Abstract
Galectin-3 is a β-galactoside-binding animal lectin having pleiotropic effects on cell growth, differentiation, and apoptosis. This lectin has been shown to be involved in phagocytosis by macrophages and in inflammation. Here we investigated an involvement of galectin-3 in the regulatory process of inflammatory bone resorption in rats with adjuvant-induced arthritis (AA rats) accompanying severe bone destruction in the ankle joints. The protein level of galectin-3 in the ankle-joint extracts was markedly augmented at week 3 after adjuvant injection, at the time when severe bone destruction was observed. Immunohistochemical analysis revealed an extremely high expression of galectin-3 in macrophages and granulocytes infiltrated in the area of severe bone destruction. To estimate the role of galectin-3 in osteoclastogenesis and osteoclastic bone resorption, recombinant galectin-3 was added to in vitro culture systems. Galectin-3 markedly inhibited the formation of osteoclasts in cultures of murine osteoclast precursor cell line as well as in rat bone marrow culture systems. This inhibition was not observed by heat-inactivated galectin-3 or by galectin-7. Although recombinant galectin-3 did not affect signaling through mitogen-activated protein kinase (MAPK) or nuclear factor-κB (NF-κB), it specifically suppressed the induction of nuclear factor of activated T-cells c1 (NFATc1). Galectin-3 significantly inhibited dentine resorption by mature osteoclasts in vitro. Furthermore, in vivo studies clearly showed a significant suppression of bone destruction and osteoclast recruitment accompanying arthritis, when galectin-3 was injected into the cavity of ankle joint of AA rats. Thus, abundant galectin-3 observed in the area of severe bone destruction may act as a negative regulator for the upregulated osteoclastogenesis accompanying inflammation to prevent excess bone destruction.
Similar content being viewed by others
Main
Galectins are a family of animal lectins having at least one carbohydrate recognition domain (CRD) with a high affinity for β-galactoside. This family now contains 15 members in mammals.1 Galectin-3 is composed of two domains; C-terminal domain, containing a carbohydrate-binding region; and the N-terminal domain, consisting mainly of tandem repeats of nine amino acids to cross-link to carbohydrate ligands or noncarbohydrate ligands, respectively.2 This protein is expressed in various inflammatory cells, including monocytes, macrophages, neutrophils, and eosinophils.3, 4, 5 The expression of galectin-3 is upregulated under inflammatory conditions, such as in tears from patients with ocular diseases and in human atherosclerotic lesions.6, 7 The expression of galectin-3 was also detected in bronchoalveolar lavage fluid obtained from mice with inflamed airways.8 These findings implicate galectin-3 expression in the pathological responses of inflammation.
Rheumatoid arthritis (RA) is a chronic inflammatory disease accompanying severe destruction of bone and articular cartilage.9 Bone destruction in RA is mainly mediated by bone-resorbing cells, osteoclasts,10, 11 multinucleated giant cells derived from hematopoietic stem cells.12 Receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL) is considered a key regulator of osteoclast differentiation and function.13 RANKL, highly expressed by synovial fibroblasts and T cells in inflammatory synovial tissues of patients with RA, is believed to be involved in the aberrant stimulation of osteoclastogenesis in inflammatory lesions.14, 15 We have previously shown lines of evidence suggesting the involvement of the chemokine MIP-1α in the process of bone destruction accompanying adjuvant-induced arthritis (AA) in rats.16 Recently, it has been reported that anti-inflammatory drugs such as leflunomide, tacrolimus, and cyclosporine A suppress bone destruction through direct inhibition of RANKL-mediated osteclastogenesis.17, 18 These findings are suggesting the availability of anti-inflammatory drugs or cytokines as the suppressor of bone destruction. Interestingly, López et al has reported that galectin-3 acts as an anti-inflammatory factor in experimental acute or chronic asthma.19, 20 In their works, gene therapy using plasmid encoding galectin-3 regulated in an improvement of cellular and functional respiratory parameters affected in the process of asthma.
