Abstract
Reduced nephron numbers may predispose to renal failure. We hypothesized that glucose transporters (GLUTs) may contribute to progression of the renal disease, as GLUTs have been implicated in diabetic glomerulosclerosis and hypertensive renal disease with mesangial cell (MC) stretch. The Os (oligosyndactyly) allele that typically reduces nephron number by ∼50%, was repeatedly backcrossed from ROP (Ra/+ (ragged), Os/+ (oligosyndactyly), and Pt/+ (pintail)) Os/+ mice more than six times into the Fvb mouse background to obtain Os/+ and +/+ mice with the Fvb background for study. Glomerular function, GLUT1, signaling, albumin excretion, and structural and ultrastructural changes were assessed. The FvbROP Os/+ mice (Fvb background) exhibited increased glomerular GLUT1, glucose uptake, VEGF, glomerular hypertrophy, hyperfiltration, extensive podocyte foot process effacement, marked albuminuria, severe extracellular matrix (ECM) protein deposition, and rapidly progressive renal failure leading to their early demise. Glomerular GLUT1 was increased 2.7-fold in the FvbROP Os/+ mice vs controls at 4 weeks of age, and glucose uptake was increased 2.7-fold. These changes were associated with the activation of glomerular PKCβ1 and NF-κB p50 which contribute to ECM accumulation. The cyclic mechanical stretch of MCs in vitro, used as a model for increased MC stretch in vivo, reproduced increased GLUT1 at 48 h, a stimulus for increased VEGF expression which followed at 72 h. VEGF was also shown to act in a positive feedback manner on MC GLUT1, increasing GLUT1 expression, glucose uptake and fibronectin (FN) accumulation in vitro, whereas antisense suppression of GLUT1 largely blocked FN upregulation by VEGF. The FvbROP Os/+ mice exhibited an early increase in glomerular GLUT1 leading to increased glomerular glucose uptake PKCβ1, and NF-κB activation, with excess ECM accumulation. A GLUT1–VEGF–GLUT1 positive feedback loop may play a key role in contributing to renal disease in this model of nondiabetic glomerulosclerosis.
Similar content being viewed by others
Main
The glomeruli constitute an important filtration barrier between the blood and urinary space, and prevention of glomerular injury is important to maintaining normal kidney function. Disparate insults resulting in an initial glomerular loss may predispose to later, additional loss of nephrons after the cessation of injury.1 Growing clinical and epidemiological evidence also indicates that an insufficient number of nephrons at birth is associated with the development of glomerulosclerosis in animals and humans, and may result in later development of end-stage kidney disease.2, 3
Brenner et al1, 3, 4 proposed that reduced nephron numbers may lead to renal failure from glomerular hypertension with glomerular hyperfiltration.3 Furthermore, previous studies have implicated glomerular hypertension in progressive renal disease after numerous different types of insults resulting in a reduced nephron number.3 Hyperfiltration may manifest as microalbuminuria and accelerated loss of renal function, and these changes are more prevalent in adults with a history of low birth weight.5 In addition, nephron deficiency accompanying low birth weight has been implicated in the development of renal failure in adulthood, cardiovascular disease, hypertension, hyperlipidemia, and diabetes.2 As noted by Drukker,2 the number of nephrons in animal and human kidneys correlates with renal mass. Accordingly, infants with low birth weights tend to be born with relatively smaller kidneys, and a nephron deficit. However, the underlying mechanism of the reduced nephron number in this situation has not yet been determined. Studies in rats and sheep have shown that a reduction in nephron mass during fetal development, or shortly after birth, favored the development of hypertension and renal disease with renal failure.6, 7 In humans, loss of sufficient kidney mass results in similar results, whereas transplantation of small kidney donor organs into large recipients increases the risk for hypertension and graft failure.8, 9 Furthermore, research in rats with oligomeganephronic hypoplastic kidneys associated with male hypogonadism showed development of glomerulosclerosis in some of the nephrons, and proteinuria.10 Humans with congenital oligomeganephronia have a reduced number of greatly enlarged glomeruli with renal hypoplasia.2 The glomeruli eventually sclerose leading to end-stage renal disease in early childhood.2 Therefore, a reduced nephron number tends to predispose to later renal damage.
In a previously described mouse model, studies have shown that ROP (Ra/+ (ragged), Os/+ (oligosyndactyly), and Pt/+ (pintail)) mice possessing the gene for oligosyndactylism (Os) represent a genetic model of reduced renal mass in which renal disease develops.11, 12, 13 Mice that carry the oligosyndactylism (Os) radiation-induced allele have a mutation in chromosome 8, with a disruption of the Anapc10 (Apc10/Doc1) gene.14 The Os allele may block progression of the cell cycle from metaphase to anaphase,15 and mice heterozygous for the Os allele have two prominent developmental defects: (1) fusion of the second and third digits of all four limbs16 and (2) a 50% reduction in the nephron number of the kidneys.11 Homozygous Os is lethal during gestation.17 Heterozygous Os mice may therefore be a useful model for identifying factors causing renal dysfunction. ROP mice heterozygous for the Os allele gradually develop moderately severe glomerulosclerosis, with increased glomerular cellularity, increased mean glomerular volume (MGV) and hypertrophic changes in the epithelium of proximal tubules and cortical collecting ducts.13 The reduced renal mass is associated with overexpression of extracellular matrix (ECM) proteins in the glomeruli, including collagen type IV and tenascin.12 The mice have not been found to develop renal failure or early death. In contrast, mice with the C57BL6 background heterozygous for the Os allele (ie, B6ROP Os/+ mice) did not develop significant renal disease. It was concluded that the development of glomerulosclerosis was dependent on the genetic background of the mice.13, 18, 19 However, the mechanisms involved in development of the renal disease remain to be determined, and multiple different genetic alterations may have roles in the glomerulosclerosis of ROP Os/+ mice.18
In this study, we generated and characterized Fvb mice with reduced renal mass due to the heterozygous Os allele. We described in a preliminary report that the FvbROP Os/+ mice have an 85% reduction in glomerular number.20 The FvbROP Os/+ mice were much more severely affected than were the C57BL6 (B6) or ROP mice carrying the Os allele. The FvbROP Os/+ mice developed marked albuminuria, rapidly progressive glomerulosclerosis, and early renal failure, which were lacking in the ROP Os/+ and B6 Os/+ mice.13, 21 The glomeruli in the FvbROP Os/+ mice are subjected to greater tension than in normal mice for a given pressure, due to their enlargement in the setting of deficient glomerular numbers and small kidneys.
Previous observations made by us and others indicated that glucose transporters (GLUTs) have been implicated in diabetic glomerulosclerosis22, 23 and in hypertensive renal disease with mesangial cell (MC) stretch.24 Changes in the expression of GLUT1 in MCs have been shown specifically to lead to parallel changes in glucose uptake and ECM production.22, 25 The GLUT1 in MCs is known to be upregulated by high extracellular glucose exposure and by cyclic mechanical stretch.24, 26 Glomerular hypertension increases glomerular GLUT1 expression, presumably by increasing the MC stretch.24 Furthermore, VEGF has been reported to be induced by MC stretch,27 and has been implicated in glomerular hypertrophy, glomerulosclerosis, and albuminuria.28, 29 We therefore hypothesized that GLUT1, glucose uptake, and VEGF could be increased in the glomeruli of FvbROP Os/+ mice, contributing to glomerulosclerosis and renal failure in this novel model.
The aims of the current studies were to characterize the new FvbROP Os/+ mouse model of rapidly progressive renal disease, and to investigate the potential roles of GLUT1 and VEGF in causing glomerular pathology in these nondiabetic, nonhypertensive mice. This new, highly susceptible mouse model should also provide clues regarding the pathogenesis of other renal diseases in which nephron numbers are reduced by various different disorders.
