production, 3.77± 0.37-fold increase with P-value≤0.01. These results clearly demonstrated that the improved NO production by both l-arginine and sepiapterin could further be augmented by salsalate to achieve maximum NO production under conditions of HG. To determine whether there are additional benefits of l-arginine, sepiapterin and salsalate treatment over NO production, alterations in superoxide generation and cytokine production were determined in HAECs treated with 2 mM l-arginine, 50 μM of salsalate, 100 μM of sepiapterin in combination in the presence or absence of HG for >48 h. As shown in Figure 5d, cells treated with l-arginine, sepiapterin and salsalate showed a 32.99±6.8% decrement of superoxide (P≤0.002) compared with untreated-HG-induced HAECs. In parallel, whereas HG-incubated cells expressed 118±8.0 pg/ml of TNF-α, cells treated with l-arginine, sepiapterin and salsalate under the influence of HG expressed 104±10 pg/ml of TNF-α (P=0.13) thus demonstrating minimal alteration in cytokine production (Figure 5e).
Supplementing l-Arginine, BH4 and Salsalate in the Diet Improves Total versus Phospho eNOS Expression in Arteries
To evaluate the effect of oral supplementation of l-arginine in combination with sepiapterin and salsalate on eNOS phosphorylation, aortae collected from mice at the time of arterial ring assay were used for immunoblotting. As shown in Figure 5f, pooled aortae from three mice in each diet group demonstrated an enhanced eNOS phosphorylation and improved total eNOS expression compared with untreated Ins2Akita+/− mice. Among the three dietary groups, while l-arginine supplementation improved phospho eNOS expression, l-arginine in combination with salsalate and sepiapterin showed pronounced increase in both total and phospho eNOS expressions.
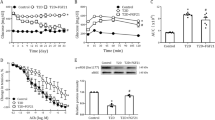
Analysis of Contractile and Relaxing Responses of Arteries from Wild Type, TgeNOS-GFP, Ins2Akita+/− and TgeNOS-GFP-Ins2Akita+/− Cross Mice
Reduced vascular function in male Ins2Akita mice has been reported especially between 12 and 24 weeks of age.1 Mice included in our study for vascular relaxation responses, as shown in Figure 6b, were fed with dietary supplements soon after weaning (3 weeks of age). Feeding of diet supplements continued for 16 weeks. Mice (both genders) were 18–20 weeks old when they were killed to perform arterial ring assay. At the end of the dietary regimen endothelial function in thoracic aortae were determined by relaxation of aortic rings to different concentrations of CCh, an endothelium-dependent vasodialator and a non-hydrolyzable analogue of acetylcholine. In comparing the untreated Ins2Akita+/− (diabetic) mice, to the wild-type control mice, wild-type tissues were more sensitive to CCh (greater relaxation) at every concentration tested (Figure 6a). The maximum average relaxation of wild-type aortic ring was 49.03±0.15% at 10 μM CCh, and the maximum average relaxation in Ins2Akita+/− aortic ring was 22 ± 3.51%. At the highest CCh concentration, wild-type relaxation ranged from 26.6 to 64.9%, and Ins2Akita+/− relaxation was between 22.9 and 49.7% in the averaged tissues.
Impairment of endothelium-dependent relaxation in the aortae of untreated Ins2Akita+/− mice and the effect of oral supplementation of l-arginine, sepiapterin and salsalate. Concentration-response curves of endothelium-dependent relaxation to CCh (0.3, 1.0, 3.0 and 10.0 μM) were analyzed at the end of diet regimen when the mice were killed at 18–20 weeks of age. Panel (a) shows endothelium-dependent relaxation of age-matched wild-type C57BL/6 mice and untreated Ins2Akita+/− diabetic mice, and panel (b) shows untreated Ins2Akita+/− mice compared with Ins2Akita+/- mice fed with l-arginine (2 mg per gram of body weight in drinking water), sepiapterin (0.1 mg per gram of body weight by oral gavage), and salsalate (0.1 g per gram of body weight mixed in ad libitum diet). The vascular relaxation responses were improved in the group of Ins2Akita+/− mice supplemented with l-arginine either independently or in combination with sepiapterin and salsalate in the ad libitum diet. Panel (c) and panel (d) show aortae collected from genetically modified TgeNOS-GFP mice and TgeNOS-GFP-Ins2Akita+/− cross mice that overexpress eNOS and subjected to isometric tension assay. Vessel relaxation values were calculated relative to the maximal changes from the contraction produced by 60 mM KCl or 1 μM phenylephrine, which was taken as 100%. Data are the means±s.e.m of 6–8 independent determinations from varied groups of mice with 4–6 animals of both genders included in each group. Statistical significance is indicated as *P <0.05.
