Abstract
Podocytes play an important role in the pathogenesis and progression of glomerulosclerosis. Recent studies indicate that aldosterone/mineralocorticoid receptor (MR) is a major contributor of chronic kidney disease (CKD) progression. Aldosterone/MR induces glomerular podocyte injury, causing the disruption of the glomerular filtration barrier and proteinuria. The present study investigated the mechanisms by which aldosterone/MR mediated podocyte injury, focusing on the involvement of oxidative stress, endoplasmic reticulum (ER) stress, and autophagy. We observed that aldosterone/MR induced ER stress and podocyte injury both in vivo and in vitro. Blockade of ER stress significantly reduced aldosterone/MR-induced podocyte injury. In addition, we found that ER stress-induced podocyte injury was mediated by CCAAT/enhancer-binding protein (C/EBP) homologous protein (Chop). Interestingly, autophagy was also enhanced by aldosterone/MR. Pharmacological inhibition of autophagy resulted in increased apoptosis. Inhibition of ER stress significantly reduced aldosterone/MR-induced autophagy. In addition, the activation of ER stress increased the formation of autophagy, which protected podocytes from apoptosis. Moreover, we observed that the addition of ROS scavenger, N-acetyl cystein (NAC), blocked both ER stress and autophagy by aldosterone/MR. Collectively, these results suggest that oxidant stress-mediated aldosterone/MR-induced podocyte injury via activating ER stress, which then triggers both Chop-dependent apoptosis and autophagy to cope with the injury. These findings may guide us to therapeutic strategies for glomerular diseases.
Similar content being viewed by others
Main
The podocyte is one of the components of the glomerular filtration barrier and serves to prevent filtration of protein from the blood. Podocyte injury or depletion plays a pivotal role in the onset of proteinuria and progression of glomerular diseases.1 Aldosterone is one of the most important factors contributing to podocyte injury. Aldosterone mediates its classic actions by acting through the mineralocorticoid receptor (MR), a member of the nuclear receptor family of proteins that function as ligand-dependent transcription factors.2 The role of aldosterone and MR in the pathogenesis of proteinuria and chronic kidney disease has been the subject of recent research. MR blockade prevented both the podocyte injury and the proteinuria in aldosterone-infused rats.3 It has also been reported that aldosterone/MR activation alters the adhesive capacity of podocytes.4 However, the detailed mechanisms of aldosterone/MR-induced podocyte injury remain elusive.
The endoplasmic reticulum (ER) performs several cellular functions, including the regulation of protein biosynthesis, folding, trafficking, and modification.5 The accumulation of unfolded proteins constitutes a form of cellular stress that has been termed ER stress. ER stress activates a signaling network called the unfolded protein response (UPR), which serves as an adaptive response and will also induce apoptosis in cells under severe or prolonged ER stress.6 The ER lumen is rich in molecular chaperones, such as glucose-regulated protein 78 (GRP78, also called Bip: immunoglobulin heavy chain-binding protein), GRP94, calnexin, and calreticulin. These chaperones used as markers of ER stress that prevent unfolded proteins from aggregating and provide an environment conducive to protein folding.7 In addition, apoptotic pathways are activated to eliminate the damaged cells if these adaptive responses fail to alleviate the stress. CCAAT/enhancer-binding protein (C/EBP) homologous protein (Chop) is thought to be the critical mediator of ER stress-induced apoptosis.8 ER stress has been associated with the development of several human chronic diseases. Recent evidence demonstrates that ER stress is a significant contributor to cardiovascular and renal disease.9 ER stress in podocytes has been associated with cellular injury, as demonstrated in various models of glomerulopathies, ageing, and tubular toxicity of proteinuria.10
Accumulating data indicate that ER stress may trigger autophagy.11 Autophagy is a multi-step process of self-degradation of cellular components in which proteins and organelles are sequestered and modified within cytosolic double-membrane vesicles, the autophagosomes, and subsequently transferred to the lysosome.12 Autophagy can promote either cell survival or cell death, depending on the type of cellular stress.13 Recent studies have shed light on the essential role of constitutive autophagy for the homeostasis of podocytes in health and disease. Autophagy appears to be a predominantly cytoprotective process that mediates protective effects in podocyte injury.14 Our previous study demonstrated that the early activation of autophagy conferred a protective effects in aldosterone-induced podocyte injury.15 However, the mechanism of aldosterone-induced autophagy was not investigated.
Reactive oxygen species (ROS) are common by-products of the cellular metabolism and play an important role in a variety of processes, including proliferation, senescence, ageing, as well as carcinogenesis.16 Our previous study found that ROS contributed to aldosterone-induced podocyte injury.17 Recently, oxidative stress has been shown to be an initiator and major contributor to both ER stress and autophagy,18 although the underlying mechanisms responsible for these events are still unknown.
The objectives of the present study were to investigate the effects of MR activation on aldosterone-induced podocyte injury, and the involvement of ER stress in this process both in vivo and in vitro. We also assessed whether autophagy was triggered by ER stress and conferred a protective role in aldosterone-induced podocyte damage. Finally, we detected whether ROS induced by aldosterone could cause ER stress and autophagy in cultured podocytes. These results indicate that the key components of ROS, ER stress, and autophagy signaling pathways are potential therapeutic targets for glomerular diseases.
MATERIALS AND METHODS
Reagents and Antibodies
4-Phenylbutyrate (PBA), spironolactone (Spi), aldosterone, 3-methyladenine (3-MA), N-acetyl cysteine (NAC), rapamycin (Rapa), and trehalose (Tre) were obtained from Sigma-Aldrich (St Louis, MO, USA). Anti-nephrin and anti-podocin antibodies were obtained from Abcam (Cambridge, MA, USA). Anti-Bip, anti-grp94, anti-Chop, and anti-β-actin antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-LC3 was purchased from Sigma-Aldrich.
