Abstract
Recent studies sight β-adrenergic receptor (AR) antagonists as novel therapeutic agents for melanoma, as they may reduce disease progression. Here within, we evaluated the expression of β-ARs in a series of human cutaneous melanocytic lesions, and studied the effect of their endogenous agonists, norepinephrine (NE) and epinephrine (E), on primary and metastatic human melanoma cell lines. Using immunohistochemistry, we found that both β1- and β2-ARs are expressed in tissues from benign melanocytic naevi, atypical naevi and malignant melanomas and that expression was significantly higher in malignant tumours. Melanoma cell lines (human A375 primary melanoma cell line and human Hs29-4T metastatic melanoma cell lines) also expressed β1- and β2-ARs by measuring transcripts and proteins. NE or E increased metalloprotease-dependent motility, released interleukin-6 and 8 (IL-6, IL-8) and vascular endothelial growth factor (VEGF). These effects of catecholamines were inhibited by the unselective β-AR antagonist propranolol. The role of soluble factors elicited by catecholamines seemed pleiotropic as VEGF synergized with NE increased melanoma invasiveness through 3D barriers, while IL-6 participated in stromal fibroblast activation towards a myofibroblastic phenotype. Our results indicate that NE and E produce in vitro via β-ARs activation a number of biological responses that may exert a pro-tumorigenic effect in melanoma cell lines. The observation that β-ARs are upregulated in malignant melanoma tissues support the hypothesis that circulating catecholamines NE and E, by activating their receptors, favour melanoma progression in vivo.
Similar content being viewed by others
Main
Melanoma represents the most aggressive type of skin cancer, with an increasing incidence found especially in young adults. A significant reduction in mortality has been not observed, despite a noteworthy improvement in early diagnosis achieved in recent years.1 At present, no medical option can cure metastatic melanoma (MM) and the only effective treatment for the eradication of the disease is early-phase surgery.2 Hence, increased knowledge of the biological pathways underlying the process of melanoma dissemination and metastasis is crucial in order to identify new therapeutic targets.
Previous studies have shown that various human solid tumours, such as breast, colon, prostatic, ovary, nasopharyngeal and oral cancer, express β2-adrenoceptor (β2-AR), raising the possibility that such receptors may affect invasion and dissemination processes.3, 4, 5, 6, 7, 8 Moreover, some stress neurotransmitters, such as norepinephrine (NE) and epinephrine (E), have been demonstrated to contribute to the regulation of tumour cell invasion, at least in part through β-AR activation.6, 7, 9 Interactions between tumour cells and soluble factors originated from the nervous system has recently been proposed to favour metastasis formation.10 Improved survival rates have been demonstrated in mice with metastatic tumour by combined administration of β-AR antagonists.11 In addition, recent evidence suggests a dramatic role of β-AR blockers in reducing metastases, tumour recurrence and specific mortality in breast cancer patients.12 More recently, the use of β-blockers for concomitant disease was associated with a reduced risk of progression of thick melanoma13 and with an increased survival time of melanoma patients,14 suggesting that the interaction of catecholamines with β-ARs could be a useful target in this disease.
Expression of β-ARs has been found in melanoma cell lines and in human melanoma biopsies and NE was demonstrated to enhance cytokine production from melanoma cells.15 However, no detailed information regarding β-ARs expression in human cutaneous benign and malignant melanocytic lesions or catecholamine influence on melanoma cell migration has been provided so far. Our aim was to evaluate the expression of β-ARs on a series of human cutaneous melanocytic naevi and malignant melanoma, and assess the potential influence of NE and E on the malignant behaviour of human melanoma cell lines. We could demonstrate a significant upregulation of β-ARs expression in melanoma in vivo and the activation of pro-tumorigenic biological responses induced by NE and E in vitro.
MATERIALS AND METHODS
Histologic Samples
Forty human cutaneous melanocytic lesions from 40 different patients were evaluated. Tissue samples were retrieved from the archives of the Division of Pathological Anatomy, Department of Critical Care Medicine and Surgery, University of Florence, Florence, and from the Division of Pathology, Pistoia Hospital, Pistoia, Italy.
The study series included five common melanocytic naevi (CN) (two females, three males, age 28–54 years, mean 35.8 years); five atypical (so-called dysplastic) melanocytic naevi (AN) (two females, three males, age 30–47 years, mean 40.4 years); five in situ primary melanoma (PM) (two females, three males, age 37–55 years, mean 44.2 years; site: three trunk, one lower extremity); nine superficial spreading (SS) PM (seven females, two males, age 41–82 years, mean 58.4 years; site: four trunk, three leg, two arm; thickness 0.30–1.90 mm, mean 0.82 mm; five level II, three level III); six nodular (N) PM (three females, three males, age 53–76 years, mean 61.5 years; site: three trunk, one leg, two arm; thickness 1.40–17 mm, mean 5.2 mm; two level III, three level IV, one level V), ten MM, five cutaneous and five lymph-nodal (one female, nine males, age 59–87 years, mean 77.1 years).