Aortae from l-arginine-fed mice showed greater relaxation to CCh than tissues from untreated diabetic mice. At 1 μM CCh, the difference in sensitivity from Ins2Akita+/− mice was considered significant (P≤0.02). At 10 μM CCh, l-arginine-treated tissues relaxed between 20.3 and 90.0% to give an average relaxation of 55.9±14.6% (Figure 6b). Aortae from mice fed with l-arginine in combination with sepiapterin-fed mice showed improved sensitivity to increasing CCh dose compared with aortic tissues from untreated mice, suggesting a benefit from l-arginine and sepiapterin diet supplementation. Relaxation was improved at all the concentrations of CCh that were tested, and the maximum average percent relaxation in l-arginine plus sepiapterin-fed aortae of 53.3±18.3% occurred with 3 μM CCh (Figure 6b). Tissues from diabetic mice treated with l-arginine, sepiapterin, and salsalate experienced a greater average relaxation than tissues from untreated diabetic mice during exposure to all CCh concentrations. Whereas statistical significance was achieved at 1 μM CCh and at 10 μM CCh, relaxation of the treated tissues ranged from 28.7 to 123.3%. The maximum average relaxation observed in these tissues was 68.8±15.5% with 10 μM CCh (Figure 6b).
In addition, vascular relaxation assays were also performed in genetically modified mice including TgeNOS-GFP mice and the novel TgeNOS-GFP-Ins2Akita+/− diabetic mice that we generated. As reported earlier,11 the TgeNOS-GFP mice aortae relaxed less than wild-type vessels for all the concentrations of CCh tested; this difference was significant at 3 μM CCh when the average relaxation in TgeNOS-GFP mice was only 19.1±0.1%. In the TgeNOS-GFP mice, the maximum average percent relaxation observed was 37.9±11.7% with 10 μM CCh, a concentration that caused relaxation ranging from 0 to 98.3% in these tissues (Figure 6c).
Aortae from TgeNOS-GFP-Ins2Akita+/− cross mice also consistently relaxed less than Ins2Akita+/− vessels when exposed to the same concentrations of CCh, significantly less at 3 and 10 μM. The maximum average percent relaxation of these tissues was 20.8% at 3 μM CCh. The range of relaxation at 10 μM was 0–60.7% relaxation. Sensitivity of these tissues to CCh was reduced compared with both WT and untreated Ins2Akita+/− control tissues. Both TgeNOS-GFP and TgeNOS-GFP-Ins2Akita+/− cross mice performed similarly between 0.3 and 3.0 μM CCh; only at 10 μM there was a divergence in the sensitivity to CCh, where the non-diabetic tissues relaxed more than the diabetic tissues (Figure 6d).
In validation assays, in both wild type and TgeNOS-GFP, a reduction in relaxation with CCh was observed following the inhibition of NOS with l-NAME (paired t-test: P=0.052 and 0.049, respectively). Relaxation with 1 μM sodium nitroprusside caused greater relaxation than CCh under normal conditions. In these tissues, relaxation with sodium nitroprusside consistently relaxed tissues more than 100% of initial contraction with 1 μM PE indicating the reactivity of the vascular smooth muscle to NO was intact in the aortae.