Animals
Four-week-old male C57BL/6 mice (20–25 g body weight) had osmotic minipumps implanted subcutaneously by an incision of the right flank region under light 3% isoflurane anesthesia. Aldosterone was infused at 300 μg/kg per day for 2 weeks. Sham-operated mice served as the controls. Aldosterone-infused mice generally treated with vehicle, PBA (1 g/kg per day) dissolved in drinking water, or Spi (20 mg/kg per day) by gastric gavage for 14 days. All mice had free access to normal diet (0.3% sodium) and water. The experimental procedures and housing conditions were approved by the Nanjing Medical University Institutional Animal Care and Use Committee.
Cell Culture
Mouse podocyte cell lines were kindly provided by Dr Peter Mundel (Albert Einstein College of Medicine, Bronx, NY) and were cultured as previously described.19 Briefly, podocytes were cultured in RPMI 1640 supplemented with recombinant mouse interferon-γ at 33 °C. After differentiating at 37 °C for 10–14 days without interferon-γ, the podocytes were used for the proposed experiments.
Western Blots
Western blot analysis was performed as described previously. Briefly, 30 μg protein extracts in Laemmli buffer containing 5% 2-mercaptoethanol were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membrane. After 1 h of incubation at room temperature in 5% dry milk powder, the membrane was incubated overnight with the primary antibody against Bip (1:1000), grp94 (1:1000), Chop (1:1000), nephrin (1:200), podocin (1:200), LC3 (1:500), or β-actin (1:1000), followed by incubation for 1 h at room temperature with the appropriate secondary antibody. After immunoblotting, the film was scanned and the intensity of immunoblot bands was detected with a Bio-Rad calibrated densitometer.
Quantitative PCR
RNA was isolated from cells using TRIzol reagent (Invitrogen, Paisley, UK) according to the manufacturer’s instructions. SYBR Green was used as a fluorogenic probe system. Primers for qRT–PCR were designed to cross exon–intron boundaries to eliminate the detection of any contaminating genomic DNA using the Primer3 software (available at http://frodo.wi.mit.edu/). The comparative ΔΔCT method of relative quantification was used to calculate for differences in gene expression using the software for ABI Prism 7500 sequence detection system (Applied Biosystems; Carlsbad, CA). mRNA levels were normalized to GAPDH. The primer pairs used were nephrin: forward 5′-TTCAGACCACACCAACATCC-3′, reverse 5′-AGCCAGGTTTCCACTCCA-3′; podocin: forward 5′-GTGAGGAGGGCACGGAAG-3′, reverse 5′-AGGGAGG;CGAGGACAAGA-3′; Bip: forward 5′-ACCTATTCCTGCGTCGGTGT-3′, reverse 5′-GCATCGAAGACCGTGTTCTC-3′; grp94: forward 5′-TGGGCCTCTGCTGTGTCCTGC-3′, reverse 5′-GGCTTTTACCCAGGTCCTCTTCCACTG-3′; Chop: forward 5′-GAGTCATTGCCTTTCTCCTTCG-3′, reverse 5′-TTTGATTCTTCCTCTTCATTTCCA-3′; and GAPDH: forward 5′-GTCTTCACTACCATGGAGAAGG-3′, reverse 5′-TCATGGATGACCTTGGCCAG-3′.
Kidney Histopathological Analysis
At the end of treatment, kidney tissues were immersion-fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS), and embedded in paraffin. Kidney sections (3 μm) were stained with periodic acid–Schiff (PAS) reagent. Estimation of mesangial area was made as previously described.20 Sections were coded and read by an observer unaware of the experimental protocol applied. Thirty different superficial glomeruli were randomly sampled for morphometric analysis. The extent of increase in mesangial matrix (defined as mesangial area) was determined by the presence of PAS-positive and nuclei-free area in the mesangium. The glomerular area was defined by tracing along the borders of the capillary loop. Relative mesangial area (defined as fraction of area of mesangial matrix area over glomerular area) was obtained using ImageJ (National Institute of Health, rsb.info.nih.gov/ij).
Apoptosis
After exposure to aldosterone, podocyte apoptosis was assessed using FACSTM annexin-V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) following the manufacturer’s protocols. In certain experiments, the fraction of apoptotic cells was also determined by terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick-end labeling (TUNEL) assay as described previously.21
Electron Microscopy
Podocytes were fixed with 2.5% glutaraldehyde/1.2% acrolein in fixative buffer (0.1 mol/l cacodylate, 0.1 mol/l sucrose, pH 7.4) and 1% osmium tetroxide. Ultrathin sections were cut using a Leica Ultracut microtome and mounted on uncoated copper grids.
Transient Transfection of Podocytes with Chop siRNA
siRNA targeting Chop was purchased from Santa Cruz Biotechnology, along with a control siRNA. Transfection of siRNA was performed using a Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. Experiments were carried out 24 h after transfection.
LysoTracker Red Staining
Following various treatments, podocytes were incubated for 30 min in 50 nM LysoTracker Red (Molecular Probes, Eugene, OR, USA, 1:1000 dilution). After incubation, the cells were washed three times in PBS. Fluorescent images were obtained by using a confocal microscope (LSM 710, Carl Zeiss, Germany).
Measurement of Intracellular ROS
ROS generation was determined using 2′,7′-dichlorofluorescein diacetate (DCF). Cells were incubated with DCF (5 mM) for 30 min at 37 °C and then washed with PBS. The immunofluorescent image was visualized and captured using a confocal microscope (LSM 710, Carl Zeiss). The fluorescence intensity was measured on a flow cytometer.
