Abstract
Tight junction has a crucial role in regulating paracellular transports (as a barrier) and in separating apical from basolateral compartments to maintain cell polarity (as a fence). Tight junction can be disrupted by various stimuli, including oxidative stress, pathogens and proinflammatory cytokines. However, association of defective tight junction with kidney stone pathogenesis remains unknown. We therefore examined whether calcium oxalate monohydrate (COM) crystals, which are the major crystalline composition in kidney stones, have any effects on expression and function of tight junction of polarized renal tubular epithelial cells. Western blot analysis revealed marked decrease in levels of occludin and zonula occludens-1 (ZO-1) in COM-treated polarized Madin-Darby canine kidney (MDCK) cells. Immunofluorescence staining revealed not only the decline of these tight junction proteins but also their redistribution and dissociation in COM-treated cells. Additionally, transepithelial resistance was significantly decreased, indicating impaired tight junction barrier and increased paracellular permeability in COM-treated cells. Subcellular fractionation followed by western blot analysis of Na+/K+-ATPase-α1 revealed that this basolateral membrane marker was also detectable in apical membrane fraction of COM-treated cells, but not in apical membrane fraction of control cells. Immunofluorescence study confirmed the translocation of Na+/K+-ATPase-α1 (from basolateral to apical membranes) in COM-treated cells, indicating impaired fence function of the tight junction. Moreover, dihydrorhodamine assay using flow cytometry revealed the significantly higher level of hydrogen peroxide in the COM-treated cells. These data provide the first evidence to demonstrate decreased expression and defective barrier and fence functions of the tight junction of renal tubular epithelial cells exposed to COM crystals that may be fundamental for subsequent renal tubulointerstitial injury, which in turn enhances the stone pathogenesis.
Similar content being viewed by others
Main
Polarized epithelial cells normally line tubular structure of internal organs, for example, renal tubules and intestines. These cells act as a selective barrier between tissues and intratubular lumens. Plasma membranes of polarized cells can be distinguished into apical and basolateral domains, each of which differs in protein compositions and functions.1 Tight junction is one of intercellular junctions that localizes between apical and basolateral membranes.2 This junction is organized as a complex of proteins comprising of transmembrane proteins, that is, occludin,3 claudins4 and junctional adhesion molecules,5 as well as cytoplasmic plaque proteins, that is, zonula occludens-1 (ZO-1),6 ZO-2,7 ZO-38 and cingulin.9 There are two main functions of tight junction, including barrier and fence functions. As a barrier, tight junction forms a complex to regulate (indeed to limit) the passage of water, ions, macromolecules and pathogens through paracellular space. As a fence, tight junction separates apical from basolateral membranes of epithelial cells to maintain cell polarity.10
Tight junction can be disrupted by oxidative stress,11, 12, 13 pathogens14 and proinflammatory cytokines.15, 16, 17 A previous study has demonstrated that mutation at C-terminus of occludin leads to increased paracellular permeability and loss of fence function.18 Occludin is a transmembrane protein that interacts with other tight junction proteins, especially ZO-1, which is the important scaffold protein that links occludin to actin cytoskeleton.19 Furthermore, there are evidences showing that hydrogen peroxide (H2O2)13 and acetaldehyde20, 21 can induce increased paracellular permeability and loss of cell polarity, which not only contribute to redistribution of occludin and ZO-1 but also dissociation of occludin/ZO-1 complex. Thus, alterations in occludin and ZO-1 can be used as the markers for determination of tight junction disruption. The disruption of tight junction is involved in several diseases, including gastrointestinal, lung and kidney diseases.22, 23 However, to the best of our knowledge, its association with kidney stone disease remains unknown.
Calcium oxalate monohydrate (COM) crystals are the major crystalline composition of kidney stone matrix. The interaction of COM crystals with renal tubular epithelial cells is important for progression of kidney stone formation. COM crystal–cell interaction leads to production of reactive oxygen species (ROS), for example, superoxide (O2˙−)24 and H2O2.25 These ROS products can mediate proinflammatory cytokines, for example, monocyte chemoattractant protein-1, leading to inflammation, epithelial cell injury and ultimately necrotic cell death.26, 27 Nevertheless, pathogenic mechanisms underlying kidney stone formation remain unclear. Therefore, understanding of cellular targets and subsequent cascades of COM crystal-induced renal tubulointerstitial injury is crucial to gain more insights into the molecular mechanisms of kidney stone disease.