MATERIALS AND METHODS
Experimental Animals
Animal studies were approved by the Institutional Animal Care and Use Committee. ROP Os/+ mice (Jackson Laboratories, Bar Harbor, ME, USA) were backcrossed into the Fvb mouse line (Taconic Farms, Rockville, MD, USA) more than six times to generate FvbROP Os/+ mice with Fvb background carrying the Os allele (Dr D Pravtcheva, New York State Institute for Research in Developmental Disabilities). All the mice were maintained on standard rodent chow (Harlan Teklad no. 2918).
Measurements of Arterial Blood Pressure
A computer-automated tail-cuff blood pressure and pulse monitoring system (Hatteras, MC-4000 Blood Pressure Analysis System) was used to determine arterial blood pressure by averaging 20 serial measurements for each mouse after initial equilibration in restraining chambers. The pulse rate was determined using a photoelectric sensor placed over the tail.
Measurements in Serum and Urine
Tail blood samples obtained from nonfasting mice were used for measurements of blood glucose concentrations using an Accucheck Simplicity Glucometer, following the manufacturer's instructions (Roche Diagnostics, Indianapolis, IN, USA). Mice were housed in individual metabolic cages for 24-h urine collections before being killed. Urine albumin concentration was determined by competitive ELISA using the Albuwell-M Test Kit (Exocell, Philadelphia, PA, USA). Urine creatinine concentrations were determined using the Creatinine Companion Kit (Exocell). Results were expressed as the urine albumin to creatinine ratio (mcg albumin/mg creatinine). Creatinine (Cr) concentration in the plasma was assayed by high performance liquid chromatography as described previously.30 Creatinine clearance (CrCl), used as a marker for glomerular filtration rate, was determined from Cr (urine) × V (urine volume)/Cr (plasma).
Glomerular Isolation and Primary MC Culture
FvbROP Os/+ and FvbROP +/+ mice were killed at 4, 8, and 12 weeks of age for the study. The right kidney was used for histological and immunohistochemical studies. The glomeruli from the left kidney were used for primary MC culture or for glomerular protein lysates. Briefly, the left renal artery was first perfused with 2 ml PBS, followed by deactivated Dynabeads (M-450, Invitrogen, Carlsbad, CA, USA) or by iron oxide particles in 10 ml Hank's buffered salt solution. The perfused kidneys were minced and passed through a 100-μm mesh sieve. The glomeruli were then collected using a magnet.31 Some of the glomeruli isolated with Dynabeads were used for cell culture, whereas others were used to prepare glomerular protein lysates for western analyses. Glomerular isolates of >95% purity were obtained with this approach.
The glomeruli isolated from FvBROP Os/+ mice and their controls, and from antisense-GLUT1 transgenic mice32 and their controls, were cultured in MEM plus F12 medium (3:1) with 10% FBS, EGF (10 ng/ml), 8 mM glucose, and penicillin (100 U/ml)/streptomycin (0.1 mg/ml) to allow the MCs to grow out. Primary culture MCs were confirmed by positive staining with α-smooth muscle actin, along with confirmation of the typical MC morphology on light microscopy.22, 33 Primary culture MCs were studied at passages 5–10. Podocytes did not survive in the MC culture conditions.
Light Microscopy and Immunohistochemistry of Kidney Tissues
Renal tissues were fixed in 4% paraformaldehyde in PBS (pH 7.4) for 24 h at 4 °C. After fixation, tissue samples were embedded in paraffin wax. Tissue sections of 5 μm thickness were used for staining with Mayer's hematoxylin, Putt's eosin, periodic acid-Schiff (PAS), or Masson's trichrome for histological and pathological examination of the glomeruli and tubulointerstitium. In addition, MGV was determined using the method described by Najafian et al,34 and mean capillary diameters (MCDs) were determined from measurements of maximum capillary loop diameters on images of PAS-stained glomeruli all at the same magnification. The mean±s.e.m. capillary diameter was then calculated for mice of each genotype for comparison.
In all, 5–8 kidney sections per group of animals were obtained for assessments of glomerulosclerosis, with ≥20 glomeruli per section. The degree of glomerulosclerosis was expressed as the mesangial index, ie, the percentage cross-sectional area of the glomerular tuft that was PAS positive. Histological preparations were examined using an Olympus BX60 microscope (Olympus, Melville, NY, USA), at magnifications of × 100 and × 400, and a photographic record was obtained using a Zeiss Axiocam camera.
Immunohistochemical staining with various antibodies including GLUT1 (1:400, Alpha Diagnostic International, San Antonio, TX, USA), VEGF (1:200, Lab Vision, CA, USA), tenascin, phospho-PKCα (p-PKCα), and PKCβ1 (all 1:100, Santa Cruz Biotechnology, CA, USA), fibronectin (FN) and laminin (both 1:100, Sigma-Aldrich, St Louis, MO, USA), collagen IV (1:100, Santa Cruz Biotechnology, against α-1, α-3, and α-5 chains), CD2AP (1:100, Abcam, Cambridge, MA, USA), nephrin and podocin (both 1:100, kind gifts from Dr L Holzman, University of Michigan) was conducted using a Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA) with some modifications. Briefly, 5 μm sections were deparaffinized, and endogenous peroxidase activity was ablated by incubation in 3% hydrogen peroxide in TBS (pH 7.4). Epitope retrieval was carried out on sections by microwave treatment (2 × 5 min in 100 mM sodium citrate, pH 6). Sections were sequentially blocked for nonspecific staining (diluted normal serum, 1:67, 60 min), and endogenous biotin (avidin/biotin blocking kit, Vector Laboratories, 30 min per step). Blocking was followed by incubation with affinity-purified antibody, control IgG, or antibody preadsorbed with blocking peptide (1 h at room temperature or overnight at 4 °C). The sections were then washed in TBS and incubated with biotinylated secondary antibody for 60 min. After further washes with TBS, the sections were incubated with an avidin-biotinylated horseradish peroxidase complex for 1 h. Finally, the sections were washed in TBS, then processed to achieve color development with 3, 3-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich), 0.0075% nickel chloride, 0.0075% cobalt chloride, and 0.0075% hydrogen peroxide for 5–10 min, and counterstained with Mayer's hematoxylin. Staining of kidney tissue from the untreated kidney or staining with nonimmune serum or rabbit IgG was performed for controls.
Detection of NF-κB p50, p65, and Nuclear Translocation in Glomerular Cells
Immunohistochemical staining for NF-κB p50 and NF-κB p65 (antibodies both 1:100, Santa Cruz Biotechnology) was performed using a Vectastain Elite ABC kit as described above. DAB staining was replaced by using the ‘Vector Red’ staining kit (Vector Laboratories). Mayer's hematoxylin stain was replaced by DAPI (Molecular Probes, Eugene, OR, USA) staining. Digital images of the Vector Red immunoperoxidase staining (NF-κB p50 or NF-κB p65) and the immunofluorescent nuclear stain DAPI (blue), were merged to identify NF-κB staining (red-brown) in the nuclei (DAPI blue) with their co-localization producing a violet color. The percentage of glomerular nuclei double stained for DAPI and NF-κB in each glomerular tuft cross-section examined was determined for the FvbROP +/+ and FvbROP Os/+ glomeruli, as a marker for NF-κB activation.
Glucose Uptake Rates in Primary MCs and Isolated Glomeruli
Glucose uptake rates of primary culture mouse MCs were determined as described previously.22, 25 Glucose uptake rates of isolated glomeruli were performed as described in our previous approach with some modifications.22 Briefly, fresh glomeruli isolated using the Dynabead method31 from individual mice were suspended in PBS with 0.1 μCi/ml 3H2-deoxyglucose (3H2-DOG, NEN-Perkin Elmer, Wellesley, MA, USA) for 10 min (linear portion of the glucose uptake curve for the freshly isolated glomeruli). An aliquot of each glomerular preparation was then taken for measuring total protein to calculate the quantity of glomerular protein in each sample. Subsequently, the glomeruli were exposed to cold stop solution with 0.1 mM phloretin, and isolated by brief centrifugation at 1000 r.p.m. (3 min). The glomeruli were then placed in scintillation cocktail for measurements of 3H activity using a Packard 1500 scintillation counter. The results of DPM per 10 min were then used to back calculate total DPM per 10 min in each sample. This number was then converted to nmol 2-DOG per 10 min, and normalized to the total glomerular protein content (μg) in the respective samples. The results of the glucose uptake rate measurements were then expressed as percentage of control, and compared between the different groups being studied.