Statistical Analysis
Data were expressed as the mean±s.e.m. All experiments were performed at least three times. Statistical analysis was performed by one way-ANOVA and Bonferroni tests. The value of P<0.05 was considered as threshold for significance.
RESULTS
Aldosterone Induced Podocyte Injury via ER Stress In Vivo
To determine whether aldosterone induced ER stress, we performed experiments where mice were infused with aldosterone. As shown in Figure 1a and b, aldosterone induced the expression of well-known UPR target genes, including Bip, grp94, and Chop. In addition, the ER stress inhibitor, PBA, inhibited aldosterone-induced upregulation of Bip, grp94, and Chop.
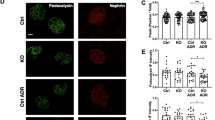
Effect of PBA on ER stress and podocyte injury in aldosterone-infused mice. (a) Bip, grp94, and Chop mRNA expression by real-time RT–PCR analysis in the kidney cortex. (b) Bip, grp94, and Chop protein expression by immunoblotting analysis in the kidney cortex. Left: representative immunoblots. Right: densitometric analysis. (c) Kidney histology (× 400). (d) Relative mesangial areas were measured in PAS-stained kidney sections as described in Materials and Methods. (e) Real-time RT–PCR analysis for nephrin and podocin in the kidney cortex. (f) Western blots for nephrin and podocin in the kidney cortex. Left: representative immunoblots. Right: densitometric analysis. Values represent the means±s.e.m. (n=6 for each group). *P<0.01 versus control; #P<0.01 versus aldosterone-infused mice. Aldo, aldosterone-infused group; Aldo+PBA, aldosterone+PBA group; ER, endoplasmic reticulum; PAS, periodic acid–Schiff; Sham, control group.
Our previous studies have demonstrated that aldosterone induced both podocyte injury and proteinuria.21 We then examined the effects of ER stress on aldosterone induced podocyte injury. Histopathologically, treatment of PBA inhibited the glomerular mesangial cell proliferation by aldosterone (Figure 1c and d). Moreover, PBA restored both the mRNA and protein levels of nephrin and podocin, which were the podocyte-specific proteins (Figure 1e and f).
Aldosterone Induced ER Stress-Dependent Podocyte Injury via MR In Vivo
To determine whether aldosterone-induced ER stress and podocyte injury was mediated by MR, we used a MR inhibitor and repeated the above-mentioned protocols in the kidney cortex. As shown in Figure 2a and b, selective MR antagonist Spi blocked the increased mRNA and protein levels of Bip, grp94, and Chop by aldosterone. Also, Spi restored the normal structure of the glomeruli (Figure 2c and d) and enhanced nephrin and podocin mRNA and protein expression (Figure 2e and f). These data indicated that the MR mediated aldosterone-induced ER stress and podocyte injury.
Effect of spironolactone on ER stress and podocyte injury in aldosterone-infused mice. (a) Bip, grp94, and Chop mRNA expression by real-time RT–PCR analysis in the kidney cortex. (b) Bip, grp94, and Chop protein expression by immunoblotting analysis in the kidney cortex. Left: representative immunoblots. Right: densitometric analysis. (c) Kidney histology (× 400). (d) Quantitative analysis for mesangial expansion. (e) Real-time RT–PCR analysis for nephrin and podocin in the kidney cortex. (f) Western blots for nephrin and podocin in the kidney cortex. Left: representative immunoblots. Right: densitometric analysis. Values represent the means±s.e.m. (n=6 for each group). *P<0.01 versus control; #P<0.01 versus aldosterone-infused mice. Aldo, aldosterone-infused group; Aldo+Spi, aldosterone+spironolactone group; ER, endoplasmic reticulum; Sham, control group.
Aldosterone Induced Podocyte Injury via ER Stress In Vitro
Consistent with in vivo findings, aldosterone induced ER stress in MPC5 mouse podocyte cells by detecting Bip, grp94, and Chop expression. Also, PBA inhibited aldosterone-induced ER stress and podocyte injury, whereas the expression of Bip, grp94, and Chop were downregulated, and the expression of nephrin and podocin were restored by the pretreatment of PBA (Figure 3a and b). PBA prevented aldosterone-induced podocyte apoptosis as assessed using annexin V/flow cytometry detection (Figure 3c). Electron microscopy analysis showed that PBA inhibited aldosterone-induced ER expansion (Figure 3d), which is a critical evidence of ER stress.22 These data indicated ER stress could induce podocyte damage in vitro.
Effect of PBA on aldosterone-induced podocyte injury and ER stress. Podocytes were pretreated with PBA (5 mmol/l) for 1 h followed by co-incubation with aldosterone (100 nmol/l) for another 12 h for real-time reverse transcriptase (RT)–PCR analysis, 24 h for immunoblotting analysis and transmission electron microscopy, or 48 h for apoptosis analysis. (a) Real-time RT–PCR analysis for nephrin, podocin, Bip, grp94, and Chop. (b) Western blots for nephrin, podocin, Bip, grp94, and Chop. β-Actin serves as a loading control. Upper: representative immunoblots. Lower: densitometric analysis. (c) Quantification of apoptotic cells by flow cytometry. (d) ER ultrastructure morphology (× 50 000). Arrows indicate representative examples for expanded ER. Values are means±s.e.m. from three independent experiments. *P<0.01 versus control; #P<0.01 versus aldosterone treatment group. Aldo, aldosterone treatment group; Aldo+PBA, aldosterone+PBA group; Cntl, control group; ER, endoplasmic reticulum; PBA, 4-Phenylbutyrate.