We have hypothesized that COM crystals may also induce renal tubular epithelial cell injury through disruption of tight junction. In this study, we therefore evaluated the effects of COM crystals on expression and function of tight junction in polarized Madin-Darby canine kidney (MDCK) cells, which were originated from distal nephron. Western blot analysis and immunofluorescence assay were performed to determine expression levels, localization and interaction of two tight junction proteins, occludin and ZO-1. Tight junction barrier function was evaluated by measurement of transepithelial resistance (TER), whereas its fence function was examined by detection of translocation of basolateral protein, Na+/K+-ATPase-α1, at apical membrane fraction.
MATERIALS AND METHODS
Preparation of COM Crystals
COM crystals were prepared as described previously.28 Briefly, 10 mM calcium chloride dihydrate (CaCl2·H2O) was mixed with 10 mM sodium oxalate (Na2C2O4) to make up their final concentrations to 5 and 0.5 mM, respectively, in basic buffer containing 90 mM Tris-HCl (pH 7.4) and 10 mM sodium chloride (NaCl). Then, the mixture was incubated at room temperature overnight followed by centrifugation at 3000 r.p.m. for 5 min. The supernatant was discarded and COM crystals were washed with methanol. After another centrifugation at 3000 r.p.m. for 5 min, methanol was removed and COM crystals were dried overnight at 37°C. Then, COM crystals were decontaminated by UV light radiation for 30 min.
Cell Culture, Polarization and COM Treatment
MDCK cells were grown in Eagle's minimum essential medium (MEM; GIBCO, Invitrogen, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, 1.2% penicillin-G/streptomycin and 2 mM L-glutamine, and maintained in a humidified incubator at 37°C with 5% CO2. Polarization was achieved by using Transwells (Costar, Cambridge, MA USA). Briefly, cells at a density of 5.0–7.5 × 104 cells per ml were plated and grown on prewetted collagen-coated permeable polycarbonate membrane insert (0.4 μm pore size) for 4 days. The culture medium was refreshed every other day. The collagen was coated onto the permeable polycarbonate membrane by dissolving collagen type IV powder (Sigma, St Louis, MO, USA) in 0.25% acetic acid at 4°C. The collagen solution was then added onto membrane insert (6–10 μg/cm2) to cover whole surface area of the membrane and incubated at 4°C overnight. Polarized MDCK cell monolayers were then maintained in COM-containing or COM-free medium for 48 h, and then subjected to subsequent investigations. For COM-containing medium, COM crystals were added to complete Eagle's MEM to obtain a final concentration of 100 μg/ml. For COM-free medium, 100 μg/ml COM crystals were added into the medium for 30 min, but were finally removed from the medium (as to obtain identical concentrations of free calcium and oxalate ions in the medium compared with those of COM-containing medium).
Flow Cytometric Analysis of Cell Death (Annexin V/Propidium Iodide Double Staining)
MDCK cells from the monolayer (including both adherent and floating cells) were trypsinized with 3 ml of 0.1% trypsin in 2.5 mM EDTA and resuspended in 10 ml MEM. The harvested cells were centrifuged at 1500 r.p.m. at 4°C for 5 min, and washed with PBS. Cell pellets were resuspended in annexin V buffer (10 mM HEPES, 140 mM NaCl and 2.5 mM CaCl2.2H2O; pH 7.4) at a final concentration of 5 × 105 cells per ml and then incubated with FITC-labeled annexin V (BD Biosciences, San Jose, CA, USA) on ice for 15 min in the dark. Propidium iodide (BD Biosciences) was added to the samples at a final concentration of 10 μl/ml before analysis. The cells were then analyzed by flow cytometry (FACScan, Becton Dickinson Immunocytometry System, San Jose, CA, USA) and MDCK cells treated with 2 μg/ml camptothecin were used as a positive control. This experiment was performed in triplicate. Percentage of total cell death (% cell death)=((number of both apoptotic and necrotic cells/number of all cells) × 100%).