Materials
Rabbit polyclonal anti-β1- or anti-β2-AR were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) or Chemicon (Temecula, CA, USA), strepatdvidin-biotin peroxidase complex from Ultravision (LabVision, Fremont, CA, USA), DAKO EnVision System HRP from Dako (Carpenteria, CA, USA). For in vitro experiments, unless specified all reagents were obtained from Sigma (St Louis, MO) except PVDF membrane (Millipore, Bedford, MA); Matrigel (BD Biosciences, Bedford, MA); Diff-Quik staining kit (Medion Diagnostics, Miami, FL); Transwell (Corning Incorporated, Corning, NY); ilomastat (Chemicon International, Bedford, MA); p38 MAPK (mitogen-activated protein kinase), phospho-p38 MAPK (Thr180/Tyr182), p44/p42 MAPK and phospho-p44/p42 (T202/Y204) monoclonal antibodies (Cell Signalling, Danvers, MA). The Amplite TM Universal Fluorimetric matrix metalloproteinase (MMP) Activity Assay Kit-Red Fluorescence was supplied by AAT Bioquest, Sunnyvale, CA.
Immunohistochemistry
The specimens were obtained by surgical resection in all cases and fixed in 10% formalin before being processed in paraffin. Haematoxylin-eosin stained sections from each histological specimen were reviewed to confirm the histological diagnosis. The protocol was approved by the Institutional Review Board for use of human tissues.
For immunohistochemical analyses, a representative section of 3 μm for each lesion was selected. All sections were deparaffined in Bio-Clear (Bio-Optica, Mi, Italy) and hydrated with grade ethanol concentrations until distilled water. Antigen retrieval was performed by calibrated water bath capable of maintaining the Epitope Retrieval Solution EDTA (pH 9.0) at 97 °C for 15 min. The sections were then allowed to cool down to room temperature (RT) for 20 min. To block endogenous peroxidase activity, slides were treated with 3.0% hydrogen peroxidase in distilled water for 10 min and subsequently washed two or three times with PBS. Then polyclonal antibodies anti-β1- or anti-β2-AR were incubated for 1 h at RT at 1:100 dilution or at 1:30 dilution, respectively. Immunohistochemical analysis was performed using the streptavidin-biotin peroxidase complex for β2-AR, or DAKO EnVision System HRP for β1-AR. Finally, aminoethilcarbazole (LabVision) was applied for 5 min as chromogen.
Normal eccrine sweat glands intensely express β2-AR, and this parameter was used as a positive internal control.16 Negative control was performed by substituting the primary antibody with a non-immune serum at the same concentration. The control sections were treated in parallel with the samples. The sections were lightly counterstained with Mayer’s haematoxylin.
Immunostaining was independently assessed by two observers (DM, SM). Discrepancies in the reading were resolved by a second parallel reading of the slides. The percentage of positive cells per lesion was scored according to semi-quantitative criteria. As the percentage of positive naevus melanocytes/melanoma cells was always higher than 50%, semi-quantitative results were expressed as score 1 (50–80% positive naevus melanocytes/melanoma cells), score 2 (81–90% naevus melanocytes/melanoma cell staining) and score 3 (91–100% melanoma cell staining). The cell staining intensity was scored on a scale as weak, moderate, strong, very strong.
For statistical analysis, non-parametric tests were used to determine significant differences between groups. The distribution of the scored values of positive lesions after immunological staining in each group was the unit of analysis. Groups were: naevi, PM, MM. Statistical evaluation was performed also comparing CN vs AN, naevi vs malignant lesions, and in situ PM plus SSPM vs NPM and MM. Differences were assessed using the non-parametric Mann–Whitney U-test for independent samples and were considered significant at P≤0.05.
Cell Lines and Culture Conditions
Human dermal fibroblasts (HDFs) isolated from a surgical explantation taken from healthy patients, human A375 PM cell line and human Hs29-4T MM cell line were cultivated in DMEM supplemented with 10% FCS at 37 °C in a 5% CO2 humidified atmosphere. All experiments were performed with 70–80% confluent cultures, following 18-h incubation in serum-free culture medium. Cells were then stimulated with NE or E, at 1 μM concentration. Where needed, cells were pre-treated with unselective β-AR antagonist propranolol (Sigma-Aldrich) (1 μM). After 1 h, medium was removed and cells were stimulated with NE 1 μM with or without propranolol 1 μM. For fibroblast activation, cells were grown to sub-confluence and treated for 24 h with the indicated cytokines. Fresh serum-free medium was added for an additional 24 h before collection of conditioned medium (CM), in order to obtain CM free from cytokines (but conditioned by their earlier administration). HDFs cells were then incubated with the obtained CM for 24 h and then used for western blot analyses.