Aldosterone Induced ER Stress-Dependent Podocyte Injury via MR In Vitro
Consistently, as shown in Figure 4a and b, MR inhibitor also attenuated the induction of UPR and the reduction of podocyte markers by aldosterone in MPC5 mouse podocyte cells. Flow cytometric assays for apoptosis showed Spi blocked aldosterone-induced podocyte apoptosis (Figure 4c). The inhibition of ER stress by MR inhibitor is also reconfirmed by transmission electron microscopy by observing the volume of ER (Figure 4d). Thus MR might mediate aldosterone-induced ER stress and podocyte injury in vitro.
Effect of spironolactone on aldosterone-induced podocyte injury and ER stress. Podocytes were pretreated with spironolactone (1 μmol/l) for 1 h followed by co-incubation with aldosterone (100 nmol/l) for another 12 h for real-time reverse transcriptase (RT)–PCR analysis, 24 h for immunoblotting analysis and transmission electron microscopy, or 48 h for apoptosis analysis. (a) Real-time RT–PCR analysis for nephrin, podocin, Bip, grp94, and Chop. (b) Western blots for nephrin, podocin, Bip, grp94, and Chop. β-Actin serves as a loading control. Upper: representative immunoblots. Lower: densitometric analysis. (c) Quantification of apoptotic cells by flow cytometry. (d) ER ultrastructure morphology (× 50 000). Arrows indicate representative examples for expanded ER. Values are means±s.e.m. from three independent experiments. *P<0.01 versus control; #P<0.01 versus aldosterone treatment group. Aldo, aldosterone treatment group; Aldo+Spi, aldosterone+spironolactone group; Cntl, control group; ER, endoplasmic reticulum.
Chop Mediated ER Stress-Induced Podocyte Injury
Several studies implicate that Chop protein plays an important role in ER stress-induced programmed cell death.23 To investigate a role for Chop in aldosterone-induced apoptotic signaling, we transfected podocytes with siRNAs targeted to Chop or non-specific control siRNA. As shown in Figure 5a and b, real-time PCR and western blot analyses revealed a significant reduction in Chop protein expression in MPC5 mouse podocytes transfected with Chop siRNA as compared to the control. Importantly, concomitant with Chop reduction, the aldosterone-induced reductions of nephrin and podocin expression were partially blocked in Chop siRNA-transfected cells (Figure 5c and d). In addition, downregulation of Chop by siRNA attenuated aldosterone-induced podocyte apoptosis, which were determined by flow cytometry (Figure 5e) and the TUNEL assay (Figure 5f).
Effect of Chop silencing on aldosterone-induced podocyte injury and ER stress. (a and b) The inhibition efficiency of siRNAs against Chop. Podocytes were transfected with siRNAs targeting Chop for 24 h, and the mRNA and protein levels of Chop were determined by real-time reverse transcriptase (RT)–PCR (a) and western blot (b). (c–f) The effect of aldosterone (100 nmol/l) on podocyte injury in podocytes transfected with siChop. Podocytes were transfected with Chop siRNA for 24 h and then treated with aldosterone (100 nmol/l) for another 12 h for RT–PCR analysis, 24 h for immunoblotting analysis, or 48 h for apoptosis analysis. (c) RT–PCR analysis. (d) Western blot analysis. Left: representative immunoblots. Right: densitometric analysis. (e) Quantification of apoptosis by flow cytometry. (f) Representative photographs of double-fluorescence labeling of Hoechst nuclear staining (blue) and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL; green). Data are expressed as the means±s.e.m. (n=6). *P<0.01 versus control; #P<0.01 versus aldosterone treatment group. Cntl, control group; ER, endoplasmic reticulum; Vehi, scrambled siRNA group.
Autophagy Triggered by ER Stress Protects Against Aldosterone/MR-Induced Podocyte Injury
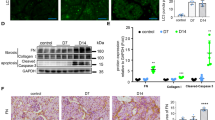
To determine whether autophagy could be induced after aldosterone treatment, first we morphologically confirmed the induction of autophagy using LysoTracker Red staining, which is an autophagy marker. As expected, the number of LysoTracker Red-positive puncta was markedly increased by aldosterone, and was suppressed by Spi or PBA, suggesting that the increase of autophagy by aldosterone was induced by MR and ER stress. Pretreatment with autophagy inhibitor 3-MA also reduced aldosterone-induced autophagy (Figure 6a and b). Next, we investigated the ratio of LC3II/LC3I to actin, which is considered an accurate indicator of autophagy.24 As shown in Figure 6c, aldosterone promoted the conversion of LC3-I to LC3-II, which was also blocked by Spi, PBA, and 3-MA. Moreover, treatment with 3-MA enhanced aldosterone-induced podocyte damage accessed by nephrin downregulation (Figure 6c and d) and apoptosis (Figure 6e). These studies suggested that the induction of autophagy provided a prosurvival role during aldosterone-induced injury to cultured podocytes.
Effect of autophagy inhibitor on aldosterone-induced podocyte injury. Confluent podocytes were pretreated with spironolactone (1 μmol/l), PBA (5 mmol/l) or 3-MA (2 mmol/l) for 1 h, and then co-stimulated by 100 nmol/l aldosterone. (a) Representative images of podocytes stained with LysoTracker Red to detect autophagic activity (× 400). (b) Quantification of LysoTracker Red fluorescence by flow cytometry. (c) Western blot analysis for nephrin and LC3 conversion. Left: representative immunoblots. Right: densitometric analysis. (d) Nephrin messenger RNA levels assessed by real-time RT–PCR. (e) Quantification of apoptosis. Values represent means±s.d., n=6. Three separate experiments were performed with comparable results. *P<0.01 versus control; #P<0.01 versus aldosterone treatment group; ΔP<0.01 versus aldosterone plus Spi or aldosterone plus PBA treatment group. Aldo, aldosterone treatment group; Aldo+3-MA, aldosterone+3-MA group; Aldo+PBA, aldosterone+PBA group; Aldo+Spi, aldosterone+spironolactone group; Cntl, control group; PBA, 4-Phenylbutyrate.