Single and Double Immunofluorescence Staining and Imaging
MDCK cells, ∼1 × 105 cells, were cultured on cover slips (cleaved mica disk diameter: 9.5 mm, V-1 grade, SPI supplier, Toronto, Canada). After the cells were maintained with or without COM crystals (100 μg/ml) for 48 h, the cells were rinsed with ice-cold membrane-preserving buffer. The cells were then fixed with 3.7% formaldehyde for 15 min and permeabilized with 0.1% Triton X-100 for 15 min. After another washing step with ice-cold membrane-preserving buffer, the fixed cells were incubated with primary antibody. For single immunofluorescence staining, the cells were incubated with mouse monoclonal Na+/K+-ATPase-α1 (1:50 in 1% skim milk/PBS; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 37°C for 1 h. The cells were then incubated with AlexaFluor-488-conjugated goat anti-mouse IgG (1:5000 in 1% skim milk/PBS; Invitrogen/Molecular Probes, Burlington, Canada) and Hoechst dye (1:1000 in PBS for nuclear stain; Invitrogen/Molecular Probes) at 37°C for 1 h. For double immunofluorescence staining, the cells were incubated with mouse monoclonal anti-ZO-1 (Zymed, San Francisco, CA, USA) and rabbit polyclonal anti-occludin (Santa Cruz Biotechnology; both at a dilution factor of 1:50 in 1% skim milk/PBS) at 37°C for 1 h. The cells were then incubated with AlexaFluor-488-conjugated goat anti-mouse IgG (1:5000 in 1% skim milk/PBS; Invitrogen/Molecular Probes), Cy3-conjugated donkey anti-rabbit IgG (1:5000 in 1% skim milk/PBS; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and Hoechst dye (1:1000 in PBS for nuclear stain; Invitrogen/Molecular Probes) at 37°C for 1 h. Thereafter, the cover slips were mounted with 50% glycerol/PBS and then visualized by using a laser-scanning confocal microscope equipped with LSM5 Image Browser (LSM 510 META, Carl Zeiss, Oberkochen, Germany).
Measurement of TER
TER was measured after the polarized MDCK cells were maintained in the medium with or without COM crystals for 0, 3, 6, 9, 12, 24 and 48 h using a Millicell-ERS resistance system (Millipore, Bedford, MA, USA), as detailed in manufacturer's instruction. TER was measured at three different sites in each Transwell and the resistance of polycarbonate membrane was subtracted.
Fractionation of Apical and Basolateral Membranes
Apical membranes of the polarized MDCK cells were isolated by a peeling method, which is based on the principle of hydrous affinity and/or ionic interaction between these surfaces and apical membranes, as described previously.29 After incubation with or without COM crystals for 48 h, the culture medium was removed and the polarized cells were rinsed twice with ice-cold membrane-preserving buffer (1 mM MgCl2 and 0.1 mM CaCl2 in PBS). Thereafter, Whatman filter paper (0.18-mm thick, Whatman International, Maidstone, UK), prewetted with deionized water, was placed onto the polarized cell monolayer. After a 5-min incubation period, the filter paper was peeled out and the apical membranes retained at the surfaces after peeling were harvested by rehydration using deionized water and gentle scrapping. The remaining cell shafts were also scrapped and resuspended in deionized water. These apical and basolateral membrane-enriched fractions were then lyophilized and subjected to protein extraction and western blot analysis.
Western Blot Analyses
Whole cells, apical membranes or basolateral membranes were resuspended in Laemmli's buffer and proteins recovered from individual fractions were measured by Bradford's method using Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA). The recovered proteins were then resolved by 10% SDS-PAGE with an equal amount of total protein (30 μg) in each lane for western blot analysis of occludin and ZO-1, and with an equal number of priming cells (3.0 × 105 cells) in each lane for western blot analysis of Na+/K+-ATPase-α1. The separated proteins were transferred onto nitrocellulose membrane and nonspecific bindings were blocked with 5% skimmed milk in PBS at room temperature for 1 h. The membrane was then incubated at 4°C overnight with rabbit polyclonal anti-occludin (1:1000 in 1% skim milk/PBS; Santa Cruz Biotechnology), mouse monoclonal anti-ZO-1 (1:1000 in 1% skim milk/PBS; Zymed), mouse monoclonal anti-Na+/K+-ATPase-α1 (1:1000 in 1% skim milk/PBS; Santa Cruz Biotechnology) or mouse monoclonal anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase; 1:1000 in 1% skim milk/PBS; Santa Cruz Biotechnology). After washing with PBS three times, the membrane was then incubated with corresponding secondary antibody conjugated with horseradish peroxidase (1:2000 in 1% skim milk/PBS) at room temperature for 1 h. Immunoreactive bands were detected by SuperSignal West Pico chemiluminescence substrate (Pierce Biotechnology, Rockford, IL, USA) and autoradiogram.