It has been reported that a high level of galectin-3 expression is associated with synovial tissues of patients with RA.21 In these patients, the protein level of galectin-3 is also significantly elevated in sera and synovial fluid. Ortega et al22 reported that galectin-3 can inhibit osteoclast recruitment at the chondro-osseous boundary. However, it is unclear how galectin-3 is involved in the regulation of bone destruction associated with RA. Galectin-3, also designated Mac-2, previously identified as a marker for murine activated macrophages,23 was also shown to be expressed in osteoclasts,24 suggesting the possible involvement of galectin-3 in the regulation of osteoclastic bone resorption.
In this study, we focused on investigating the possible involvement of galectin-3 in the regulation of inflammatory bone destruction using rats with AA accompanying severe bone destruction around the ankle joints, considered to be experimental model for human RA. We detected abundant galectin-3 expression in inflammatory cells infiltrated into areas of severe bone destruction, whereas only a low level of galectin-3 was detected in osteoclasts. We further examined the precise effects of administrated galectin-3 on inflammatory bone destruction, using an in vivo system of inflammatory bone destruction in AA rats as well as using an in vitro culture system for evaluating osteoclastogenesis and pit formation.
MATERIALS AND METHODS
Animals and Reagents
Sprague–Dawley (SD) rats and Lewis rats were obtained from Kyudo (Tosu, Japan). Heat-killed Mycobacterium butyricum and mineral oil were obtained from Difco Laboratories (Detroit, MI, USA). Recombinant mouse galectin-3 was purchased from R&D Systems (Minneapolis, MN, USA). Recombinant human soluble RANKL was from PeproTech (London, UK), and recombinant human TNF-α was obtained from Roche Molecular Biochemical (Mannheim, Germany). 1α,25-Dihydroxy vitamin D3 (1α,25(OH)2D3) was purchased from Biomol Research Laboratories (Plymouth Meeting, UK). Leukocyte acid phosphatase kit and β-lactose were obtained from Sigma (St Louis, MO, USA). Anti-phospho-extracellular signal-regulated kinase (ERK), jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK) and anti-ERK, JNK, p38 MAPK, and NF-κB inhibitor (IκB) antibodies (Abs) were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-galectin-3, nuclear factor of activated T-cells c1 (NFATc1), actin, and galectin-3 siRNA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Peroxidase-conjugated anti-mouse and anti-rabbit IgG Abs and enhanced chemiluminescence (ECL) kits were purchased from Amersham Biosciences (Buckinghamshire, UK).
Induction of Adjuvant Arthritis
Adjuvant arthritis was induced as described previously.25, 26 Briefly, female Lewis rats at 5 weeks were intradermally injected at the base of the tail with complete adjuvant consisting of 25 mg/kg heat-killed M. butyricum suspended in mineral oil. For the control experiments, rats were injected with mineral oil alone. The rats were killed on day 21 after the adjuvant injection and processed for immunohistochemical analysis. In the case of galectin-3 administration, the recombinant galectin-3 or vehicle (PBS) was injected into the ankle-joint cavities of adjuvant-injected rats. Thirty-gauge needles were used to inject 5 μg recombinant galectin-3 in 30 μl PBS into the left ankle joint. As an internal control, 30 μl PBS was injected into the right ankle joint. The injections were performed twice a week beginning on day 6 after the adjuvant injection. The rats were killed on day 14 after the first injection of recombinant galectin-3 or PBS. The hind paws (tarsal bones and tibia) of rats were collected and imaged on ultraspeed radiographic film (Kodak, Rochester, NY, USA). The level of bone destruction was analyzed by a soft X-ray analysis system (SOFRON: SRO-M 50; Sofron Inc., Tokyo, Japan). All animal treatments were performed according to the guidelines for the care and use of laboratory animals at Kyushu University.