Western Blot Analysis
Cells were lysed for 20 min on ice in 500 μl of complete RIPA lysis buffer (50 mmol/l Tris-HCl (pH 7.5), 150 mmol/l NaCl, 1% NP40, 2 mmol/l EGTA, 1 mmol/l sodium orthovanadate, 1 mmol/l phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin). Lysates were clarified by centrifuging, separated by SDS-PAGE and transferred onto nitrocellulose. The immunoblots were incubated in 3% bovine serum albumin, 10 mmol/l Tris-HCl (pH 7.5), 1 mmol/l EDTA and 0.1% Tween 20 for 1 h at RT and were probed first with specific antibodies and then with secondary antibodies. For chemiluminescence detection, we used Gel Logic 2002 Kodak Imaging System, equipped with a charge-coupled device camera, which guarantees high linearity. Quantity One software (Bio-Rad) was used to obtain quantitative analyses.
Invasion Assay
Transwell system, equipped with 8-μm pore polyvinylpirrolidone-free polycarbonate filters, was used. Cells (5 × 104 in 300 μl) were loaded into the upper compartment in serum-free growth medium with or without 50 μmol/l ilomastat. The upper sides of the porous polycarbonate filters were coated with 50 μg/cm2 of reconstituted Matrigel basement membrane and placed into six-well culture dishes containing 1 ml of complete growth medium. After 24 h of incubation at 37 °C, noninvading cells and the Matrigel layer were mechanically removed using cotton swabs, and the microporous membrane was fixed in 96% methanol and stained with Diff-Quick solution. Chemotaxis was evaluated by counting the cells that migrated to the lower surface of the polycarbonate filters (six randomly chosen fields, mean±s.d.).
Real-Time PCR
Total RNA was extracted from Hs29-4T and A375 derived from our experimental conditions using the RNeasy Minikit kit. Total RNA (1 μg) was reverse-transcribed using the Quantitect Reverse Transcription Kit. Reverse transcription was performed in a final volume of 20 μl containing reverse transcriptase, real-time buffer 1 × and real-time primer mix. The amplification was carried out at 42 °C for 2 min, then 42 °C for 15 min and 95 °C for 3 min. Measurement of gene expression was performed by quantitative real-time PCR (7500 Fast Real-Time PCR System, Applied Biosystems), using the Qiagen Quantifast SYBR Green PCR kit. For each sample, 1 μg of cDNA was added to 25 μl of PCR mix. The samples were then subjected to 40 cycles of amplification at 95 °C for 10 s and 60 °C for 30 s. RNeasy Minikit, Quantitect Reverse Transcription Kit, all primer/probe mixes and Qiagen Quantifast SYBR Green PCR Kit were from Qiagen, except the following primers:
ADRB1 FW: 5′-CAGGTGAACTCGAAGCCCAC-3′
ADRB1 REV: 5′-CTCCCATCCCTTCCCAAACT-3′
ADRB2 FW: 5′-ACGCAGTGCGCTCACCTGCCAGACT-3′
ADRB2 REV: 5′-GCTCGAACTTGGCAATGGCTGTGA-3′
VEGF FW: 5′-TACCTCCACCATGCCAAGTG-3′
VEGF REV: 5′-ATGATTCTCCCTCCTCCTTC-3′
IL-8 FW: 5′-CTGGCCGTGGCTCTCTTG-3′
IL-8 REV: 5′-TTAGCACTCCTTGGCAAAACTG-3′
MMP-2 FW: 5′-ACGACCGCGACAAGAAGTTAT-3′
MMP-2 REV: 5′-ATTTGTTGCCCAGGAAAGTG-5′ (NM_00453; Digestive and liver disease 37 (2005) 584–592)
MMP-9 FW: 5′-GACAAGCTCTTCGGCTTCTG-3′
MMP-9 REV: 5′-TCGCTGGTACAGGTCGAGTA-5′
IL-6 FW: 5′-AGTTCCTGCAGTCCAGCC-3′
IL-6 REV: 5′-TCAAACTGCATAGCCACTTTC-3′.
Quantitative MMP Activity Assay
MMPs activity was measured with Amplite TM Universal Fluorimetric MMP Activity Assay Kit according to the manufacturer’s instructions. Briefly, serum-free medium from confluent monolayer of cells was collected and 5 μl were added to 4-aminophenylmercuric acetate (AMPA; 1 nmol/l) at 37 °C for 1 h to detect MMP-2 activity and at 37 °C for 2 h to detect MMP-9 activity. A 50 μl portion of the mixture was then added to 50 μl of MMP Red substrate solution. After 60 min of incubation the signal was read by fluorescence microplate reader with excitation (Ex)/emission (Em)=540 nm/590 nm.
Statistical Analysis
In vitro data are presented as means±s.d. from at least three experiments. Results were normalized vs control expression levels. Statistical analysis of the data were performed by Student’s t-test. P≤0.05 was considered statistically significant.