Flow Cytometric Analysis of ROS Production (Dihydrorhodamine Assay)
MDCK cells were preincubated with 10 μM dihydrorhodamine 123 (DHR123; Sigma) at 37°C for 5 min in culture medium before an exposure to COM crystals (100 μg/ml). The cells were then trypsinized, washed and resuspended in the fresh culture medium. The fluorescence signal was measured by flow cytometry (FACScan, Becton Dickinson Immunocytometry System). The MDCK cells treated with phorbol myristate acetate were used as positive control. This experiment was performed in triplicate independently.
Statistical Analysis
All quantitative data are presented as mean±s.e.m. Comparisons between two sets of samples were performed using unpaired Student's t-test. P-values <0.05 was considered statistically significant.
RESULTS
Justification of the Model and Experimental Intervention
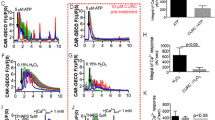
This study was carefully designed by avoiding any possible pitfalls/biases. First, control (COM-free) medium was also exposed to an equal amount (100 μg/ml) of crystals, which were subsequently removed from the medium after 30-min incubation, to obtain comparable levels of free calcium and oxalate ions in the medium to that of COM-containing medium. Second, polarization of MDCK cells was confirmed by confocal microscopic examination of gp135 and Na+/K+-ATPase-α1, which served as the markers for apical and basolateral membranes, respectively (data not shown). Finally, severe cytotoxicity was excluded from our study. We initially screened for the optimal dosage and incubation time point, as various doses of COM crystals were used and multiple time points were evaluated. We performed flow cytometric analysis to quantitate apoptotic and necrotic cells using annexin V/propidium iodide double staining. The data showed that using high doses (>100 μg/ml) and prolonged incubation (>48 h) were associated with profound cytotoxicity (data not shown), whereas the dosage of COM crystals at 100 μg/ml and 48-h incubation revealed comparable percentage of total cell death in COM-treated and control cells (Figure 1). In contrast, the lower dosages and shorter incubation time points provided no changes (or if any, undetectable by methods used herein) of expression and function of tight junction. Therefore, we selected the dosage at 100 μg/ml and 48-h incubation time point as the optimal conditions for all subsequent experiments, and effects of COM crystals on tight junction proteins reflected response in the cells to COM crystals, and not the effects of severe cytotoxicity or cell death.
Flow cytometric data of cell death after the polarized MDCK cells were exposed to COM crystals (100 μg/ml) for 48 h. Apoptosis and necrosis of these cells were evaluated using annexin V/propidium iodide double staining. Percentage of total cell death (% cell death)=((number of both apoptotic and necrotic cells/number of all cells) × 100%). Lower panel shows mean (+s.e.m.) of cell death data obtained from three independent experiments. NS=not significant.
Alterations in Tight Junction Protein Expression by COM Crystals
Effect of COM crystals on levels and expression patterns (organization/colocalization) of tight junction proteins was examined. First, we performed western blot analysis to evaluate changes in levels of two major tight junction proteins, occludin and ZO-1. After an exposure to COM crystals for 48 h, levels of both occludin and ZO-1were markedly decreased in polarized MDCK renal tubular epithelial cells (Figure 2). These two proteins normally form a complex with other tight junction proteins, and previous studies have demonstrated that disruption of tight junction causes redistribution and dissociation of occludin and ZO-1.13, 30 We thus examined expression pattern and colocalization of occludin and ZO-1. Immunofluorescence study showed that these two proteins were sharply, continuously and homogeneously expressed at paracellular junctions with ‘honeycomb’ appearance in control cells exposed to COM-free medium, whereas COM-treated cells showed disruption and redistribution of both occludin and ZO-1 with some cytoplasmic expression (Figure 3). Moreover, dissociation of these two proteins was also observed, particularly in nonmembrane compartments.
COM crystals induced downregulation of occludin and ZO-1. Proteins derived from whole cell lysate of control or COM-treated cells were equally loaded into each lane (30 μg) and resolved by 10% SDS-PAGE. The resolved proteins were then transferred onto a nitrocellulose membrane. After blocking, the membrane was incubated with rabbit polyclonal anti-occludin or mouse monoclonal anti-ZO-1, and then with corresponding secondary antibody conjugated with horseradish peroxidase. The immunoreactive bands were visualized by chemiluminescence and autoradiography. GAPDH was also examined to confirm equal loading of total protein in each lane.