Cyclic Mechanical Stretch of Cultured MCs
Rat MCs derived from the continuous line 16KC222 were cultured in flexible 6-well plates (FLEX-1) coated with type-1 collagen. Mouse MCs from the FvbROP Os/+ mice were found to be very difficult to grow in culture. Therefore, rat MCs were used for in vitro stretch studies. Rat MCs were maintained in RPMI 1640 medium (Gibco) supplemented with 20% NuSerum IV, 100 U/ml penicillin, 0.1 mg/ml streptomycin (Life Technologies, Gaithersburg, MD, USA), and D-glucose to a final concentration of 8 mM. After the cells reached 80% confluence, the NuSerum IV was decreased to 1% for 24 h to arrest cell proliferation before beginning the stretch experiments. MCs were stretched in a Flexercell® system at 60 cycles per minute for 48 and 72 h with 10% linear elongation. The cells were then washed with cold PBS, and total proteins were harvested with lysis buffer A (50 mM Tris HCl pH 8.0, 2 mM EDTA, 1% Triton-X100, 312.5 mM N-ethylmalemide, and 0.2% SDS), supplemented with protease inhibitor cocktail (Complete Mini, Roche Diagnostics) and PMSF. The cells were vortexed for 30 s and the lysate was centrifuged at 14 000 r.p.m. for 15 min; the supernatant was then collected and frozen at −80 °C until performance of Western analyses.
Immunostaining for VEGF Receptors in Cultured Mouse MCs
Antibodies specific for VEGFR1 and VEGFR2 receptor proteins were obtained from Abcam and Cell Signaling Technology (Danvers, MA, USA), respectively. The antibodies were each diluted 1:50 for immunostaining in the cultured MCs. The MCs were seeded to 8-well glass chamber slides (1 × 104 cells per well) and allowed to attach overnight. On the subsequent day, nonconfluent primary culture mouse MCs were fixed in acetone and stained for the different receptors using a method described previously.25
Treatment of Cultured MCs with VEGF-A165
Primary culture mouse MCs from C57BL6 mice and antisense-GLUT1 transgenic mice with the C57BL6 background were maintained in MEM+F12 medium, (3:1, Gibco/Invitrogen), 10% FBS, EGF (10 ng/ml, Sigma-Aldrich), 8 mM glucose, and (100 U/ml) penicillin/(0.1 mg/ml) streptomycin (Life Technologies). The antisense-GLUT1 MCs were isolated from our GT1AS antisense-GLUT1 transgenic mouse line with the C57BL6 background.32 The glomeruli of these mice have a 50% reduction in GLUT1 protein. The MCs from these antisense-GLUT1 mice also exhibit an ∼50% reduction in GLUT1 protein and a similar reduction in glucose uptake rate (unpublished data). The cells were grown to ∼80% confluence; the serum was then decreased to 1% for 24 h to arrest cell growth. The MCs were treated with or without 2 ng/ml of mouse recombinant VEGF-A165 protein (Alpha Diagnostic International) for 48 h, adding fresh VEGF-A165 every 24 h, then washed with cold PBS, and the cell proteins were harvested with lysis buffer A containing PMSF. In separate experiments, 3H2-deoxyglucose uptake rates were determined in MCs±VEGF-A165 (2 ng/ml).
Western Analyses
Isolated glomeruli and harvested cells were homogenized with lysis buffer A plus Cocktail Inhibitor of Protein Complete Mini (Roche Diagnostics) with PMSF. Protein concentrations were determined by the bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL, USA). Equal amounts of protein from the samples were subjected to SDS-PAGE and electrophoretically transferred into a polyvinylidene diflouride membrane (Millipore, Bedford, MA, USA). The membranes were then blocked with 5% nonfat dry milk in TBST for 1 h at room temperature and incubated with primary antibody in TBST, anti-GLUT1 antibody (1:2500 dilution, Alpha Diagnostic International), anti-VEGF antibody (1:1000 dilution, Lab Vision), anti-FN antibody (1:1000 dilution, Sigma-Aldrich), anti-collagen IV antibody (1:1000 dilution, Santa Cruz Biotechnology), anti-PKCβ1 antibody (1:500 dilution, Santa Cruz Biotechnology), anti-PKCα antibody (1:500 dilution, Santa Cruz Biotechnology), or anti-tubulin antibody (1:1000, Santa Cruz Biotechnology), followed by incubation with horseradish peroxidase-conjugated anti-rabbit IgG (1:1000 dilution; Pierce Biotechnology) and washed with TBST. The signals were detected with SuperSignal West Dura Extended Duration Substrate (Pierce Biotechnology). Endogenous β-tubulin protein was used as an internal control. Signals for each protein were quantified with the NIH image v.1.62 program.
TEM to Analyze Glomeruli
The renal cortical tissue was diced into 1 mm blocks for electron microscopy, fixed overnight at 4 °C by immersion in half-strength Karnovsky's solution (2.5% glutaraldehyde–2% paraformaldehyde), and then transferred into 0.1 M cacodylate buffer for storage (at 4 °C). The EM tissue was embedded in Epon and processed in house. Samples were stained with uranyl acetate and lead citrate. Sections were examined using an Hitachi HS-9 electron microscope (Hitachi, Pleasanton, CA, USA) at 60 kV.
Measurements of Glomerular Basement Membrane Thickness
Random transmission electron microscopy (TEM) samples of the glomeruli were used to measure thickness of the glomerular basement membrane (GBM) in FvbROP +/+ and Os/+ mice at 4 weeks of age using orthogonal intercepts.35 More than 160 serial measurements of GBM width by orthogonal intercepts on randomly chosen samples were performed for each genotype of mouse, and the mean±s.e.m. was determined for each.
Assessments of Podocyte Foot Process Effacement
Random TEM samples from FvbROP +/+ and Os/+ mice were used to examine the extent of podocyte foot process effacement at 4 weeks of age. All capillary loops imaged from the examined glomeruli were evaluated for the presence or absence of this abnormality, with n=19–28 capillary loops for each genotype from the TEM images. The percentage of capillary loops affected by the foot process effacement was determined for mice of each genotype for comparison.
Statistics
Power analyses were performed using the G*Power3 software program. Data are reported as the mean±s.e.m. for individual groups. ANOVA was performed for analysis of serial data groups. Results that provided a significant F-statistic were pursued further with Student's t-test. Intergroup comparisons were made by Student's t-test or by χ2 analysis, where appropriate. Student's t-test was used to compare results obtained from two different groups, where n≥3. A formula designed for experiments with small sample sizes was used: t=mean1−mean2/square root of ((s.d.1)2/n1+(s.d.2)2/n2), where indicated. P<0.05 was considered significant for statistical comparisons.
RESULTS
Kidney Development, Glomerular Morphometrics, and Metabolic and Physiological Studies
The Os allele produced a 43% reduction in kidney weight in the FvbROP Os/+ mice at 8 weeks of age compared with the FvbROP +/+ control mice (P<0.0001, Figure 1a). Despite the reduced kidney mass, FvbROP Os/+ mice were neither hypertensive nor diabetic (Figure 1b). The MCD and MGV were both increased more than 1.5-fold when compared with the FvbROP +/+ control mice (Figure 1c and d). The body weights in the FvbROP Os/+ mice were not significantly different from the FvbROP +/+ mice at 4 and 8 weeks of age (Table 1). However, kidney weight/body weight ratios were significantly lower in the FvbROP Os/+ mice (Table 1).