Autophagy Inducers Alleviated Aldosterone-Induced Podocyte Injury
Previous experiments have shown that autophagy helps podocytes cope with adverse environmental stress and allows them to recover from injury. Moreover, autophagy inducers can reduce podocyte injury by increasing autophagy levels.25, 26 The mammalian target of Rapa (mTOR) is a negative regulator of autophagy. Autophagy can be induced in an mTOR-dependent or -independent manner by diverse input signals.27 To confirm the protective effects of autophagy inducers, we pretreated podocytes with the classic mTOR pathway inhibitor Rapa and an mTOR-independent autophagy inducer Tre. As shown in Figure 7a and b, pretreatment with Rapa or Tre increased the level of autophagy, compared to the group treated with aldosterone alone. In addition, both real-time PCR (Figure 7c) and western blot (Figure 7d) showed that nephrin expression was significantly higher in the Rapa and Tre-pretreated aldosterone-stimulated cells than in the untreated aldosterone-stimulated group. Also, both Rapa and Tre decreased aldosterone-induced podocyte apoptosis (Figure 7e). These results suggest that the upregulating autophagy levels by the mTOR-dependent or mTOR-independent pathways alleviated aldosterone-induced podocyte injury.
Effect of autophagy inducer on aldosterone-induced podocyte injury. Confluent podocytes were pretreated with rapamycin (50 nmol/l) or trehalose (50 mmol/l) for 1 h, and then co-stimulated by 100 nmol/l aldosterone. (a) Representative images of podocytes stained with LysoTracker Red to detect autophagic activity (× 400). (b) Quantification of LysoTracker Red fluorescence by flow cytometry. (c) Nephrin mRNA levels assessed by real-time RT–PCR. (d) Western blot analysis for nephrin. Upper: representative immunoblots. Lower: densitometric analysis. (e) Quantification of apoptosis. Values represent means±s.d., n=6. Three separate experiments were performed with comparable results. *P<0.01 versus control; #P<0.01 versus aldosterone treatment group. Aldo, aldosterone treatment group; Aldo+Rapa, aldosterone+rapamycin group; Aldo+Tre, aldosterone+trehalose group; Cntl, control group.
Combination of Chop Silencing and Autophagy Activation Markedly Improved Aldosterone-Induced Podocyte Injury
Given that both Chop siRNA and autophagy inducer showed cytoprotective effects in aldosterone-treated podocytes. Then, we evaluated the effect of the combination of Chop silencing and autophagy activation on podocyte injury. As expected, compared to Chop siRNA monotherapy, combination treatment significantly suppressed both the decrease in nephrin expression (Figure 8a and b) and the elevation of apoptosis (Figure 8c) by aldosterone. Therefore, the additional treatment with Chop siRNA with Rapa elicits better renoprotective effects than monotherapy with either drug.
Effect of cotreatment with Chop silencing and rapamycin on aldosterone-induced podocyte injury. Podocytes were transfected with siRNAs targeting Chop for 24 h, followed by rapamycin (50 nmol/l) for 1 h and aldosterone (100 nmol/l) for another 12 h for real-time RT–PCR analysis, 24 h for immunoblotting analysis, or 48 h for apoptosis analysis. (a) Nephrin mRNA levels assessed by real-time RT–PCR. (b) Western blotting analysis for nephrin. Upper: representative immunoblots. Lower: densitometric analysis. (c) Quantification of apoptosis. Values represent means±s.d., n=6. Three separate experiments were performed with comparable results. *P<0.01 versus control; #P<0.01 versus aldosterone treatment group; ΔP<0.01 versus aldosterone plus Chop siRNA group. Aldo, aldosterone; Rapa, rapamycin.
Aldosterone-Induced ROS Caused ER Stress and Autophagy
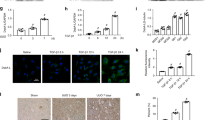
Consistent with previous results,19 exposure of cultured podocytes to aldosterone resulted in ROS generation. Prevention of oxidative stress with the antioxidant NAC attenuated podocyte injury by restoring nephrin expression and preventing apoptosis (Figure 9). We further examined whether ROS was able to induce ER stress and autophagy. As shown in Figure 10a and b, NAC blocked aldosterone-induced Bip, grp94 and Chop mRNA, and protein expression. In addition, treatment of NAC inhibited aldosterone-induced autophagy by using LysoTracker Red staining (Figure 10c and d) and western blot for LC3 conversion (Figure 10e). These results indicated that the elevated levels of intracellular ROS positively regulated aldosterone-induced ER stress and autophagy.
Effect of ROS on aldosterone-induced podocyte injury. (a) Representative images of podocytes stained with dichlorodihydrofluorescein diacetate (DCF). Confluent podocytes were pretreated with NAC (5 mmol/l) for 30 min, and then co-stimulated with 100 nM aldosterone for another 2 h in the presence of DCF. (b) Quantification of DCF fluorescence by flow cytometry. (c–e) Confluent podocytes were pretreated with NAC and then incubated with aldosterone for the indicated periods of time. (c) Nephrin mRNA expression. (d) Nephrin protein expression. Left: representative immunoblots. Right: densitometric analysis. (e) Quantification of apoptosis. Values represent means±s.d., n=6. Three separate experiments were performed with comparable results. *P<0.01 versus control; #P<0.01 versus aldosterone treatment group. Aldo, aldosterone treatment group; Aldo+NAC, aldosterone+NAC group; Cntl, control group; NAC, N-acetyl cysteine; ROS, reactive oxygen species.