COM crystals induced disruption of tight junction, redistribution and dissociation of occludin and ZO-1. The polarized MDCK cells were incubated with 100 μg/ml COM crystals for 48 h and then processed for double immunofluorescence staining for occludin (in red) and ZO-1 (in green). In merged view (right column), colocalization of occludin and ZO-1 was observed in yellow. The polarized MDCK cells grown in COM-free medium (to which 100 μg/ml COM crystals were added and incubated for 30 min, but were then removed) served as control cells. Original magnification was × 200 for all panels.
Effect of COM Crystals on Barrier Function of Tight Junction
One of the major functions of tight junction is to serve as a barrier to control paracellular permeability. We examined whether COM crystals had any effects on paracellular permeability, as determined by measurement of TER. The results showed that COM crystals caused marked decrease in TER at all time points, as compared with basal TER of COM-treated cells and also TER of control cells at all matched time points (Figure 4).
Defective barrier function of tight junction induced by COM crystals. Polarized MDCK cells were grown in Transwells, and transepithelial resistance (TER) was measured at various time points after exposure to 100 μg/ml COM crystals. The polarized MDCK cells grown in COM-free medium (to which 100 μg/ml COM crystals were added and incubated for 30 min, but were then removed) served as the controlled cells. The data are reported as mean±s.e.m. (n=3 independent experiments for each data point). * Represents P<0.005 and ** represents P<0.0005 compared with TER of controlled cells at the matched time point, whereas # represents P<0.005 compared with the basal TER level.
Effect of COM Crystals on Fence Function of Tight Junction
Fence function is another important function of tight junction, which separates basolateral from apical membranes, thereby preventing translocation of membrane proteins from one to another site. Membrane proteins of polarized MDCK cells were fractionated into apical and basolateral membrane compartments using the recently established peeling method.29 Western blot analysis of Na+/K+-ATPase-α1 revealed that this basolateral marker protein was present not only in the whole cell lysate and basolateral membrane fraction in control cells but was also detectable in apical membrane fraction in COM-treated cells, indicating impaired fence function of tight junction (Figure 5a). This defect in fence function was confirmed by immunofluorescence study, which revealed translocation of Na+/K+-ATPase-α1 from basolateral to apical membranes in COM-treated cells (Figure 5b). Using autoreflection of COM crystals (which was detected in red at λ633 nm), the data also revealed some internalized COM crystals within MDCK cells, whereas their majority remained adherent on the apical surface.
Defective fence function of tight junction induced by COM crystals. (a) Proteins derived from whole cell lysate, apical membrane fraction and basolateral membrane fraction of controlled and COM-treated cells were subjected to western blot analysis using mouse monoclonal anti-Na+/K+ATPase-α1 as primary antibody. Each lane was loaded with proteins derived from equal number of cells (3.0 × 105 cells per lane). Translocation of Na+/K+ATPase-α1 (a marker of basolateral membrane) from basolateral to apical membrane compartment was observed in the COM-treated cells, but not in the control cells. (b) The polarized MDCK cells were incubated with 100 μg/ml COM crystals for 48 h and then processed for immunofluorescence staining of Na+/K+-ATPase-α1 (in green) and nucleus (in blue). The COM crystals were detected in red by autoreflection at λ633 nm and the images with different color detection were integrated. The polarized MDCK cells grown in COM-free medium (to which 100 μg/ml COM crystals were added and incubated for 30 min, but were then removed) served as the control cells.
ROS Production
Oxidative stress has been reported to be involved in cell injury and tight junction disruption.11 We therefore evaluated the ROS production in COM-treated cells using dihydrorhodamine assay and flow cytometry. The data showed significantly greater level of hydrogen peroxide in COM-treated cells as compared with the control cells (Figure 6).
COM crystals induced ROS production. Intracellular hydrogen peroxide level of polarized MDCK cells with or without treatment with COM crystals (100 μg/ml) was evaluated by dihydrorhodamine assay using flow cytometry. Integrated mean fluorescent intensity (iMFI) of intracellular hydrogen peroxide=(Percentage of hydrogen peroxide-producing cells) × (Mean fluorescent intensity). The data are reported as mean±s.e.m. (n=3 independent experiments for each data set). * Represents P<0.05.