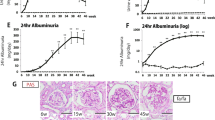
(a–e) Effects of the Os allele on kidney size, hemodynamic, and metabolic parameters. (Panel a) Representative images of kidney sizes in FvbROP Os/+ mice and FvbROP +/+ control mice. (b) Graph of arterial blood pressures (mm Hg) and blood glucose levels (mg/dl) at 4 weeks of age for FvbROP +/+ controls and FvbROP Os/+ mice (n=6–9 mice in each group). (c) MCD in the glomeruli is compared between FvbROP +/+ and FvbROP Os/+ mice at 4 weeks of age (n=3 mice in each group, n=22–28 measurements per mouse). (d) MGV is compared between FvbROP +/+ and FvbROP Os/+ mice at 4 weeks of age (n=3 mice in each group, n=23 measurements per mouse). (e) Changes in creatinine clearance (CrCl), CrCl/g kidney, and plasma creatinine concentration in FvbROP Os/+ mice and FvbROP +/+ control mice at ages 4 and 8 weeks. CrCl was already reduced at 4 weeks of age in FvbROP Os/+ mice, and was further reduced at 8 weeks of age. The CrCl/g kidney was significantly increased in FvbROP Os/+ mice at 4 weeks of age, suggesting hyperfiltration in the remaining glomeruli. By 8 weeks of age, this hyperfiltration had disappeared. The plasma creatinine was increased 60% at 4 weeks of age in FvbROP Os/+ mice, and increased further to 100% above the FvbROP +/+ controls by 8 weeks of age, with P<0.03 vs the plasma creatinine in FvbROP Os/+ mice at 4 weeks of age (n=3 mice in each group). *P<0.001 and **P<0.05 vs controls in all measurements.
Food intake was similar between the different types of mice; however, both urine output and fluid intake were significantly higher in the FvbROP Os/+ mice (Table 1), a finding noted with the Os allele on another mouse background.36 Specifically, the FvbROP Os/+ mice drank 1.5-fold more water than did the FvbROP +/+ mice at 4 weeks of age (P=0.038), and 1.8-fold more at 8 weeks of age, (P=0.001), Table 1. The FvbROP Os/+ mice also produced 2.6-fold more urine than did the FvbROP +/+ mice at both 4 (P=0.012) and 8 weeks of age (P=0.011), as shown in Table 1.
Albuminuria and CrCl Measurements
The FvbROP Os/+ mice exhibited heavy albuminuria from the earliest time point assessed at age 4 weeks (Table 1). The ratio of albumin to creatinine in the urine of FvbROP Os/+ mice at 4 weeks of age was 188-fold higher than in the FvbROP +/+ control mice (P=0.00008), and was 94-fold higher than in the FvbROP +/+ mice at 8 weeks of age (P=0.0001, Table 1). The lesser increase in the albumin/creatinine ratio for FvbROP Os/+ mice at 8 weeks of age resulted largely from increased albuminuria in controls at that age, rather than decreased total albumin excretion in the FvbROP Os/+ mice. The FvbROP Os/+ mice also had substantial glomerular hyperfiltration at age 4 weeks (Figure 1e), which decreased over time such that by 8 weeks of age the CrCl/g kidney was back to the control level, and by 12 weeks of age the CrCl/g kidney was below control levels. Plasma creatinine levels were already elevated 60% in the FvbROP Os/+ mice at 4 weeks of age (P<0.05, Figure 1e) and were further increased to 100% above controls at 8 weeks of age (P<0.03 vs plasma creatinine at age 4 weeks, Figure 1e), preceding the death of the animals which began at ∼12 weeks of age.
Renal Histology
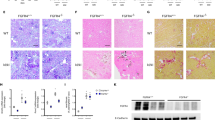
To evaluate the mice for development of glomerulosclerosis, PAS staining and Masson's trichrome staining were performed on kidney sections from FvbROP Os/+ and FvbROP +/+ mice at 4, 8, and 12 weeks of age. PAS staining indicated that the FvbROP Os/+ mice had developed severe glomerulosclerosis by 4 weeks of age, and that the matrix accumulation in the glomeruli consistently increased from 8 to 12 weeks when the mice began to die (Figure 2a and b). In addition, tubular enlargement was detected at 4 weeks of age with development of interstitial fibrosis by 8 weeks of age (not shown). Glomerulosclerosis assessed by the mesangial index in the FvbROP Os/+ mice was increased from 45 to 83% above FvbROP +/+ control levels during the period of 4, 8, and 12 weeks of age (P<0.001, Figure 2b). Masson's trichrome staining indicated that the FvbROP Os/+ mice had extensive glomerular collagen accumulation by 4 weeks of age, which was increased from 125% to more than 200% above the levels in FvbROP +/+ control mice at 4 to 12 weeks of age (Figure 2c and d). The increase in glomerulosclerosis with time in the FvbROP Os/+ mice was associated with a decrease in the CrCl/g kidney over time, and an increase in the plasma creatinine concentration. In contrast, FvbROP +/+ control mice did not develop significant glomerulosclerosis or renal failure.
(a–f) Glomerulosclerosis (GS) in FvbROP mice with and without the Os allele. (Panel a) PAS-stained kidneys from FvbROP +/+ and FvbROP Os/+ mice at ages 4, 8, and 12 weeks (n=4–6 mice in each group). (b) Graph of the mesangial index (glomerulosclerosis) for FvbROP +/+ and FvbROP Os/+ mice at 4, 8, and 12 weeks of age (n=4–6 mice in each group). (c) Masson's trichrome-stained kidneys from FvbROP +/+ and FvbROP Os/+ mice at ages 4, 8, and 12 weeks (n=4–6 mice in each group). (d) Graph of glomerular collagen scoers for FvbROP +/+ and FvbROP Os/+ mice at 4, 8, and 12 weeks of age (n=4–6 mice in each group). (e) Western blot of glomerular FN in FvbROP Oscores/+ mice at 4 weeks of age (n=3–6 mice in each group). (f) Graph of western blot data for the glomerular FN protein level in FvbROP Os/+ and FvbROP +/+ mice at age 4 weeks. In all measurements, *P<0.01 and **P<0.05 vs control.
ECM Protein Deposition in the Glomeruli
Deposition of individual ECM proteins in the glomeruli was determined by Western and immunohistochemical analyses. As noted above, the FvbROP Os/+ mice rapidly developed heavy albuminuria and glomerulosclerosis by 4 weeks of age. In this study, the kidneys were assessed for specific ECM proteins that could contribute to this glomerulosclerosis. The glomeruli were analyzed by both immunolabeling of fixed kidney sections and by Western analyses of proteins from the isolated glomeruli. FN had substantially accumulated in the glomeruli of the FvbROP Os/+ mice by 4 weeks of age. In addition, collagen accumulation in the glomeruli of the FvbROP Os/+ mice, as assessed by Masson's trichrome staining, was also quite substantial by this time point, and also at 8 and 12 weeks of age. However, type IV collagen was not increased, and the glomerular collagen which had accumulated was therefore of another type. Glomerular tenascin and laminin were also found not to be increased in the FvbROP Os/+ mice. Glomerular FN protein was increased ∼3.5-fold at 4 weeks of age (P<0.01) in the FvbROP Os/+ mice, as determined by Western analysis (Figure 2e and f).
TEM of the Kidneys
The kidney tissues of FvbROP Os/+ mice at 4 weeks of age were used for analysis by TEM, which detected heavy matrix deposition in the glomeruli of FvbROP Os/+ mice (Figure 3b). Effacement of glomerular podocytes was also very prominent in the glomeruli of FvbROP Os/+ mice at 4 weeks of age, and this change was accompanied by a 10-fold greater frequency of glomerular capillary loops exhibiting podocyte foot process effacement (P<0.01, Figure 3d and e). Moreover, quantitation of GBM thickness by the orthogonal intercept method detected a 65% increase in FvbROP Os/+ mice (P<0.01 vs FvbROP +/+ mice, Figure 3g and h). All these changes were associated with marked albuminuria in the FvbROP Os/+ mice as described earlier.