Effect of ROS on aldosterone-induced ER stress and autophagy. Confluent podocytes were pretreated with NAC (5 mmol/l) for 30 min, and then co-stimulated by 100 nmol/l aldosterone. (a) Real-time RT–PCR analysis for Bip, grp94, and Chop. (b) Western blots for Bip, grp94, and Chop. Left: representative immunoblots. Right: densitometric analysis. (c) Representative images of podocytes stained with LysoTracker Red (× 400). (d) Quantification of LysoTracker Red fluorescence by flow cytometry. (e) Western blot analysis for LC3 conversion. Left: representative immunoblots. Right: densitometric analysis. Values represent means±s.d., n=6. Three separate experiments were performed with comparable results. *P<0.01 versus control; #P<0.01 versus aldosterone treatment group. Aldo, aldosterone treatment group; Aldo+NAC, aldosterone+NAC group; Cntl, control group; ER, endoplasmic reticulum; NAC, N-acetyl cysteine; ROS, reactive oxygen species.
DISCUSSION
In this study, we analyzed MR, ROS, ER stress, and autophagy in aldosterone-induced podocyte injury. We demonstrate that MR antagonist and ER stress inhibitor ameliorated the damaging effects of aldosterone both in vivo and in vitro. In addition, our data show that Chop blockage using specific siRNA abolished apoptosis induced by aldosterone in podocytes. The activation of ER stress also increased the formation of autophagy, which protects podocytes from apoptosis. Finally, our study provides a mechanistic link between ROS, ER stress, and autophagy activation in podocyte injury induced by aldosterone/MR signaling (Figure 11).
A schematic model of the proposed links between aldosterone/MR-induced ROS, ER stress, and autophagy in podocytes. The ROS generation induced by aldosterone/MR activates ER stress in podocytes, contributing to podocyte injury via a Chop-dependent manner. Activation of UPR also triggers autophagy to cope with the injury. MR, ROS, ER stress, Chop, and autophagy are potential therapeutic targets for glomerular diseases; ER, endoplasmic reticulum; MR, mineralocorticoid receptor; ROS, reactive oxygen species; UPR, unfolded protein response.
Aldosterone, a mineralocorticoid hormone primarily synthesized in the adrenal gland, is a major regulator of extracellular fluid volume and sodium and potassium balance.28 Studies over the past two decades have shown that aldosterone plays an independent role as a mediator of kidney injury and progression of chronic kidney disease.29 Aldosterone has been shown to exert its effects through genomic or non-genomic pathway.30 Genomic effects of aldosterone are generally thought to be mediated by the MR and involve transcription, while aldosterone can also exert rapid nongenomic effects that are not blocked by inhibitors of transcription.31 Numerous cell culture, animal, and human studies have shown aldosterone and the MR are implicated in activation of a variety of pathologic processes including inflammation, remodelling, and fibrosis in several target organs.32 MR antagonists conferred protection against podocyte injury and proteinuria in a variety of animal models.33 Our study found that aldosterone induced ER stress, autophagy, and podocyte injury were inhibited by an MR antagonist, which supports an MR-dependent mechanism.
Several studies have demonstrated increased expression of ER stress proteins in human kidney biopsies consistent with findings in experimental animal models.34 Our study also revealed that Bip and grp94, components of the ER chaperone system,35 were exclusively upregulated during aldosterone treatment. Increasing evidence supports that ER stress-induced apoptosis is an important pathogenic factor in a vast number of diseases.36 Chop is the major proapoptotic transcription factor upregulated by ER stress.37 Chop is ubiquitously expressed at very low levels, but is robustly expressed by perturbations that induce stress in a wide variety of cells. The present study found that aldosterone exposure obviously upregulated Chop expression, and knockdown of Chop by siRNA decreased the induction of apoptosis. How Chop contributes to aldosterone-evoked and ER stress-mediated podocyte apoptosis remains obscure. Several earlier reports showed that Chop-induced apoptosis involves interaction with members of the BCL-2 family and induction of the calcium signaling pathway.38 However, the exact mechanism of this effect is still under investigation. In addition, mouse embryonic fibroblasts derived from Chop-knockout mice exhibit only partial resistance to ER stress-driven apoptosis, indicating that Chop is not the only death pathway.39 Thus, further studies are necessary to establish such notions in podocytes. Previously, several studies have shown PBA, a well-known chemical chaperone, inhibited ER stress-mediated apoptosis.40, 41 In accordance with these reports, we showed that PBA alleviates ER stress response induced by aldosterone in podocytes. Treatment with PBA downregulated UPR-related proteins including Bip, grp94, and Chop.