DISCUSSION
COM is the main and most important crystalline composition of kidney stones. The interaction of renal tubular epithelial cells and COM crystals provokes cellular injury, which in turn enhances kidney stone formation. Nevertheless, the detailed mechanisms and subcellular targets that are involved in COM crystal-induced renal tubular epithelial cellular injury remain unclear. Several studies have demonstrated that disruption of tight junction is one of important mechanisms involving various models of stimuli-induced epithelial cellular injury.15, 31 We therefore hypothesized that COM crystals might also induce renal tubular epithelial cellular injury through disruption or impairment of tight junction. To address our hypothesis, we performed both expression and functional studies on tight junction of polarized MDCK cells, which were originated from distal nephron,32 the initiating site of kidney stone formation.
We first examined levels and expression pattern of occludin and ZO-1. Occludin is one of the main tight junction proteins that interacts with another occludin molecule in the adjacent cell and with other subcellular scaffold proteins within the same cell.33 ZO-1 acts as a linker between occludin and actin cytoskeleton, and serves as a core protein to interact with other proteins in the tight junction complex.19 Knockdown of these proteins leads to diverse phenotypic alterations in epithelial cells34 and their expression levels are correlated with integrity of epithelial cells.18, 35 We therefore focused on these two important markers of tight junction in this study.
Western blot analysis clearly demonstrated that levels of occludin and ZO-1 were markedly decreased after the cells were exposed to COM crystals for 48 h (Figure 2). Our data were consistent to those reported in previous studies demonstrating that various stimuli (including oxidative stress, proinflammatory cytokines and pathogens) caused downregulation of occludin and ZO-1 in MDCK and Caco-2 cells.17, 30, 36, 37, 38 Immunofluorescence studies revealed not only downregulation but also redistribution and dissociation of these two proteins (Figure 3), indicating disruption of tight junction complex. These data were also in concordance with findings of a recent study illustrating that H2O2-induced disruption of tight junction caused redistribution of occludin and ZO-1 from intercellular junction into cytoplasmic compartment of Caco-2 and T84 cells.13, 30 Furthermore, mutation at C-terminus of occludin in MDCK cells exhibited a discontinuous junctional staining pattern,18 which was similar to the findings in our study.
To demonstrate functional deterioration of tight junction after the cells were exposed to COM crystals, we focused our attention onto both barrier and fence functions. As a barrier, tight junction has crucial role in regulation of paracellular transport of water, ions, macromolecules and other substances. Loss or impairment of this function would lead to leakage of these substances from intratubular space to interstitial compartment and ultimately to tissue injury/inflammation. TER is a perfect parameter representing tight junction integrity and epithelial permeability to ions, as it correlates with the flux of small molecules across the tissue.39 Our study clearly demonstrated that TER was markedly decreased in polarized MDCK cells exposed to COM crystals compared with the basal TER and TER at all time points of the control cells (Figure 4). Similarly, a large number of previous studies have also reported that disruption of tight junction by oxidants, pathogens and proinflammatory cytokines in various epithelial cells caused declined TER, which was associated with downregulation of occludin and ZO-1.12, 15, 21, 30, 36, 37, 38 Our findings indicated that COM crystals caused increased permeability and defective barrier function of tight junction of renal tubular epithelial cells.
As a fence, tight junction prevents translocation of proteins from basolateral membrane to apical membrane (or vice versa) and maintains the cell polarity. Some investigators have proposed that fence and barrier functions of epithelial tight junction are not coordinated by showing that energy depletion in MDCK cells caused reduction only in TER but did not affect its fence function.40 However, many lines of evidences have suggested that occludin is involved in both barrier and fence functions.18, 36 We thus examined whether COM crystals also affected the fence function of tight junction. We employed a recently established method to isolate and purify apical membranes using peeling strategy based on physical property of filter paper.29 This peeling method is very simple, easy to follow (even by inexperienced hands), time saving and cost effective with a higher efficiency (as compared with the conventional methods) for isolation of apical membrane from polarized epithelial cells.29 Western blot analysis clearly confirmed that the fence function of tight junction was also affected by COM crystals, as Na+/K+-ATPase-α1, which is a marker for basolateral membrane, was also detectable in apical membrane compartment after the cells were exposed to COM crystals (Figure 5a). The defective fence function induced by COM crystals was also confirmed by immunofluorescence study (Figure 5b).