RESULTS
Expression of β-ARs in Tissue Samples
The presence of β-ARs was demonstrated in all melanocytic lesions examined. The staining for β1-AR was confined to the cell cytoplasm in naevus melanocytes and melanoma cells; the reaction for β2-AR was also confined to the cell cytoplasm in all cases, with an additional peripheral membrane pattern in some AN melanocytes and malignant cells. The cell staining intensity was always weak with regard to the reaction for β1-AR, whereas the immunostaining for β2-AR appeared to be weak in CN, moderate (except one very-strong reaction), in AN; moderate or strong in in situ PM; from weak to moderate or strong in SSPM, and from strong to very strong in NPM and MM, with no difference between cutaneous and nodal metastasis.
The immunostaining of each lesion taking into account both reaction intensity and percentage of positive cells is shown in Figure 1. In regards to β1-AR expression (Figure 1a), score 1 was evaluated in both CN and AN, score 2 was found in a minority (3 in situ and 2 SS) of PM, whereas in the other PM and MM score 3 was detected. β1-AR expression was significantly higher in malignant than in benign lesions (P≤0.0001) and in PM or MM than in naevi (P≤0.0001 and P≤0.0001). No difference was observed between CN and AN, or between in situ/SSPM compared with NPM/MM.
Immunohistochemical expression of β1-AR (a) and β2-AR (b) in cutaneous human melanocytic lesions: percentage of positivity and staining intensity in each lesion. Each circle represents the percentage of stained cells for one lesion. A quarter-black circle indicates positive weak staining; a half-black circle indicates positive moderate staining; a three-quarter-black circle indicates positive strong staining; a solid-black circle indicates very-strong staining.
With regards to β2-AR expression (Figure 1b), score 1 was observed in CN, score 2 in AN and score 3 was detected in all PM and MM but one (SSPM), which exhibited score 2. β2-AR reactivity was significantly higher in malignant lesions than in naevi (P≤0.0001), and in PM or MM, respectively, than in naevi (P≤0.0001 and P≤0.0001). AN exhibited a significantly higher reactivity compared with CN (P≤0.003), and no difference was observed between in situ/SSPM and NPM/MM.
In addition, no significant difference was detected between PM and MM for both receptors.
Examples of reactions of melanocytic lesions for β1- and β2-AR are shown in Figure 2. Epidermal keratinocytes were lightly coloured for β2-AR, as previously described.17 Endothelial and stromal cells exhibited heavy reactivity for β2-AR in malignant lesions, and to some extent, in AN.
Expression of β1-AR and β2-AR in human cutaneous melanocytic lesions. β1-AR immunostaining in CN (a), AN (b), in situ PM (c), SSPM (d), NPM (e), nodal MM (f): all lesions show a low reaction intensity, confined to the cell cytoplasm. β2-AR immunoreactivity in CN (g), AN (h), in situ PM (i), SSPM (j), NPM (k), nodal MM (l): except the weak staining intensity observed in CN, a moderate to strong or very-strong reactivity intensity is detected in all the other lesions. While CN cells show only cytoplasmic staining for β2-AR, AN and melanoma cells show cytoplasmic and membranous positivity; stromal cells show some reactivity in malignant lesions and AN (Scale bar, 50 μm).
Taken together, our data showed that β1- and β2-ARs were variably expressed in human melanocytic lesions with a significant upregulation in PM and MM, and, at least for β2-AR, a significant upregulation was also observed in AN vs CN.
Effects of Catecholamines on Cancer Cell Motility
In order to confirm the correlation between sensitivity to catecholamines and progression towards a malignant phenotype of melanoma cells, we treated with NE or E two human melanoma cell lines, namely Hs29-4T cells, selected from a metastatic lesion, and A375 cells, derived from PM. We observed that both cell lines express low and comparable levels of β1-AR, as shown in Figure 3, while they both express higher amounts of β2-AR (Figures 4a and b), with the primary A375 melanoma cell line exhibiting a significantly higher expression of β2-AR compared with the metastatic Hs29-4T cell line. Both cell lines are able to respond to catecholamine stimulation with protein kinase A (PKA) phosphorylation, a known trait of β-AR stimulation (Figures 4c and d). In addition, we evaluated the effects of both catecholamines on the MAPK pathways, as few studies until now have investigated the effects of β-adrenergic signalling on these molecular pathways in melanoma models. As shown in Figure 5, both NE and E are able to induce activation, although with different kinetics, of p42-p44 and p38 MAPK in both cell lines (Figures 5a and b).
Expression of β1-AR in melanoma cell lines. (a) Analysis of β1-AR expression by immunoblot in primary (A375) and metastatic (Hs29-4T) melanoma cell lines. (b) Amount of ADRB1 mRNA by real-time PCR. The amount of target, normalized to the endogenous reference (18S RNA), was given by the 2−ΔΔct calculation and was reported as 2−ΔΔct. Both immunoblots and real-time PCR are the mean of three independent assays.