(a–h) TEM images of glomeruli. (Panels a and b) Arrows indicate substantial mesangial matrix accumulation in FvbROP Os/+ mice. (c and d). Arrows indicate glomerular podocyte foot processes, where podocyte foot process effacement was far greater in FvbROP Os/+ glomeruli. (e) Graph of the frequency of glomerular capillary involvement with podocyte foot process effacement in FvbROP +/+ and FvbROP Os/+ mice (n=3 mice for each group, n=6–8 glomerular capillaries counted/mouse). (f and g) Arrows indicate GBM in the affected mice. (h) Graph of GBM thickness (n=3 mice for each group, n=2–3 glomerular basement membrane loops measured per mouse). *P<0.001 vs control.
Glomerular Expression of GLUT1, VEGF, PKCβ1, p-PKCα, PKCα, and TGFβ1
Severe glomerulosclerosis developing in FvbROP Os/+ mice indicated activation of prosclerotic factors. Therefore, we investigated the expression of candidate proteins that might be involved in this process. We found increases in glomerular GLUT1, VEGF, and PKCβ1 expression by both Western analyses of glomerular proteins (Figures 4a and c) and immunolabeling of kidney tissue sections (Figures 4b). Western analyses of glomerular GLUT1 showed 2.7- (P<0.05) and 1.9-fold (P<0.05) increases at ages 4 and 8 weeks (not shown), respectively in the FvbROP Os/+ mice (Figure 4a and c). The glomerular glucose uptake rate was increased 2.7-fold in the FvbROP Os/+ mice (Figure 4d) in association with the increased GLUT1. PKCβ1 protein was increased in the glomeruli of FvbROP Os/+ mice, with a perinuclear pattern of localization, indicating its activation there (Figure 4b). Western analyses for total glomerular PKCβ1 showed a 3.5-fold increase in this protein in FvbROP Os/+ mice at 4 weeks of age, which returned to baseline by 8 weeks of age (Figure 4a and c). Active PKCα (p-PKCα) exhibited a nonsignificant trend toward increase in FvbROP Os/+ mice, and total PKCα was also not significantly increased (Figure 4a and b). Furthermore, glomerular TGFβ1 expression and PCNA immunolabeling were not significantly changed in the FvbROP Os/+ mice at 4 weeks of age (not shown). In contrast, glomerular VEGF (Figure 4a and c) was increased ∼2.9-fold above controls in the FvbROP Os/+ mice at 4 weeks of age (P<0.05), but was not significantly increased at 8 weeks of age (P>0.05).
(a–d) Glomerular gene expression and glucose uptake rates in FvbROP Os/+ and FvbROP +/+ mice. (a) Western analyses of glomerular GLUT1, VEGF, PKCβ1, and PKCα in FvbROP+/+ and FvbROP Os/+ mice at 4 weeks of age (n=3–4 mice in each group). (b) Immunostaining for VEGF, PKCβ1, and active p-PKCα protein in FvbROP +/+ and FvbROP Os/+ mice at 4 week of age. (c) Bar graph of Western blot data for glomerular GLUT1, VEGF, and PKCβ1 in FvbROP +/+ and FvbROP Os/+ mice at 4 and 8 weeks of age (Western blots of the above proteins from mice at 8 weeks of age are not shown). (d) Glomerular glucose uptake rates (3H2-DOG) in FvbROP +/+ and FvbROP Os/+ mice at 4 weeks of age (n=3–4 mice in each group). *P<0.001 and **P<0.05 vs control.
As the FvbROP Os/+ mice developed marked albuminuria at 4 weeks of age, selected podocyte proteins in the slit diaphragm and proteins involved in attaching podocytes to the GBM were investigated in the FvbROP Os/+ vs FvbROP +/+ mice. No significant changes were detected for nephrin or podocin in the FvbROP Os/+ mice at 2 weeks of age (not shown), or for CD2AP protein at 4 weeks of age (not shown), by immunolabeling and assessment of the glomeruli.
Examination of NF-κB and its Activation in the Glomeruli
The transcription factor NF-κB may respond to both PKC and VEGF; therefore, we investigated its potential activation in the FvbROP Os/+ mice at 4 weeks of age. The glomeruli of both FvbROP +/+ and FvbROP Os/+ mice exhibited substantial immunolabeling for NF-κB p50, and much less labeling for NF-κB p65. Translocation of the NF-κB subunits to the nucleus of glomerular cells (ie, activation) was investigated by double immunolabeling for NF-κB subunits (Vector Red immunoperoxidase, red-brown) and cell nuclei (DAPI, blue). NF-κB p50 was found to exhibit 70% greater activation (nuclear localization, P<0.01) in glomerular cells of the FvbROP Os/+ mice than in the FvbROP +/+ mice (Figure 5a and b), and NF-κB p50 was activated to a greater extent than was NF-κB p65 (Figure 5b). The glomeruli of FvbROP Os/+ mice by age 4 weeks also had 27% more cells/tuft cross-section than did the glomeruli from FvbROP +/+ control mice, when assessed by numbers of nuclei (P<0.05, not shown). However, PCNA immunolabeling to assess for cell proliferation in the glomeruli was not significantly different between the FvbROP Os/+ and FvbROP +/+ mice at this time point (not shown).
(a and b). Activation of glomerular NF-κB in FvbROP mice with the Os allele. (a) Immunostaining of NF-κB p50 and p65 in both the FvbROP +/+ and FvbROP Os/+ mice at 4 weeks of age. The FvbROP Os/+ mice exhibited 70% increased nuclear translocation (activation) of NF-κB p50 in their glomeruli as evidenced by the overlapping immunolabeling for p50 (red-brown) and DAPI (blue) to produce a violet signal. The low level of active NF-κB p65 in FvbROP +/+ glomeruli did not appear to increase in the FvbROP Os/+ mice (n=17 glomerular measurements in each group). (b) Bar graph of nuclear translocation of NF-κB subunits p50 and p65 in the glomeruli of FvbROP +/+ and FvbROP Os/+ mice at 4 weeks of age. **P<0.05 vs control.
MC Expression of GLUT-1, VEGF, and ECM Proteins in Response to Cyclic Mechanical Stretch In Vitro
The FvbROP Os/+ mice have a reduced nephron number due to the Os allele, resulting in glomerular hypertrophy with increased volume, increased capillary diameter, and hyperfiltration. Increased capillary diameter associated with glomerular hypertrophy increases MC tension and stretch for any given pressure, as described by LaPlace's law and Kriz' description of glomerular architecture.37, 38 Therefore, to examine the relationships between MC GLUT1, VEGF, and ECM expression in response to stretch, we used a Flexercell® system programmed to induce a cyclic mechanical stretch of cultured MCs in vitro as described in the ‘Materials and methods’ section for 48 and 72 h. MCs isolated from the FvbROP Os/+ and FvbROP +/+ mice revealed that the former were very difficult to maintain and grow in culture. The FvbROP Os/+ MCs, when they survived, grew far slower than did the FvbROP +/+ MCs. This characteristic might result from the known effect of the Os allele to impair progression of cell division from metaphase to anaphase.39 Therefore, we instead used a line of rat MCs for the in vitro cell stretch studies (see the section ‘Materials and methods’) and these cells attached well to the FlexCell plates.