We also confirmed that COM crystals could induce oxidative stress to renal tubular epithelial cells, as the dihydrorhodamine assay revealed the significantly higher level of hydrogen peroxide in COM-treated cells (Figure 6). Interestingly, we observed that some COM crystals were internalized into polarized MDCK cells, whereas the majority remained adherent at apical surface (Figure 5b). Whether the intracellular or extracellular crystals triggered the cellular response remained to be elucidated. However, we hypothesize that the cellular responses observed in our study (that is, oxidative stress and defective tight junction) should be affected by both intracellular and extracellular crystals, which triggers oxidative stress. Subsequently, the oxidative stress or crystals (both intracellular and extracellular) per se cause defective tight junction, which leads to intercellular (paracellular) migration of intratubular COM crystals, and of calcium, oxalate and phosphate ions to the interstitium to initiate tubulointerstitial injury, inflammation and Randall's plaque formation.26, 41, 42
In summary, we report herein for the first time that COM crystals caused disruption of tight junction, accompanied with impairment of its barrier and fence functions. These data may, at least in part, explain renal tubulointerstitial injury/inflammation in kidney stone disease. Moreover, the defective tight junction may be a reasonable factor to link between ‘intratubular’ and ‘interstitial’ (Randall's plaque) theories of kidney stone disease.
References
Yeaman C, Grindstaff KK, Nelson WJ . New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev 1999;79:73–98.
Matter K, Balda MS . Signalling to and from tight junctions. Nat Rev Mol Cell Biol 2003;4:225–236.
Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993;123:1777–1788.
Morita K, Furuse M, Fujimoto K, et al. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 1999;96:511–516.
Martin-Padura I, Lostaglio S, Schneemann M, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 1998;142:117–127.
Stevenson BR, Siliciano JD, Mooseker MS, et al. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 1986;103:755–766.
Jesaitis LA, Goodenough DA . Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol 1994;124:949–961.
Haskins J, Gu L, Wittchen ES, et al. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol 1998;141:199–208.
Citi S, Sabanay H, Jakes R, et al. Cingulin, a new peripheral component of tight junctions. Nature 1988;333:272–276.
Matter K, Balda MS . Functional analysis of tight junctions. Methods 2003;30:228–234.
Rao RK, Baker RD, Baker SS, et al. Oxidant-induced disruption of intestinal epithelial barrier function: role of protein tyrosine phosphorylation. Am J Physiol 1997;273:G812–G823.
Sheth P, Basuroy S, Li C, et al. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem 2003;278:49239–49245.
Basuroy S, Seth A, Elias B, et al. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J 2006;393:69–77.
Li Q, Zhang Q, Wang C, et al. Disruption of tight junctions during polymicrobial sepsis in vivo. J Pathol 2009;218:210–221.
Rodriguez P, Heyman M, Candalh C, et al. Tumour necrosis factor-alpha induces morphological and functional alterations of intestinal HT29 cl.19A cell monolayers. Cytokine 1995;7:441–448.
Tazuke Y, Drongowski RA, Teitelbaum DH, et al. Interleukin-6 changes tight junction permeability and intracellular phospholipid content in a human enterocyte cell culture model. Pediatr Surg Int 2003;19:321–325.
Al Sadi RM, Ma TY . IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol 2007;178:4641–4649.
Balda MS, Whitney JA, Flores C, et al. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 1996;134:1031–1049.
Fanning AS, Jameson BJ, Jesaitis LA, et al. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 1998;273:29745–29753.
Rao RK . Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res 1998;22:1724–1730.
Atkinson KJ, Rao RK . Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol 2001;280:G1280–G1288.
Sawada N, Murata M, Kikuchi K, et al. Tight junctions and human diseases. Med Electron Microsc 2003;36:147–156.
Lee DB, Huang E, Ward HJ . Tight junction biology and kidney dysfunction. Am J Physiol Renal Physiol 2006;290:F20–F34.
Patel AB, Robertson WG, Choong S, et al. Heat-shock protein 25 ameliorates calcium oxalate crystal-mediated oxidative stress in renal epithelial cells. BJU Int 2006;98:1094–1099.
Schepers MS, van Ballegooijen ES, Bangma CH, et al. Crystals cause acute necrotic cell death in renal proximal tubule cells, but not in collecting tubule cells. Kidney Int 2005;68:1543–1553.