Expression of β2-AR in melanoma cell lines and analysis of the signalling pathway activated by NE stimulation. (a) Analysis of β2-AR expression by immunoblot in primary (A375) and metastatic (Hs29-4T) melanoma cell lines. (b) Amount of ADRB2 mRNA by real-time PCR. The amount of target was given by the 2−ΔΔct calculation and was reported as 2−ΔΔct. Immunoblot and real-time PCR are the mean of three different experiments. (c, d) Analysis of activation of PKA. Melanoma cell lines were serum-deprived overnight and then stimulated with NE (1 μM) for the indicated period and an immunoblot analysis for the detection of the phosphorylation level of p-PKA was shown. Actin immunoblot was used for normalization. The bar graph below represents the phosphorylation level of PKA in four different experiments. *P<0.005.
Analysis of activation of p42/p44 and p38 MAPK. Melanoma cell lines were serum-deprived overnight and then stimulated with E (a) or NE (1 μM) (b) for the indicated period and an immunoblot analysis for the detection of the phosphorylation level of MAPKs were shown. Total p42/p44 and p38 MAPK immunoblot were used for normalization. The bar graphs below represent the phosphorylation level of MAPKs in four different experiments. *P<0.005.
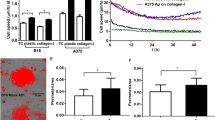
We then analysed the 3D motility of these cells upon catecholamine stimulation. The invasion assay, carried out by Boyden chambers covered with Matrigel to mimic a 3D barrier, revealed that both NE and E are able to elicit invasive behaviour in both metastatic or PM cells. Both NE and E appear to be more effective in PM cells with respect to MM cells. In addition, E is the most efficient catecholamine in eliciting invasiveness of the metastatic Hs29-4T cell line (Figures 6a and b). In both cells lines the increase in 3D invasiveness is sensible to treatment with ilomastat, a broad range inhibitor of MMPs (Figures 6a and b). The pro-invasive effect of both NE and E is strongly sensitive to propranolol, thereby confirming the involvement of β subtypes of AR. The last finding suggests the involvement of a proteolytic degradation of the Matrigel barrier during invasion. We, therefore, analysed by Real-Time PCR the expression of MMP-2 and MMP-9, the main proteolytc enzymes expressed by Hs29-4T and A375 cell lines, during stimulation with catecholamines. Figures 6c and d reveal that, while NE and E do not influence MMP-9 production, the expression of MMP-2 is increased by NE in A375 PM cells and by E in metastatic Hs29-4T. More importantly, both NE and E are able to maintain a high activation state of secreted MMP-2 and MMP-9 following catecholamines stimulation for 24 h on both cell lines (Figures 6e and f).
Effects of E and NE stimulation on melanoma cell lines invasion. Melanoma cell lines were serum-deprived overnight and then seeded in the upper Boyden chamber for assay their invasion. (a, b) NE (a) or E (b) (1 μM), in the presence or absence of ilomastat (50 μmol/l) or propranolol (1 μM), were added in the upper Boyden chamber. Bar graphs represent the mean of migrated cells counted in six different fields for each experiment. *P<0.005 vs untreated. (c, d). Expression of MMP-2 (c) and MMP-9 (d) mRNA by real-time PCR. Melanoma cell lines were serum-deprived overnight and then stimulated with E or NE (1 μM) for 24 h. The amount of target, normalized to the GAPDH mRNA amounts, was given by the 2−ΔΔCT calculation and was reported as arbitrary units (a.u.). The graphs report data as the mean of three independent assays. (e, f) MMP-2 and MMP-9 activity assay. Melanoma cell lines were serum-deprived overnight and then stimulated with NE or E (1 μM) for 24 h. The media obtained were then tested for MMPs activity with a fluorimetric kit, following the manufacturer’s instructions (see Materials and Methods). Data are presented as RFU vs concentration of test compounds. The graphs report data as the mean of four independent experiments. *P<0.005.
Effects of Catecholamines on Cytokine Production and Tumour Microenvironment
Cancer cells secrete many cytokines, chemokines and growth factors that can affect their own aggressiveness, in terms of proliferation, invasion or survival, as well the reactivity of the surrounding stroma. To address the role of catecholamine stimulation in these features we first analysed the expression of a panel of cytokines/growth factors by real-time PCR. We found that catecholamine stimulation leads to an increase in the expression of VEGF, IL-6 and IL-8 (Figures 7a–c). Interestingly the two catecholamines used show differential effects for VEGF, IL-6 and IL-8 in Hs29-4T and A375 cells. In A375 PM cells both NE or E are able to elicit expression of VEGF, IL-6 and IL-8. Conversely, the Hs29-4T metastatic cell line senses NE to increase expression of IL-6 and IL-8, and E to express VEGF.