DISCUSSION
Endothelial dysfunction though known as the underlying cause that initiates various complications of diabetes involving different organ systems, definitive treatment strategies to rescue from its effect in terms of either preventing or lessening the severity of complications are still unavailable.20 The beneficial effects of NO that are generated by eNOS activity in maintaining vascular health and function have been well established.4 These reports have demonstrated that restoring eNOS activity to enhance bioavailability of NO is a promising treatment strategy to alleviate macro- and micro-vascular complications of diabetes. In the present study, we have primarily investigated the effect of enhancing eNOS expression/activity in a type-1 diabetic Ins2Akita+/− mouse model. We introduced a genetic modification of overexpressing human eNOS-GFP fusion gene targeting predominantly the vascular endothelial cells of the Ins2Akita+/− mouse. As stated in the methods, this was achieved by crossbreeding the Ins2Akita+/− mice with TgeNOS-GFP mice. As an alternative approach, we adopted a nutrition-based preventive and therapeutic strategy, where Ins2Akita+/− mice were fed with l-arginine, sepiapterin independently or in combination with salsalate, a known inhibitor of inhibitor kappa B kinase, and an upstream regulator of the inflammatory mediator nuclear factor kappa B. Results of the present investigation demonstrate eNOS as the chief source of superoxide in HAECs incubated with HG and in intact vessels from diabetic mice. Supplementing l-arginine in combination with sepiapterin, an immediate precursor of BH4, with salsalate increased phospho eNOS expression, NO production in HAECs that were cultured in HG medium for >48 h. In diabetic mice, the diet supplementation for >16 weeks significantly enhanced phosphorylation of eNOS in the vessels and improved vascular relaxation to CCh induction compared with untreated diabetic mice.
Generation of the novel TgeNOS-GFP-Ins2Akita+/− cross mice was attempted with the anticipation that enhanced eNOS expression will improve endothelial cell function in type-1 diabetes. Unexpectedly, the expression levels of total eNOS/phosphoeNOS in Ins2Akita+/− and the TgeNOS-GFP-Ins2Akita+/− cross mice were significantly reduced (Figures 4a and b) even though the TgeNOS-GFP mice without diabetes exhibited enhanced eNOS expression. This probably could be attributed to the increased blood-glucose levels. These results are in agreement with results reported by Toque et al,1 a study that included only the Ins2Akita+/− diabetic mice and demonstrated that progression of age over 12 weeks significantly reduced phospho/versus total eNOS expression, NO production and subsequent vasorelaxation responses. However, a single report from Sasaki et al31 demonstrated the generation of streptozotocin (STZ)-induced diabetic mice that overexpresses (bovine) eNOS. Unlike our observation and the report by Toque et al,1 aortic total eNOS expression in this STZ model was high. The phospho eNOS expression was not determined in this study. Discrepancy in the levels of total eNOS expression between our model and the STZ model could be due to the characteristics of STZ. Outcomes are different due to the doses of STZ used for diabetic induction. Use of STZ enhances superoxide generation with a marked increase in the expression of pro-inflammatory cytokines resulting in confounding data. These disadvantages of STZ are eliminated in our model as diabetes in Ins2Akita+/− mice results due to a genetic mutation.
However, in support of our observation that there is a lack of protection in TgeNOS-GFP-Ins2Akita+/− cross mice, overexpression of eNOS in STZ-induced diabetes augmented neo-intima formation by carotid artery ligation. Also, as shown in Figures 3a and b in our study, there was a robust superoxide generation in the vessel wall, which could be blocked by l-NAME indicating eNOS as a chief source of superoxide. HAECs incubated with HG also demonstrated enhanced HG-induced superoxide generation, which was significantly blocked by l-NAME thus corroborating the in vivo results. Paradoxically, the TgeNOS-GFP overexpressing mice in the absence of diabetes also showed increased superoxide generation and exhibited poor vascular relaxation responses compared with wild-type mice (Figure 6d). These results were unexpected as it is widely accepted or anticipated that increasing eNOS expression and activity enhances NO production and vasorelaxation. Earlier studies18, 19 using transgenic mice overexpressing eNOS have demonstrated the NO-induced resistance to NO-mediated vasodilators. We believe that the long duration of eNOS overexpression could cause this resistance, which could be essentially mediated by reduced soluble guanylate cyclase and the cyclic GMP-dependent protein kinase G. Alternatively, the enhanced expression and the activity of eNOS in TgeNOS mice (demonstrated in Figures 4a and b) could cause an imbalance between eNOS, l-arginine (substrate of eNOS) and BH4 (cofactor of eNOS) levels within the vessel wall, thus resulting in uncoupling of eNOS, which produces superoxide rather than NO. This robust generation of superoxide, which scavenges on the inadequately generated NO leading to peroxynitrite (ONOO−) formation could negatively influence soluble guanylate cyclase activity thus limiting the relaxation of vascular smooth muscle cells. These possible mechanisms may be largely responsible for similar vasodilation characteristics in both TgeNOS and TgeNOS-Ins2Akita+/- mice though their eNOS expression/activity levels are different in the arteries. The presence of hyperglycemia could exacerbate the mechanisms discussed above thus demonstrating reduced vascular response to NO/cGMP pathway. These results indicate that nutrition-based diet-supplementation strategies are more beneficial than genetic modification.