Western analyses of MC proteins from different time points showed an early three-fold increase in MC GLUT1 protein at 48 h (P<0.05) (Figure 6a and c), which was sustained at 72 h (not shown). The increase in GLUT1 preceded an increase in VEGF (2.2-fold, P<0.01) which became detectable at 72 h, but not at the earlier 48 h time point (Figure 6b and c). Subsequent examination for expression of the VEGF receptors, VEGFR1 and VEGFR2, in MCs by immunolabeling with specific antibodies (Abcam, Cambridge, England and Cell Signaling Technology) showed they were both expressed in primary culture mouse MCs (not shown).
(a–c) Cyclic mechanical stretch induction of MC GLUT1 and VEGF expression. (a) GLUT1 protein levels were increased at 48 h of stretch, and remained elevated at 72 h (not shown). (b) VEGF protein expression was increased at 72 h of stretch, but not at 48 h of stretch (not shown). (c) Graph of GLUT1 protein and VEGF with and without cyclic mechanical stretch in cultured MCs. **P<0.05 vs controls.
As VEGF was increased in the glomeruli of FvbROP Os/+ mice, and the GLUT1 gene is known to contain growth factor-responsive elements, we investigated the potential responses of mouse MC GLUT1 and glucose uptake, plus FN, to VEGF treatment. We have previously shown that high GLUT1 expression induces FN expression in GLUT1-transduced MC.22 As FvbROP Os/+ MC did not grow well in culture, we used C57BL6 mouse MC. These primary culture mouse MCs were serum-deprived then treated with or without VEGF-A165, (2 ng/ml) for 48 h. This was a time point by which MC responses could be detected. MC GLUT1 increased 2.1-fold in response to VEGF-A165 (Figure 7a and b) and the glucose uptake rate increased 3.6-fold (Figure 7c), whereas FN expression increased 3.3-fold (Figure 7d and f). Next, primary culture MCs from antisense-GLUT1 transgenic mice (C57BL6 background) were treated with or without VEGF-A165, 2 ng/ml), for 48 h. FN expression increased only 1.6-fold in the GLUT1-deficient cells (Figure 7e and f), indicating a role for GLUT1 in VEGF-induced MC FN expression.
(a–f) Role of GLUT1 in VEGF-induced MC FN production. (a) Primary culture mouse MCs with the C57BL6 background were studied in culture with and without VEGF-A165 peptide (2 ng/ml) for 48 h. VEGF-A165 stimulated a two-fold increase in MC GLUT1 protein. (b) Graph of western blot data from MCs treated with VEGF. **P<0.05, n=4 in each group). (c) Graph of 3H2-deoxyglucose (DOG) uptake rates in cultured MCs treated with or without VEGF-A165 2 ng/ml for 48 h, **P<0.05 for VEGF-A165-treated cells vs controls, n=4 samples per group. The glucose uptake rate increased 3.6-fold in response to treatment with VEGF-A165. (d) FN protein increased 3.3-fold in response to the VEGF-A165 treatment, n=3 in each group. (e) In comparison, FN increased only 1.6-fold in cultured MCs from antisense-GLUT1 transgenic mice in response to the 2 ng/ml VEGF-A165 treatment for 48 h. (f) Graph of western blot data from MCs isolated from normal and antisense-GLUT1 transgenic mice, treated with VEGF. *P<0.01 and **P<0.05 vs controls.
DISCUSSION
Chronic renal failure is a disorder achieving epidemic proportions.40, 41 Once a critical mass of nephrons is lost, the kidneys tend to lose additional nephrons over time which can lead to progressive renal failure and the need for dialysis or transplantation.2, 3, 4, 42 The causes of this relentless progression to end-stage renal disease have been the subject of intensive study over recent decades, and glomerular hypertension with hyperfiltration has been implicated in a progressive glomerular scarring process over time.1, 3 The use of ACE inhibitors and angiotensin receptor blockers (ARBs) has been promoted to slow the progression of chronic renal disease, particularly that due to diabetes mellitus, but they also seem to have a benefit in chronic nondiabetic renal disease.43, 44, 45 Much of the data supporting this mechanism comes from the remnant kidney model in rats,1, 42, 46, 47 and also in mice.47 Although the treatment of glomerular hypertension and hyperfiltration with ACE inhibitors or ARBs seems to be helpful in slowing the progression of renal disease in some disorders, these treatments are not curative. Other factors are likely involved in the progression of chronic renal disease and some of those potential factors have been addressed in the present studies. In this study, we produced and characterized a new model of rapidly progressive, nondiabetic glomerulosclerosis on the Fvb mouse background. We examined the expression of candidate glomerular proteins involved in the process, and identified a GLUT1-VEGF positive feedback mechanism which may be important in the pathogenesis of progressive renal disease resulting from reduced nephron number.
The new FvbROP Os/+ mouse model described in this study is a model of reduced nephron number and reduced kidney mass resulting from the presence of the Os allele on the Fvb background. These mice are unique in that they develop a rapidly progressive glomerulosclerosis with severe albuminuria and early renal failure, ending in their premature demise. Such severe renal disease has not been observed when the Os allele is present on the C57BL6 or ROP genetic backgrounds.19 Only when the Os allele was mated from the ROP background into the Fvb background did the severe phenotype develop. C57BL6 mice develop little to no renal pathology with the heterozygous Os allele,13 and ROP mice heterozygous for Os develop a moderately severe glomerulosclerosis which does not progress to renal failure, shorten the lifespan, or produce marked albuminuria.11, 13, 19 Therefore, there is something uniquely different about the Fvb background which makes the mice much more susceptible to the Os allele with reduced nephron number. In reviewing the literature, even ROP mice surgically treated to produce a remnant kidney model (5/6 nephrectomy or ∼83% reduction in nephrons) 47 did not develop such severe disease as that observed in the FvbROP Os/+ mice described in this study. Although a reduced nephron number may act as a stimulus to further renal disease, the genetic background of the animals seems to determine the extent of renal disease that develops.
In the studies reported herein, the Os allele led to reduced kidney mass as expected, with glomerular hypertrophy and glomerular hyperfiltration. The FvbROP Os/+ mice exhibited early increases in glomerular GLUT1, glucose uptake, and VEGF that preceded the development of severe glomerulosclerosis, and likely contributed to glomerular pathology. GLUT1 and VEGF have both been implicated in the development of glomerular disease in diabetes mellitus, and more recently in hypertensive renal disease (GLUT1) or the remnant kidney (VEGF).24, 26, 28 In contrast, glomerular TGFβ1 was not increased at either 4 or 8 weeks of age in the FvbROP Os/+ mice. VEGF has previously been implicated in albuminuria, although the results have been variable.28, 29, 48 In this study, its early, high expression in the FvbROP Os/+ mice was associated with a marked albuminuria at 4 weeks of age, and the decrease in glomerular VEGF by 8 weeks of age was associated with an insignificant trend for reduction in the albuminuria. The glomerular GLUT1 level remained elevated when reassessed at 8 weeks and 3 months of age as the glomerular disease progressed, whereas the elevation of glomerular VEGF was no longer statistically significant by 8 weeks of age. The differences in GLUT1 and VEGF expression between the FvbROP Os/+ mice and FvbROP +/+ control mice was greatest at 4 weeks of age.
We have previously found the PKC signaling system to be activated by overexpression of GLUT1 in kidney MCs.49, 50 Furthermore, PKCβ and NF-κB have been found to be activated under high glucose conditions,51 a situation in which MC GLUT1 is increased.26 Therefore, we examined this signaling system in the FvbROP Os/+ mouse glomeruli, which have high GLUT1 expression and glucose uptake. We found that similar to our cultured MCs,50 PKCβ1 was increased in the glomeruli in a perinuclear pattern, indicating activation.51 PKCα exhibited a nonsignificant trend for increased activation in the glomeruli of FvbROP Os/+ mice, whereas total PKCα (active and inactive) was also not significantly increased.