Consistent with a recent study,42 our data showed that autophagy was activated in podocytes by aldosterone. Previous studies have highlighted the essential role of autophagy for the cellular homeostasis of podocytes in health and disease. We found that the inhibition of basal levels of autophagy in cultured podocyte by 3-MA or chloroquine induced podocyte injury (Supplementary Data). Autophagy appears to be an important cytoprotective process that mediates protective effects in podocyte injury and glomerular diseases.43, 44 The results of the present study showed inhibition of autophagy by 3-MA exacerbated aldosterone-induced podocyte injury. In addition, both the mTOR-dependent and mTOR-independent autophagy inducers alleviated aldosterone induced podocyte injury via upregulating autophagy levels. In other cases, autophagy is also known to be involved in cell death under the influence of developmental or stress signals.45 Moreover, our data are consistent with the findings of other studies that autophagy could be triggered by ER stress.46 Therefore, ER stress may cause autophagy to either protect or kill cells in different environments. However, the precise role of autophagy in ER stress is far from clear. It is possible that when the proteasome-mediated degradation system is overloaded by the accumulation of abnormal proteins in the ER, autophagy would be activated to assist in removing them, thus serving as an ER protein quality system.47
ROS overproduction has been well correlated with many podocyte injury models in vitro and in experimental diseases including diabetic nephropathy, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis.48 Consistent with these previous reports, we confirmed that inhibition of ROS with NAC, a widely used antioxidant, significantly attenuated the cytotoxicity of aldosterone. Oxidative stress is a known inducer of ER stress49 and autophagy.50 As expected, the current study revealed the enhanced effect of aldosterone-induced podocyte ER stress and autophagy was inhibited by antioxidants. We therefore conclude that aldosterone promotes podocyte ER stress and autophagy through the generation of ROS. Conversely, it has been proven that prolonged ER stress can induce the generation of ROS.51 Furthermore, autophagy inhibition led to enhanced ROS generation in several cell lines.52, 53 Therefore, the crosstalk among ROS, ER stress, and autophagy is quite complex. Elucidating the correlation represents a major area for our future research.
In conclusion, our data support the idea that oxidant stress perturbs ER homeostasis and activates ER stress in podocytes contributing to aldosterone/MR-induced podocyte injury. Activation of UPR triggers both the Chop-dependent apoptosis and autophagy to cope with the injury. Although the detailed mechanisms through which ROS induced by aldosterone mediates ER stress and autophagy remain to be elucidated, these findings provide important insight into the response of podocytes to aldosterone.
References
Mundel P, Reiser J . Proteinuria: an enzymatic disease of the podocyte? Kidney Int 2010;77:571–580.
Stow LR, Gumz ML, Lynch IJ et al. Aldosterone modulates steroid receptor binding to the endothelin-1 gene (edn1). J Biol Chem 2009;284:30087–30096.
Shibata S, Nagase M, Yoshida S et al. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension 2007;49:355–364.
Lin S, Li D, Jia J et al. Spironolactone ameliorates podocytic adhesive capacity via restoring integrin alpha 3 expression in streptozotocin-induced diabetic rats. J Renin Angiotensin Aldosterone Syst 2010;11:149–157.
Inagi R . Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron Exp Nephrol 2009;112:e1–e9.
Oslowski CM, Urano F . Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol 2011;490:71–92.
Boot-Handford RP, Briggs MD . The unfolded protein response and its relevance to connective tissue diseases. Cell Tissue Res 2010;339:197–211.
Ghosh AP, Klocke BJ, Ballestas ME et al. CHOP potentially co-operates with FOXO3a in neuronal cells to regulate PUMA and BIM expression in response to ER stress. PLoS One 2012;7:e39586.
Santos CX, Nabeebaccus AA, Shah AM et al. Endoplasmic reticulum stress and nox-mediated reactive oxygen species signaling in the peripheral vasculature: potential role in hypertension. Antioxid Redox Signal 2014;20:121–134.
Pallet N . New insights on stress-induced epithelial phenotypic changes. Nephrol Dial Transplant 2012;27:483–485.
Cybulsky AV . The intersecting roles of endoplasmic reticulum stress, ubiquitin-proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int 2013;84:25–33.
Huber TB, Walz G, Kuehn EW . mTOR and rapamycin in the kidney: signaling and therapeutic implications beyond immunosuppression. Kidney Int 2011;79:502–511.
Mizushima N, Levine B, Cuervo AM et al. Autophagy fights disease through cellular self-digestion. Nature 2008;451:1069–1075.
Huber TB, Edelstein CL, Hartleben B et al. Emerging role of autophagy in kidney function, diseases and aging. Autophagy 2012;8:1009–1031.
Wang W, Ding G, Yuan Y et al. Early autophagy activation inhibits podocytes from apoptosis induced by aldosterone. Chin J Nephrol; 28:835–839.
Benz CC, Yau C . Ageing, oxidative stress and cancer: paradigms in parallax. Nat Rev Cancer 2008;8:875–879.
Zhu C, Huang S, Yuan Y et al. Mitochondrial dysfunction mediates aldosterone-induced podocyte damage: a therapeutic target of PPARgamma. Am J Pathol 2011;178:2020–2031.
Yuzefovych LV, Ledoux SP, Wilson GL et al. Mitochondrial DNA damage via augmented oxidative stress regulates endoplasmic reticulum stress and autophagy: crosstalk, links and signaling. PLoS One 2013;8:e83349.
Su M, Dhoopun AR, Yuan Y et al. Mitochondrial dysfunction is an early event in aldosterone-induced podocyte injury. Am J Physiol Renal Physiol 2013;305:F520–F531.
Mallipattu SK, Liu R, Zhong Y et al. Expression of HIV transgene aggravates kidney injury in diabetic mice. Kidney Int 2013;83:626–634.
Yuan Y, Huang S, Wang W et al. Activation of peroxisome proliferator-activated receptor-gamma coactivator 1alpha ameliorates mitochondrial dysfunction and protects podocytes from aldosterone-induced injury. Kidney Int 2012;82:771–789.
Carpenter JE, Jackson W, Benetti L et al. Autophagosome formation during varicella-zoster virus infection following endoplasmic reticulum stress and the unfolded protein response. J Virol 2011;85:9414–9424.
Lee J, Ozcan U . Unfolded protein response signaling and metabolic diseases. J Biol Chem 2014;289:1203–1211.
Kabeya Y, Mizushima N, Ueno T et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000;19:5720–5728.