However, the transgenic overexpression of eNOS has been shown to be beneficial in specific organs under certain pathological conditions. For example, studies on TgeNOS overexpressing mice have been shown to be protective against endotoxin shock and displays systemic hypotension.7, 18, 21 Overexpression of eNOS has been shown to limit neo-intimal lesion formation and medial thickening in vascular remodeling.8 It also protects against skeletal muscle ischemia/reperfusion injury and offers significant cardioprotection in particular in cardiomyocyte-specific eNOS expression.10 Expression of eNOS-GFP in apo-E knockout mice reduced plasma cholesterol and contributed to anti-atherogenic properties of elevated eNOS expression.11 Therefore, it seems necessary to evaluate the effect of eNOS overexpression independently in other organs under different physiopathological conditions to validate the model in completion.
Beneficial effects of increasing NO production by l-arginine treatment has been reported earlier in type-122 and type-213 diabetic mice, rats with chronic renal failure23, 24 and also in diabetic patients.25 In STZ-treated type-1 diabetic mice, endothelial cell activation and inflammation was abolished by l-arginine treatment. However, the available literature on the beneficial effects of l-arginine is inconclusive mainly due to inconsistent concentrations of l-arginine used and the duration of treatment.26 A combination of sepiapterin and l-arginine diet supplementation has been shown to decrease progression of glomerular injury.13 Improved vasorelaxation of aortae from apolipoprotein E and LDL-receptor knockout mice were achieved by treating the vessels (not through feeding) with a combination of l-arginine and BH4.27 However, paradoxical reports show that BH4 supplementation in the diet can worsen rather than improve endothelial dysfunction indicates limited long-term benefit in improving eNOS coupling.28, 29 On the other hand, dietary supplementation of salsalate had a favorable effect on eNOS/phospho eNOS expression and upregulation of anti-inflammatory/antioxidant activity in Zucker fatty rats.14 On the basis of these prior reports and our novel finding that IKKβ blocks eNOS QUOTE activity,6 a combination of l-arginine, sepiapterin and salsalate diet regimen was formulated and the Ins2Akita+/− diabetic mice were fed with this combination diet for 16 weeks. l-arginine supplementation with sepiapterin and salsalate significantly improved vasorelaxation and their corresponding phospho eNOS expressions in the aortae with a little influence on blood-glucose levels. These results were in support of the in vitro cell-based assays, where HAECs grown in HG exhibited enhanced phospho eNOS expression, NO production when they were treated with l-arginine in combination with salsalate. Indeed, as shown in Figure 5c, incubation of cells with salsalate independently enhanced significant NO production thus demonstrating the ability of dietary supplementation of salsalate to substitute l-arginine and BH4. However, even though supplementing salsalate is highly beneficial, gaining knowledge on its long-term effect on NO signaling is highly warranted.
The phospho eNOS expression levels were inconsistent in l-arginine plus sepiapterin treatment group in the cell-based assays thus supporting earlier reports demonstrating the limited long-term benefits of BH4.28, 30 Nevertheless, there could be an added advantage of supplementing l-arginine, sepiapterin and salsalate in addition to enhancing NO production. We observed simultaneous reduction of superoxide generation as demonstrated in Figure 5d. However, this supplementation did not have influence on generation of inflammatory mediators as shown in Figure 5e. This could be due to the specific influence of the supplements primarily on NO/ROS signaling than the secondary mediators of ROS such as TNF-α.