Expression of VEGF, IL-6 and IL-8 in E- and NE-treated melanoma cell lines. Melanoma cell lines were serum-deprived overnight and then stimulated with E or NE (1 μM). Total RNA was used for the amplification of mRNA of IL-6 (a), VEGF (b) and IL-8 (c), using as housekeeping gene GAPDH mRNA. The amount of target, normalized to the GAPDH mRNA amounts, was given by the 2−ΔΔCT calculation and was reported as arbitrary units (a.u.). The graphs report data as mean of three independent assays. *P<0.005.
The role of VEGF, IL-6 and IL-8 in tumour progression varies from increase in invasiveness/scattering and growth of angiogenic sprouting for VEGF and IL-8, to activation of stromal and/or inflammatory cells for IL-6.18, 19, 20 We observed that VEGF stimulation increases the invasive spur induced by NE in A375 PM cells, while IL-6 does not have a role (Figure 8a). On the other side, we analysed the ability of IL-6, in association with NE, to activate dermal fibroblasts. We observed that IL-6 is able to activate dermal fibroblasts, as demonstrated by their ability to express α-smooth muscle actin (α-SMA), an acknowledged marker of myofibroblasts.21, 22 In addition, in dermal fibroblasts, the CM of NE-treated A375 cells elicits an activation state very similar with respect to treatment with IL-6 alone. Conversely, VEGF treatment is almost ineffective in eliciting a reactivity of fibroblasts (Figure 8b).
Synergy among NE, cytokines and tumour microenvironment. (a) A375 primary melanoma cells were serum-deprived overnight and then seeded in the upper Boyden chamber for assaying their invasion. NE (1 μM), IL-6 (50 ng/μl), VEGF (20 ng/μl), in combination with NE or alone, were added in the upper Boyden chamber. Bar graph represents the mean of migrated cells counted in six different fields for each experiment. *P<0.005 vs untreated. (b) Analysis of human dermal fibroblasts (HDFs) activation state, through evaluation of α-SMA expression, after treatment with conditioned medium (CM) from NE/IL-6/VEGF-treated A375 cells. A375 cells were grown to sub-confluence and treated for 24 h with 1 μM NE, IL-6 (50 ng/μl) or VEGF (20 ng/μl). Fresh serum-free medium was added for an additional 24 h before collection of CM, in order to obtain CM free from NE, IL-6 or VEGF (but conditioned by their earlier administration). HDFs were then incubated with the obtained CM for 24 h and then used for western blot analysis of α-SMA expression. Actin immunoblot was used for normalization. The blot is representative of three different experiments.
Taken together, these data suggest that in vitro the treatment of human melanoma cells with catecholamines dramatically affects their aggressiveness, inducing expression of MMP-2, VEGF, IL-6 and IL-8. These factors orchestrate a feed-forward loop leading to increase of proteolytic invasiveness of tumour cells, as well as activating surrounding fibroblasts.
Discussion
The present study shows that the immunohistochemical expression of β-1 and β-2 ARs is significantly upregulated in melanoma tissues. Interestingly, all tested melanocytic lesions exhibited some immunoreactivity, suggesting that both benign and malignant lesions can theoretically be affected by catecholamines in vivo. However, since β-ARs staining significantly increases in PM and MM compared with melanocytic naevi, it appears that malignant lesions can be more deeply influenced by catecholamines than benign counterparts. The staining intensity for β2-AR progressively increased from CN, towards AN, to PM and MM, whereas the reaction intensity for β1-AR was weaker, in all groups of lesions. Such a difference can rely on a different reactivity of the used antibodies, but it is likely that β1-AR is expressed on naevus melanocytes and melanoma cells of sections at a lower level than β2-AR. This hypothesis was supported by PCR and western blot analysis of primary and MM cell lines, both of which exhibited a lower expression of β1-AR vs β2-AR.
In melanoma tissue sections a strong reactivity for β2-AR was also observed in most endothelial and stromal cells, including macrophages, suggesting the possible influence of catecholamines on cells of the tumour microenvironment and the chance of further potential biologic loops capable of affecting metastatic behaviour of neoplastic cells.
We also demonstrated that A375 primary and Hs29-4T MM cell lines respond to catecholamine stimulation enhancing motility and invasion, and producing molecules closely related to neoplastic progression. In keeping, we show that NE and E are able to elicit activation of p42/p44 and p38 MAPKs, acknowledged to have mandatory roles for cell growth, survival and invasive ability, in both primary and metastatic cell lines. This observation is in agreement with the findings of Pak et al.,23 also indicating the Ras-MAPKs pathway as a target of β-adrenoceptors. These data could be of striking interest in order to find new strategies for melanoma treatment. In fact, Meier et al.24 showed that combined targeting of the p42/p44 and Akt signalling pathways significantly inhibited growth and enhanced apoptosis in melanoma cell cultures.