Wu L, Feng Z, Cui S et al. Rapamycin upregulates autophagy by inhibiting the mTOR-ULK1 pathway, resulting in reduced podocyte injury. PLoS One 2013;8:e63799.
Kang YL, Saleem MA, Chan KW et al. Trehalose, an mTOR independent autophagy inducer, alleviates human podocyte injury after puromycin aminonucleoside treatment. PLoS One 2014;9:e113520.
Sarkar S, Ravikumar B, Floto RA et al. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ 2009;16:46–56.
Zhang Q, Lin L, Lu Y et al. Interaction between nitric oxide and superoxide in the macula densa in aldosterone-induced alterations of tubuloglomerular feedback. Am J Physiol Renal Physiol 2013;304:F326–F332.
Shavit L, Lifschitz MD, Epstein M . Aldosterone blockade and the mineralocorticoid receptor in the management of chronic kidney disease: current concepts and emerging treatment paradigms. Kidney Int 2012;81:955–968.
Oshima N, Onimaru H, Takechi H et al. Aldosterone is synthesized in and activates bulbospinal neurons through mineralocorticoid receptors and ENaCs in the RVLM. Hypertens Res 2013;36:504–512.
Brown NJ . Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol 2013;9:459–469.
Guichard JL, Clark D 3rd, Calhoun DA et al. Aldosterone receptor antagonists: current perspectives and therapies. Vasc Health Risk Manag 2013;9:321–331.
Nagase M, Fujita T . Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol 2013;9:86–98.
Cybulsky AV . Endoplasmic reticulum stress in proteinuric kidney disease. Kidney Int 2010;77:187–193.
Ma Y, Hendershot LM . ER chaperone functions during normal and stress conditions. J Chem Neuroanat 2004;28:51–65.
Sanchez-Lopez E, Zimmerman T, Gomez del Pulgar T et al. Choline kinase inhibition induces exacerbated endoplasmic reticulum stress and triggers apoptosis via CHOP in cancer cells. Cell Death Dis 2013;4:e933.
Zinszner H, Kuroda M, Wang X et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 1998;12:982–995.
Scull CM, Tabas I . Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol 2011;31:2792–2797.
Vandewynckel YP, Laukens D, Geerts A et al. The paradox of the unfolded protein response in cancer. Anticancer Res 2013;33:4683–4694.
Wiley JC, Meabon JS, Frankowski H et al. Phenylbutyric acid rescues endoplasmic reticulum stress-induced suppression of APP proteolysis and prevents apoptosis in neuronal cells. PLoS One 2010;5:e9135.
Hart LS, Cunningham JT, Datta T et al. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Invest 2012;122:4621–4634.
Mao N, Cheng Y, Shi XL et al. Ginsenoside Rg1 protects mouse podocytes from aldosterone-induced injury in vitro. Acta Pharmacol Sin 2014;35:513–522.
Hartleben B, Wanner N, Huber TB . Autophagy in glomerular health and disease. Semin Nephrol 2014;34:42–52.
Takabatake Y, Kimura T, Takahashi A et al. Autophagy and the kidney: health and disease. Nephrol Dial Transplant 2014;29:1639–1647.
Kim KW, Moretti L, Mitchell LR et al. Endoplasmic reticulum stress mediates radiation-induced autophagy by perk-eIF2alpha in caspase-3/7-deficient cells. Oncogene 2010;29:3241–3251.
Lin CJ, Lee CC, Shih YL et al. Inhibition of mitochondria- and endoplasmic reticulum stress-mediated autophagy augments temozolomide-induced apoptosis in glioma cells. PLoS One 2012;7:e38706.
Verfaillie T, Garg AD, Agostinis P . Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett 2013;332:249–264.
Chen S, Meng XF, Zhang C . Role of NADPH oxidase-mediated reactive oxygen species in podocyte injury. Biomed Res Int 2013;2013:839761.
Gorlach A, Klappa P, Kietzmann T . The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal 2006;8:1391–1418.
Azad MB, Chen Y, Gibson SB . Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 2009;11:777–790.
Malhotra JD, Kaufman RJ . The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 2007;18:716–731.
Guo XL, Li D, Sun K et al. Inhibition of autophagy enhances anticancer effects of bevacizumab in hepatocarcinoma. J Mol Med (Berl) 2013;91:473–483.
Kaminskyy VO, Piskunova T, Zborovskaya IB et al. Suppression of basal autophagy reduces lung cancer cell proliferation and enhances caspase-dependent and -independent apoptosis by stimulating ROS formation. Autophagy 2012;8:1032–1044.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (no. 81300573, 81100512, and 81170660), the Natural Science Foundation of Jiangsu Province (no. BK20131030), the Clinic Research Center of Jiangsu Province (No. BL2014080), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institution.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website
Supplementary information
Rights and permissions
About this article
Cite this article
Yuan, Y., Xu, X., Zhao, C. et al. The roles of oxidative stress, endoplasmic reticulum stress, and autophagy in aldosterone/mineralocorticoid receptor-induced podocyte injury. Lab Invest 95, 1374–1386 (2015). https://doi.org/10.1038/labinvest.2015.118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2015.118
This article is cited by
-
Recent progress in unraveling cardiovascular complications associated with primary aldosteronism: a succinct review
Hypertension Research (2024)
-
Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases
Nature Reviews Nephrology (2017)
-
Natriuretic peptide receptor guanylyl cyclase-A pathway counteracts glomerular injury evoked by aldosterone through p38 mitogen-activated protein kinase inhibition
Scientific Reports (2017)
-
Chrysin ameliorates podocyte injury and slit diaphragm protein loss via inhibition of the PERK-eIF2α-ATF-CHOP pathway in diabetic mice
Acta Pharmacologica Sinica (2017)