The outcome of TgeNOS-GFP-Ins2Akita+/− cross mice obtained by crossbreeding Ins2Akita+/− with TgeNOS-GFP mice was limited with 20% success rate. The poor vasorelaxation responses to CCh and the enhanced l-NAME-inhibitable superoxide generation in these mice could be attributed to decreased BH4 levels and BH4/BH2 ratio.31 Due to limited availability of aortic tissue and number of mice, BH4 levels were not determined. It is compelling to speculate that enhanced vasorelaxation could be achieved in TgeNOS-GFP-Ins2Akita+/− cross mice, if they were subjected to diet supplementation, which would enhance eNOS coupling.
In conclusion, further studies are required having a large cohort under long-term diet supplementation and treatment strategies that will meet with the requirement of local demand for NO within the diabetic setting. Even though overexpression of eNOS offers (1) cardioprotection in MI/R injury,9 (2) protection against endotoxin shock7 and (3) limits neo-intimal lesion formation and medial thickening in a model of vascular remodeling,8 the reduced vasodilatory responses and enhanced superoxide generation in the artery as demonstrated in our study reveals the need to investigate its effect in other organ systems especially under the influence of diabetes. Results of our unpublished studies show unexpected lack of protection with overexpression of eNOS in renal and retinal complications of diabetes. In this context, exploring the diet-based treatment modality may prove more beneficial over gene-therapy approach.
References
Toque HA, Nunes KP, Yao L et al. Akita spontaneously type 1 diabetic mice exhibit elevated vascular arginase and impaired vascular endothelial and nitrergic function. PLoS One 2013;8:e72277.
Anea CB, Cheng B, Sharma S et al. Increased superoxide and endothelial no synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ Res 2012;111:1157–1165.
Marcovecchio ML, Widmer B, Dunger DB et al. Effect of acute variations of insulin and glucose on plasma concentrations of asymmetric dimethylarginine in young people with type 1 diabetes. Clin Sci (Lond) 2008;115:361–369.
Forstermann U, Munzel T . Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 2006;113:1708–1714.
Chen W, Xiao H, Rizzo AN et al. Endothelial nitric oxide synthase dimerization is regulated by heat shock protein 90 rather than by phosphorylation. PLoS One 2014;9:e105479.
Mohan S, Konopinski R, Yan B et al. High glucose-induced IKK-Hsp-90 interaction contributes to endothelial dysfunction. Am J Physiol Cell Physiol 2009;296:C182–C192.
Yamashita T, Kawashima S, Ohashi Y et al. Resistance to endotoxin shock in transgenic mice overexpressing endothelial nitric oxide synthase. Circulation 2000;101:931–937.
Kawashima S, Yamashita T, Ozaki M et al. Endothelial no synthase overexpression inhibits lesion formation in mouse model of vascular remodeling. Arterioscler Thromb Vasc Biol 2001;21:201–207.
Jones SP, Greer JJ, Kakkar AK et al. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol 2004;286:H276–H282.
Brunner F, Maier R, Andrew P et al. Attenuation of myocardial ischemia/reperfusion injury in mice with myocyte-specific overexpression of endothelial nitric oxide synthase. Cardiovasc Res 2003;57:55–62.
van Haperen R, Cheng C, Mees BM et al. Functional expression of endothelial nitric oxide synthase fused to green fluorescent protein in transgenic mice. Am J Pathol 2003;163:1677–1686.
Suschek CV, Schnorr O, Hemmrich K et al. Critical role of L-arginine in endothelial cell survival during oxidative stress. Circulation 2003;107:2607–2614.
Cheng H, Wang H, Fan X et al. Improvement of endothelial nitric oxide synthase activity retards the progression of diabetic nephropathy in Db/Db mice. Kidney Int 2012;82:1176–1183.
Murthy SN, Desouza CV, Bost NW et al. Effects of salsalate therapy on recovery from vascular injury in female Zucker fatty rats. Diabetes 2010;59:3240–3246.
Bredt DS, Snyder SH . Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 1990;87:682–685.
Horvath B, Orsy P, Benyo Z . Endothelial NOS-mediated relaxations of isolated thoracic aorta of the C57bl/6j mouse: a methodological study. J Cardiovasc Pharmacol 2005;45:225–231.