Moreover, NE and E exhibited a diverse stimulation capacity on the two cell lines. Concerning the invasion assay, the primary cell line responded to NE (and, at a lesser degree, to E), while the metastatic cell line showed a clear reaction only to E. The inhibition induced by ilomastat strongly suggests a proteolytic degradation of Matrigel and the probable intervention of MMPs. In fact, we demonstrate that MMP-2, rather than MMP-9, is produced at a significantly higher level compared with baseline, by A375 cells under NE stimulus and by Hs29-4T cells under E stimulation. Furthermore, our data show that both NE and E are able to elicit sustained activation of MMP-2 and MMP-9 both in primary and metastatic cell lines. Thus, increased motility of melanoma cells seems to be due to a proteolytic invasive capacity, typical of a mesenchymal phenotype,25, 26 and catecholamines seem able to influence MMPs activity both at a transcriptional and at a post-translational level. The migration of neoplastic cells appears to be increased through activation of β-ARs, because it is completely abolished by propranolol.
With regard to the production of cytokine transcripts, A375 cells significantly increase levels of IL-6 and VEGF under NE and E challenge, whereas Hs29-4T cells increase IL-6 expression under NE stimulus, and produce significant amounts of VEGF, especially under E activation. Concerning the expression of IL-8, the primary cell line responded to both NE and E at a significantly higher degree compared with the metastatic one, and both cell lines reacted more intensely to NE. This result is in agreement with the angiogenic role of IL-8, as de novo angiogenesis is particularly useful for primary tumours to escape the hostile microenvironment and disseminate metastasis.27 In addition, the catecholamine-induced IL-8 enhancement is in agreement with the described IL-8 stimulation produced by NE in prostate cancer.10
We do not know exactly why such a discrepancy exists between primary and metastatic cell line response, but it is possible that at least in part this difference is due to a higher expression of β2-ARs, assessed as protein and transcript, on the PM cell line. Another possibility is that IL-6 and IL-8, whose expression was associated with early malignancy of melanoma in vivo28 are actually secreted more efficiently by a cell line derived from a PM.
In vitro experiments clearly show that catecholamines can augment the malignant behaviour of melanoma cells affecting both invasion capacity and cytokine production.
Our in vitro experiments suggest that some pro-metastatic loops could work in melanoma in vivo too. We demonstrated that IL-6 and NE in melanoma cells can activate dermal fibroblasts towards a myofibroblastic phenotype, identified by α-SMA expression.21 It is well known that stromal fibroblasts within tumours undergo a process, commonly called mesenchymal–mesenchymal transition to myofibroblasts, leading them to achieve a more contractile phenotype and allowing a cross talk with tumour cells dramatically increasing their aggressiveness.29, 30 In turn, activated fibroblasts can secrete other pro-metastatic cytokines, such as VEGF,31 capable of inducing further tumour angiogenesis. In addition, in our experiments, VEGF can increase, particularly when associated with NE, melanoma cell migration and hence invasion capacity.
Previous data support the hypothesis that various types of stress, such as surgical procedure and the immediate post operative period, or neuroendocrine stress due to psycho-social factors, can stimulate tumour progression both in animals and humans.10 This seems to be true also for melanoma at least in in vivo models.32, 33
Our work provides evidence that stress hormones like NE and E can significantly stimulate the malignancy of melanoma cells at various levels and that β-ARs, likely involved in this response, are largely expressed in melanoma cell lines and cutaneous melanomas. It is the first time, to our knowledge, that β-ARs are demonstrated in a large series of human cutaneous melanocytic lesions, and even in melanocytic naevi, suggesting a potential influence of catecholamines also in benign counterparts. Moreover, the detection of β2-AR also in the stromal cells of melanoma microenvironment implies further possible effects of catecholamines on melanoma progression. Consequently, it is possible that the interaction catecholamines-β-ARs could have a dramatic role during the clinical course of melanoma patients. The efforts to understand molecular events underlying such an interaction can be very useful for indicating new targets in therapy.
References
MacKie RM, Hauschild A, Eggermont AM . Epidemiology of invasive cutaneous melanoma. Ann Oncol 2009;20 (Suppl 6):vi1–vi7.
Atallah E, Flaherty L . Treatment of metastatic malignant melanoma. Curr Treat Options Oncol 2005;6:185–193.
Drell TL, Joseph J, Lang K et al. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res Treat 2003;80:63–70.
Masur K, Niggemann B, Zanker KS et al. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res 2001;61:2866–2869.
Palm D, Lang K, Niggemann B et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer 2006;118:2744–2749.
Sood AK, Bhatty R, Kamat AA et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res 2006;12:369–375.
Yang EV, Sood AK, Chen M et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res 2006;66:10357–10364.
Shang ZJ, Liu K, Liang de F . Expression of beta2-adrenergic receptor in oral squamous cell carcinoma. J Oral Pathol Med 2009;38:371–376.
Entschladen F, Lang K, Drell TL et al. Neurotransmitters are regulators for the migration of tumor cells and leukocytes. Cancer Immunol Immunother 2002;51:467–482.
Voss MJ, Entschladen F . Tumor interactions with soluble factors and the nervous system. Cell Commun Signal 2010;8:21.
Glasner A, Avraham R, Rosenne E et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol 2010;184:2449–2457.