The activation of PKCβ1 in the FvbROP Os/+ glomeruli was detected early (at 4 weeks of age), preceding the development of severe glomerulosclerosis. Investigation of a potential downstream transcription factor, which might mediate the effects of the PKC signaling system or VEGF on ECM production, indicated that NF-κB activation was significantly increased in the FvbROP Os/+ glomeruli. Therefore, this transcription factor could have an important role in GLUT1 and VEGF stimulation of glomerular matrix accumulation in this model. Other mechanisms may also be at play, but have not yet been investigated.
GLUT1 and VEGF have both been implicated in diabetic glomerulosclerosis.26, 52, 53, 54 In this study, we investigated potential mechanisms by which glomerular GLUT1 and VEGF were increased in the nondiabetic FvbROP Os/+ glomeruli, by studying cultured mouse and rat MCs in vitro. GLUT1 and VEGF are both known individually to increase in MCs in response to mechanical stretch,24, 55, 56 and our previous work has shown that GLUT1 is a stimulus for VEGF expression in MCs.56 In this study, we investigated the relationship between MC GLUT1 and VEGF expression in response to cyclic mechanical stretch in vitro, as according to Kriz' work and LaPlace's law, glomerular hypertrophy and increased glomerular capillary diameter in the FvbROP Os/+ mice should exert a stretch force on MCs attached to the GBM.57 Both GLUT1 and VEGF were increased in MCs in vitro by mechanical stretch, and the increase in GLUT1 (48 h onward) preceded the increase in VEGF (72 h), suggesting that GLUT1 could act as a stimulus for the VEGF.
Subsequently, as the GLUT1 gene has growth factor-responsive elements in its 5′-flank,58 we tested the effect of VEGF treatment on MC GLUT1 and glucose uptake. Both were increased and VEGF was clearly a potent stimulus for these. VEGF treatment of MCs, similar to overexpression of GLUT1 in MCs,22 was also a potent stimulus to FN expression, with much of the effect attributable to the increased glucose uptake.22 Experiments in which GLUT1 was suppressed in cultured MCs indicated that GLUT1 was significantly involved in VEGF-induced FN expression. Evidence was therefore obtained for a positive feedback mechanism in MCs by which GLUT1 and VEGF may influence each other to enhance ECM production. This mechanism in nondiabetic mice might also have a role in the progression of other chronic renal diseases in which nephron number has been substantially reduced.
Electron microscopy results at 4 weeks of age showed severe ultrastructural pathology in the glomeruli of FvbROP Os/+ mice, including diffuse podocyte foot process effacement and GBM thickening. Although the current studies focus on the molecular mechanisms of glomerulosclerosis in MCs, the causes of the podocyte foot process effacement and GBM thickening will be important topics in our future investigations of the FvbROP Os/+ mice. Accumulation of mesangial matrix was easily visualized, and consistent with the detection of substantially increased glomerular FN by Western analysis, and collagen by Masson's trichrome staining. The damaged glomerular barrier may explain the concomitant marked albuminuria in the FvbROP Os/+ mice. Subsequent investigations of developing kidneys will allow for determination of the point at which glomerular pathology begins, and will show whether the alterations begin during embryogenesis, or subsequent to birth of the animals when the kidneys must function without assistance from the mother. Even ROP mice with 5/6 nephrectomy do not develop the severity of glomerular disease and albuminuria observed in the FvbROP Os/+ mice.47 The severity of albuminuria, accompanied by rapidly progressive glomerulosclerosis suggest some similarities to the congenital nephrotic syndrome in humans,59 and excess glomerular expression of VEGF has been associated with the development of a collapsing glomerulopathy.60 Future investigations should determine whether novel podocyte proteins involved in maintaining the barrier to filtration are significantly altered in the FvbROP Os/+ mice, as nephrin, podocin, and CD2AP levels tested in the studies herein were unchanged.
In summary, we report the development of a mouse model exhibiting rapidly progressive glomerulosclerosis with marked albuminuria, early renal failure, and early demise of the animals carrying the Os allele. The early increases in glomerular GLUT1 and glucose uptake likely contributed to the severe renal disease of the FvbROP Os/+ mice. Evidence was also presented supporting roles for VEGF, PKCβ1, and NF-κB in the pathogenesis of the renal disease in these mice. Additional studies investigating glomerular hemodynamics, glomerular numbers, differential gene expression, developmental stages of the renal disease, and genetic analyses to identify the inheritance of the increased susceptibility of Fvb mice to Os will further clarify the mechanisms regulating development of the severe renal pathology in these animals. Investigations of the FvbROP Os/+ mouse model may allow for detection of new and important factors regulating the development of nondiabetic glomerulosclerosis.
References
Brenner BM, Lawler EV, Mackenzie HS . The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int 1996;49:1774–1777.
Drukker A . Oligonephropathy: from a rare childhood disorder to a possible health problem in the adult. Isr Med Assoc J 2002;4:191–195.
Brenner BM, Mackenzie HS . Nephron mass as a risk factor for progression of renal disease. Kidney Int Suppl 1997;63:S124–S127.
Brenner BM, Garcia DL, Anderson S . Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1988;1:335–347.
Luyckx VA, Brenner BM . Low birth weight, nephron number, and kidney disease. Kidney Int Suppl 2005;97:S68–S77.
Woods LL . Neonatal uninephrectomy causes hypertension in adult rats. Am J Physiol 1999;276:R974–R978.
Moritz KM, Wintour EM, Dodic M . Fetal uninephrectomy leads to postnatal hypertension and compromised renal function. Hypertension 2002;39:1071–1076.
Mei-Zahav M, Korzets Z, Cohen I, et al. Ambulatory blood pressure monitoring in children with a solitary kidney—a comparison between unilateral renal agenesis and uninephrectomy. Blood Press Monit 2001;6:263–267.
Brenner BM, Milford EL . Nephron underdosing: a programmed cause of chronic renal allograft failure. Am J Kidney Dis 1993;21:66–72.
Suzuki H, Tokuriki T, Kamita H, et al. Age-related pathophysiological changes in rat oligomeganephronic hypoplastic kidney. Pediatr Nephrol 2006;21:637–642.
Zalups RK . The Os/+ mouse: a genetic animal model of reduced renal mass. Am J Physiol 1993;264:F53–F60.
He C, Zalups RK, Henderson DA, et al. Molecular analysis of spontaneous glomerulosclerosis in Os/+ mice, a model with reduced nephron mass. Am J Physiol 1995;269:F266–F273.
He C, Esposito C, Phillips C, et al. Dissociation of glomerular hypertrophy, cell proliferation, and glomerulosclerosis in mouse strains heterozygous for a mutation (Os) which induces a 50% reduction in nephron number. J Clin Invest 1996;97:1242–1249.
Wise TL, Pravtcheva DD . Oligosyndactylism mice have an inversion of chromosome 8. Genetics 2004;168:2099–2112.
Pravtcheva DD, Wise TL . Disruption of Apc10/Doc1 in three alleles of oligosyndactylism. Genomics 2001;72:78–87.
Gruneberg H . Genetical studies on the skeleton of the mouse. XXVII. The development of oligosyndactylism. Genet Res Camb 1961;2:33–42.
Gruneberg H . Genetical studies on the skeleton of the mouse. XVIII. Three genes for syndactylism. Genetics 1956;54:113–145.
Lenz O, Zheng F, Vilar J, et al. The inheritance of glomerulosclerosis in mice is controlled by multiple quantitative trait loci. Nephrol Dial Transplant 1998;13:3074–3078.
Esposito C, He CJ, Striker GE, et al. Nature and severity of the glomerular response to nephron reduction is strain-dependent in mice. Am J Pathol 1999;154:891–897.
Oppermann M, Faulhaber-Walter R, Castrop H, et al. Glomerular hypertension and hyperfiltration in young fvb.ROP Os/+ mice. FASEB J 2007;21:595.28 (abstract).
Jarad G, Lakhe-Reddy S, Blatnik J, et al. Renal phenotype is exacerbated in Os and lpr double mutant mice. Kidney Int 2004;66:1029–1035.