Khan SR . Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol 2004;8:75–88.
Habibzadegah-Tari P, Byer KG, Khan SR . Reactive oxygen species mediated calcium oxalate crystal-induced expression of MCP-1 in HK-2 cells. Urol Res 2006;34:26–36.
Thongboonkerd V, Semangoen T, Sinchaikul S, et al. Proteomic analysis of calcium oxalate monohydrate crystal-induced cytotoxicity in distal renal tubular cells. J Proteome Res 2008;7:4689–4700.
Fong-ngern K, Chiangjong W, Thongboonkerd V . Peeling as a novel, simple, and effective method for isolation of apical membrane from intact polarized epithelial cells. Anal Biochem 2009;395:25–32.
Seth A, Yan F, Polk DB, et al. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 2008;294:G1060–G1069.
Xu DZ, Lu Q, Swank GM, et al. Effect of heat shock and endotoxin stress on enterocyte viability apoptosis and function varies based on whether the cells are exposed to heat shock or endotoxin first. Arch Surg 1996;131:1222–1228.
Saier Jr MH . Growth and differentiated properties of a kidney epithelial cell line (MDCK). Am J Physiol 1981;240:C106–C109.
Feldman GJ, Mullin JM, Ryan MP . Occludin: structure, function and regulation. Adv Drug Deliv Rev 2005;57:883–917.
Yu AS, McCarthy KM, Francis SA, et al. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol 2005;288:C1231–C1241.
McCarthy KM, Skare IB, Stankewich MC, et al. Occludin is a functional component of the tight junction. J Cell Sci 1996;109 (Part 9):2287–2298.
Musch MW, Walsh-Reitz MM, Chang EB . Roles of ZO-1, occludin, and actin in oxidant-induced barrier disruption. Am J Physiol Gastrointest Liver Physiol 2006;290:G222–G231.
Patrick DM, Leone AK, Shellenberger JJ, et al. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma modulate epithelial barrier function in Madin-Darby canine kidney cells through mitogen activated protein kinase signaling. BMC Physiol 2006;6:2.
Vikstrom E, Tafazoli F, Magnusson KE . Pseudomonas aeruginosa quorum sensing molecule N-(3 oxododecanoyl)-l-homoserine lactone disrupts epithelial barrier integrity of Caco-2 cells. FEBS Lett 2006;580:6921–6928.
Pasternak AS, Miller WM . Measurement of trans-epithelial electrical resistance in perfusion: potential application for in vitro ocular toxicity testing. Biotechnol Bioeng 1996;50:568–579.
Mandel LJ, Bacallao R, Zampighi G . Uncoupling of the molecular ‘fence’ and paracellular ‘gate’ functions in epithelial tight junctions. Nature 1993;361:552–555.
Evan AP, Lingeman JE, Coe FL, et al. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 2003;111:607–616.
Bushinsky DA . Nephrolithiasis: site of the initial solid phase. J Clin Invest 2003;111:602–605.
Acknowledgements
We are grateful to Sakdithep Chaiyarit for his technical assistance. This study was supported by the Thailand Research Fund, the Commission on Higher Education and Mahidol University (to V. Thongboonkerd). P. Peerapen was supported by the Royal Golden Jubilee Ph.D. Program of the Thailand Research Fund. V. Thongboonkerd is also supported by ‘Chalermphrakiat’ Grant, Faculty of Medicine Siriraj Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Peerapen, P., Thongboonkerd, V. Effects of calcium oxalate monohydrate crystals on expression and function of tight junction of renal tubular epithelial cells. Lab Invest 91, 97–105 (2011). https://doi.org/10.1038/labinvest.2010.167
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2010.167
Keywords
This article is cited by
-
Vinegar reduced renal calcium oxalate stones by regulating acetate metabolism in gut microbiota and crystal adhesion in rats
International Urology and Nephrology (2022)
-
Alterations to microbial secretome by estrogen may contribute to sex bias in irritable bowel syndrome
Inflammopharmacology (2022)
-
More complete polarization of renal tubular epithelial cells by artificial urine
Cell Death Discovery (2018)
-
Decreased interaction between ZO-1 and occludin is involved in alteration of tight junctions in transplanted epiphora submandibular glands
Journal of Molecular Histology (2017)
-
p38 MAPK mediates calcium oxalate crystal-induced tight junction disruption in distal renal tubular epithelial cells
Scientific Reports (2013)