Powe DG, Entschladen F . Targeted therapies: using β-blockers to inhibit breast cancer progression. Nat Rev Clin Oncol 2011;8:511–512.
De Giorgi V, Grazzini M, Gandini S et al. Treatment with β-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 2011;171:779–781.
Lemeshow S, Sørensen HT, Phillips G et al. β-Blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomarkers Prev 2011;20:2273–2279.
Yang EV, Kim SJ, Donovan EL et al. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun 2009;23:267–275.
Steinkraus V, Mak JC, Pichlmeier U et al. Autoradiographic mapping of beta-adrenoceptors in human skin. Arch Dermatol Res 1996;288:549–553.
Sivamani RK, Lam ST, Isseroff RR . Beta adrenergic receptors in keratinocytes. Dermatol Clin 2007;25:643–653.
Carmeliet P, Jain RK . Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov 2011;10:417–427.
Ara T, Declerck YA . Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer 2010;46:1223–1231.
Waugh DJ, Wilson C . The interleukin-8 pathway in cancer. Clin Cancer Res 2008;14:6735–6741.
Kalluri R, Zeisberg M . Fibroblasts in cancer. Nat Rev Cancer 2006;6:392–401.
Tomasek JJ, Gabbiani G, Hinz B et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349–363.
Pak Y, Pham N, Rotin D . Direct binding of the β1 adrenergic receptor to the cyclic AMP-dependent guanine nucleotide exchange factor CNrasGEF leads to Ras activation. Mol Cell Biol 2002;22:7942–7952.
Meier F, Busch S, Lasithiotakis K et al. Combined targeting of MAPK and Akt signalling pathways is a promising strategy for melanoma treatment. Br J Dermatol 2007;156:1204–1213.
Hofmann UB, Westphal JR, Van Muijen GN et al. Matrix metalloproteinases in human melanoma. J Invest Dermatol 2000;115:337–344.
Parri M, Taddei ML, Bianchini F et al. EphA2 reexpression prompts invasion of melanoma cells shifting from mesenchymal to amoeboid-like motility style. Cancer Res 2009;69:2072–2081.
Singh RK, Varney ML . IL-8 expression in malignant melanoma: implications in growth and metastasis. Histol Histopathol 2000;15:843–849.
Moretti S, Pinzi C, Spallanzani A et al. Immunohistochemical evidence of cytokine networks during progression of human melanocytic lesions. Int J Cancer 1999;84:160–168.
De Wever O, Mareel M . Role of tissue stroma in cancer cell invasion. J Pathol 2003;200:429–447.
Dvorak HF, Weaver VM, Tlsty TD et al. Tumor microenvironment and progression. J Surg Oncol 2011;103:468–474.
Dong J, Grunstein J, Tejada M et al. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J 2004;23:2800–2810.
Azpiroz A, De Miguel Z, Fano E et al. Relations between different coping strategies for social stress, tumor development and neuroendocrine and immune activity in male mice. Brain Behav Immun 2008;22:690–698.
Vegas O, Fano E, Brain PF et al. Social stress, coping strategies and tumor development in male mice: behavioral, neuroendocrine and immunological implications. Psychoneuroendocrinology 2006;31:69–79.
Acknowledgements
This study was financially supported by fundings from Italian Association for Cancer Research, Istituto Toscano Tumori; PRIN 2008–2011, Fondazione Cassa di Risparmio di Pistoia e Pescia (Pistoia, Italy) and from Fondazione Banche di Pistoia e Vignole per la Cultura e lo Sport (Quarrata, Pistoia, Italy). These sponsors were not involved in any part of the study or in manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Both β1- and β2-adrenoceptors are significantly up-regulated in malignant melanoma compared to melanocytic naevi, and stimulation of melanoma cell lines with norepinephrine orepinephrine causes them to increase proteolytic invasiveness and secrete pro-tumorigenic cytokines. These data suggest that circulating norepinephrine and epinephrine affect melanoma progression in vivo, at least in part through the β-adrenoceptor activation.
Rights and permissions
About this article
Cite this article
Moretti, S., Massi, D., Farini, V. et al. β-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Invest 93, 279–290 (2013). https://doi.org/10.1038/labinvest.2012.175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2012.175
Keywords
This article is cited by
-
Effect of chronic stress on tumorigenesis and development
Cellular and Molecular Life Sciences (2022)
-
Obesity and cancer—extracellular matrix, angiogenesis, and adrenergic signaling as unusual suspects linking the two diseases
Cancer and Metastasis Reviews (2022)
-
Dissecting the role of cell signaling versus CD8+ T cell modulation in propranolol antitumor activity
Journal of Molecular Medicine (2022)
-
Stress and cancer: mechanisms, significance and future directions
Nature Reviews Cancer (2021)
-
β3-adrenoreceptor blockade reduces tumor growth and increases neuronal differentiation in neuroblastoma via SK2/S1P2 modulation
Oncogene (2020)