Heilig CW, Concepcion LA, Riser BL, et al. Overexpression of glucose transporters in rat mesangial cells cultured in a normal glucose milieu mimics the diabetic phenotype. J Clin Invest 1995;96:1802–1814.
Heilig CW, Brosius III FC, Cunningham C . Role for GLUT1 in diabetic glomerulosclerosis. Expert Rev Mol Med 2006;8:1–18.
Gnudi L, Viberti G, Raij L, et al. GLUT-1 overexpression: link between hemodynamic and metabolic factors in glomerular injury? Hypertension 2003;42:19–24.
Heilig CW, Kreisberg JI, Freytag S, et al. Antisense GLUT-1 protects mesangial cells from glucose induction of GLUT-1 and fibronectin expression. Am J Physiol Renal Physiol 2001;280:F657–F666.
Heilig CW, Liu Y, England RL, et al. D-glucose stimulates mesangial cell GLUT1 expression and basal and IGF-I-sensitive glucose uptake in rat mesangial cells: implications for diabetic nephropathy. Diabetes 1997;46:1030–1039.
Gruden G, Thomas S, Burt D, et al. Mechanical stretch induces vascular permeability factor in human mesangial cells: mechanisms of signal transduction. Proc Natl Acad Sci USA 1997;94:12112–12116.
Schrijvers BF, Flyvbjerg A, Tilton RG, et al. Pathophysiological role of vascular endothelial growth factor in the remnant kidney. Nephron Exp Nephrol 2005;101:e9–e15.
Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, et al. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes 2002;51:3090–3094.
Dunn SR, Qi Z, Bottinger EP, et al. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int 2004;65:1959–1967.
Takemoto M, Asker N, Gerhardt H, et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 2002;161:799–805.
Heilig CW, Saunders T, Brosius III FC, et al. Glucose transporter-1-deficient mice exhibit impaired development and deformities that are similar to diabetic embryopathy. Proc Natl Acad Sci USA 2003;100:15613–15618.
Dumler F, Cortes P . Uracil ribonucleotide metabolism in rat and human glomerular epithelial and mesangial cells. Am J Physiol 1988;255:C712–C718.
Najafian B, Basgen JM, Mauer M . Estimating mean glomerular volume using two arbitrary parallel sections. J Am Soc Nephrol 2002;13:2697–2705.
Ivanyi B, Pap R, Ondrik Z . Thin basement membrane nephropathy: diffuse and segmental types. Arch Pathol Lab Med 2006;130:1533–1537.
Stewart AD, Stewart J . Studies on syndrome of diabetes insipidus associated with oligosyndactyly in mice. Am J Physiol 1969;217:1191–1198.
Sakai T, Kriz W . The structural relationship between mesangial cells and basement membrane of the renal glomerulus. Anat Embryol (Berl) 1987;176:373–386.
Lemley KV, Elger M, Koeppen-Hagemann I, et al. The glomerular mesangium: capillary support function and its failure under experimental conditions. Clin Invest 1992;70:843–856.
Pravtcheva DD, Wise TL . A transgene-induced mitotic arrest mutation in the mouse allelic with Oligosyndactylism. Genetics 1996;144:1747–1756.
Chen RA, Scott S, Mattern WD, et al. The case for disease management in chronic kidney disease. Dis Manag 2006;9:86–92.
McClellan WM . The epidemic of renal disease–what drives it and what can be done? Nephrol Dial Transplant 2006;21:1461–1464.
Neuringer JR, Brenner BM . Glomerular hypertension: cause and consequence of renal injury. J Hypertens Suppl 1992;10:S91–S97.
Taal MW, Brenner BM . Combination ACEI and ARB therapy: additional benefit in renoprotection? Curr Opin Nephrol Hypertens 2002;11:377–381.
Taal MW, Brenner BM . Achieving maximal renal protection in nondiabetic chronic renal disease. Am J Kidney Dis 2001;38:1365–1371.
Brenner BM, Zagrobelny J . Clinical renoprotection trials involving angiotensin II-receptor antagonists and angiotensin-converting-enzyme inhibitors. Kidney Int Suppl 2003;83:S77–S85.
Hostetter TH, Olson JL, Rennke HG, et al. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol 1981;241:F85–F93.
Cheng H, Zhang M, Moeckel GW, et al. Expression of mediators of renal injury in the remnant kidney of ROP mice is attenuated by cyclooxygenase-2 inhibition. Nephron Exp Nephrol 2005;101:e75–e85.
Eremina V, Quaggin SE . The role of VEGF-A in glomerular development and function. Curr Opin Nephrol Hypertens 2004;13:9–15.
Henry DN, Busik JV, Brosius III FC, et al. Glucose transporters control gene expression of aldose reductase, PKCalpha, and GLUT1 in mesangial cells in vitro. Am J Physiol 1999;277:F97–104.
Weigert C, Brodbeck K, Brosius III FC, et al. Evidence for a novel TGF-beta1-independent mechanism of fibronectin production in mesangial cells overexpressing glucose transporters. Diabetes 2003;52:527–535.
Kumar A, Hawkins KS, Hannan MA, et al. Activation of PKC-beta(I) in glomerular mesangial cells is associated with specific NF-kappaB subunit translocation. Am J Physiol Renal Physiol 2002;281:F613–F619.
Heilig K, Chen S, Xiang M, et al. Overexpression of GLUT1 in glomeruli produces features of diabetic nephropathy (DN) in mice. (Abstr.). JASN 2004;15:263A.
Heilig CW, Brosius III FC, Henry DN . Glucose transporters of the glomerulus and the implications for diabetic nephropathy. Kidney Int 1997;60 (Suppl):S91–S99.
Lenz T, Haak T, Malek J, et al. Vascular endothelial growth factor in diabetic nephropathy. Kidney Blood Press Res 2003;26:338–343.
Gruden G, Araf S, Zonca S, et al. IGF-I induces vascular endothelial growth factor in human mesangial cells via a Src-dependent mechanism. Kidney Int 2003;63:1249–1255.
Pfafflin A, Brodbeck K, Heilig CW, et al. Increased glucose uptake and metabolism in mesangial cells overexpressing glucose transporter 1 increases interleukin-6 and vascular endothelial growth factor production: role of AP-1 and HIF-1alpha. Cell Physiol Biochem 2006;18:199–210.
Kriz W, Elger M, Lemley K, et al. Structure of the glomerular mesangium: a biomechanical interpretation. Kidney Int 1990;30 (Suppl):S2–S9.
Murakami T, Nishiyama T, Shirotani T, et al. Identification of two enhancer elements in the gene encoding the type 1 glucose transporter from the mouse which are responsive to serum, growth factor, and oncogenes. J Biol Chem 1992;267:9300–9306.
Kuusniemi AM, Merenmies J, Lahdenkari AT, et al. Glomerular sclerosis in kidneys with congenital nephrotic syndrome (NPHS1). Kidney Int 2006;70:1423–1431.
Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 2003;111:707–716.
Acknowledgements
CWH was supported by grant awards from the Juvenile Diabetes Research Foundation (no. 1-2004-698) and the American Diabetes Association ( no. 7-02-RA-34).
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, Y., Heilig, K., Minto, A. et al. Nephron-deficient Fvb mice develop rapidly progressive renal failure and heavy albuminuria involving excess glomerular GLUT1 and VEGF. Lab Invest 90, 83–97 (2010). https://doi.org/10.1038/labinvest.2009.95
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2009.95
Keywords
This article is cited by
-
Vascular endothelial growth factors: multitasking functionality in metabolism, health and disease
Journal of Inherited Metabolic Disease (2015)
-
Identification of nephropathy candidate genes by comparing sclerosis-prone and sclerosis-resistant mouse strain kidney transcriptomes
BMC Nephrology (2012)
-
Relationship between five GLUT1 gene single nucleotide polymorphisms and diabetic nephropathy: a systematic review and meta-analysis
Molecular Biology Reports (2012)