Deckert V, Lizard G, Duverger N et al. Impairment of endothelium-dependent arterial relaxation by high-fat feeding in ApoE-deficient mice: toward normalization by human ApoA-I expression. Circulation 1999;100:1230–1235.
Ohashi Y, Kawashima S, Hirata K et al. Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. J Clin Invest 1998;102:2061–2071.
Yamashita T, Kawashima S, Ohashi Y et al. Mechanisms of reduced nitric oxide/cGMP-mediated vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. Hypertension 2000;36:97–102.
Mohan S, Reddick RL, Musi N et al. Diabetic eNOS knockout mice develop distinct macro- and microvascular complications. Lab Invest 2008;88:515–528.
van Haperen R, de Waard M, van Deel E et al. Reduction of blood pressure, plasma cholesterol, and atherosclerosis by elevated endothelial nitric oxide. J Biol Chem 2002;277:48803–48807.
West MB, Ramana KV, Kaiserova K et al. L-Arginine prevents metabolic effects of high glucose in diabetic mice. FEBS Lett 2008;582:2609–2614.
Nesher N, Frolkis I, Schwartz D et al. L-Arginine improves endothelial function, independently of arginine uptake, in aortas from chronic renal failure female rats. Am J Physiol Renal Physiol 2014;306:F449–F456.
Savard S, Lavoie P, Villeneuve C et al eNOS gene delivery prevents hypertension and reduces renal failure and injury in rats with reduced renal mass. Nephrol Dial Transplant 2012;27:2182–2190.
Lucotti P, Setola E, Monti LD et al. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab 2006;291:E906–E912.
Hoang HH, Padgham SV, Meininger CJ . L-Arginine, tetrahydrobiopterin, nitric oxide and diabetes. Curr Opin Clin Nutr Metab Care 2013;16:76–82.
Jiang J, Valen G, Tokuno S et al. Endothelial dysfunction in atherosclerotic mice: improved relaxation by combined supplementation with L-arginine-tetrahydrobiopterin and enhanced vasoconstriction by endothelin. Br J Pharmacol 2000;131:1255–1261.
Crabtree MJ, Smith CL, Lam G et al. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol 2008;294:H1530–H1540.
Takaya T, Hirata K, Yamashita T et al. A specific role for eNOS-derived reactive oxygen species in atherosclerosis progression. Arterioscler Thromb Vasc Biol 2007;27:1632–1637.
Badal SS, Danesh FR . Strategies to reverse endothelial dysfunction in diabetic nephropathy. Kidney Int 2012;82:1151–1154.
Sasaki N, Yamashita T, Takaya T et al. Augmentation of vascular remodeling by uncoupled endothelial nitric oxide synthase in a mouse model of diabetes mellitus. Arterioscler Thromb Vasc Biol 2008;28:1068–1076.
Acknowledgements
We would like to thank Ms Rosie Kryczka from IPS Therapeutic and Ms Colleen Gaudette, Research Assistant and Zheng Hongying, Research Scientist (UTHSCSA) for their excellent technical support. This study was supported by the NIH/NIDDK grant 5 RO1 DK096119 (SM), and the National Space Biomedical Research Institute (NSBRI) grant CA02802 through NASA NCC 9-58-298 (MN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
In diabetes, endothelial nitric oxide synthase (eNOS) produces superoxide rather than NO. This paper describes a treatment for enhancing NO availability in the type- 1 diabetic Ins2Akita+/− mouse model. Addition of salsalate, a negative regulator of inhibitor kappa B kinase, to a diet supplemented with L-arginine and sepiapterin, a precursor of the eNOS cofactor tetrahydrobiopterin, significantly improves vasorelaxation of thoracic/abdominal aorta in these mice.
Rights and permissions
About this article
Cite this article
Krishnan, M., Janardhanan, P., Roman, L. et al. Enhancing eNOS activity with simultaneous inhibition of IKKβ restores vascular function in Ins2Akita+/− type-1 diabetic mice. Lab Invest 95, 1092–1104 (2015). https://doi.org/10.1038/labinvest.2015.96
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2015.96