Immunohistochemistry and Histological Analysis
After fixation by perfusion with 4% paraformaldehyde/PBS, tissue blocks were taken from the hind paws and immersed in the same fixative for 6 h at 4°C, followed by washing in PBS overnight at 4°C. After decalcification in 10% EDTA for 3 weeks at 4°C, the tissue blocks were embedded in paraffin. Sections (6 μm) of ankle joints including tibia-tarsal-calcaneus bones were prepared and immunostained. After blocking the nonspecific binding sites with 10% donkey serum for 30 min at room temperature, sections were incubated with rabbit polyclonal anti-rat galectin-3 antibody (1:100 dilution) in a humidified chamber overnight at 4°C. Sections were incubated with biotinylated donkey anti-rabbit IgG (1:200 dilution; Jackson Immunoresearch Laboratories, West Grove, PA, USA) for 1 h at room temperature, followed by reaction with peroxidase-conjugated streptoavidin (1:300 dilution; Dako Japan, Kyoto, Japan) for 1 h at room temperature. Color development was performed using a DAB substrate kit (Vector Laboratories, Burlingame, CA, USA). The sections were also stained for tartrate-resistant acid phosphatase (TRAP). The mean number of osteoclasts in each unit area (0.1452 mm2) was determined in metatarsal bones using TRAP-stained sections.
Double-Immunofluorescence Staining
The sections were hydrated and pretreated with 10% normal donkey serum for 1 h at room temperature. Sections were incubated with primary Abs, which were rabbit polyclonal anti-rat galectin-3 Ab (1:100 dilution) mixed with mouse monoclonal anti-macrophage Ab ED1 (1:200 dilution; BMA Biomedicals, Augst, Switzerland) or goat polyclonal antineutrophil elastase (1:100 dilution; Santa Cruz Biotechnology) in a humidified chamber overnight at 4°C. After washing with PBS, the sections were incubated with a mixture of FITC-conjugated donkey anti-rabbit IgG (1:200 dilution; Jackson) with rhodamine-conjugated donkey antimouse IgG (1:200 dilution; Jackson) or Cy3-conjugated mouse anti-goat IgG (1:100 dilution; Jackson) for 1 h at room temperature. After washing with PBS, the sections were observed under a fluorescence microscope (Olympus; AX70, Tokyo, Japan).
Cell Culture and Differentiation of Osteoclasts
Formation of osteoclasts from rat bone marrow cells was performed mainly as described previously.27 Briefly, bone marrow cells were obtained from the tibia and femur of male SD rats of 4 weeks old. Bone marrow stromal cells were depleted with a Sephadex G10 column. These cells were cultured in 96-well plates (4 × 105 cells per well) in α-MEM (Gibco, Grand Island, NY, USA) containing 15% FBS (Biosource, Rockville, MD, USA) in the presence of 10−8 M 1α,25(OH)2D3, 20 ng/ml of RANKL, and 10% heat-treated ROS17/2.8 cell-conditioned medium (htROSCM), as described previously.28 Various concentrations of recombinant galectin-3 or β-lactose was added to these cultures. After 4 days of culture, the cells were fixed and stained for TRAP. TRAP-positive multinucleated cells (TRAP+ MNCs) containing three or more nuclei were then counted.
The osteoclast precursor cell line, RAW-D, is a subclone of murine macrophage cell line RAW264 cells and has an extremely high potential ability to differentiate into osteoclasts.29 RAW-D cells were cultured in 96-well plates (6.8 × 103 cells per well) in α-MEM containing 10% FBS for 3 days in the presence of RANKL (20 ng/ml), TNF-α (1 ng/ml), and various concentrations of recombinant galectin-3.
Western Blotting
RAW-D cells were starved in α-MEM containing 0.5% FBS for 5 h, then incubated with or without recombinant galectin-3 for 60 min. These cells were stimulated with 100 ng/ml RANKL and further incubated for the indicated times. Thereafter, the cells were harvested and lysed in lysis buffer containing 1% Triton X-100, 0.5% NP-40, 150 mM NaCl, 50 mM Tris–HCl (pH 7.4), and protease inhibitor cocktail (Sigma). The lysates, containing equal amounts of proteins, were separated by 10% SDS–PAGE and transferred to nitrocellulose membranes. After blocking with 5% nonfat dry milk, the membranes were probed with anti-phospho ERK1/2, JNK1/2, and p38 Abs and then reacted with peroxidase-conjugated anti-mouse or anti-rabbit IgG Abs. The same membranes were stripped and reprobed with anti-ERK1/2, JNK1/2, p38, IκB, NFATc1 or actin, and the proteins were visualized by ECL kit.
Areas of distal tibia were dissected from AA rats and control rats and then frozen immediately in liquid nitrogen. Tissues were reduced to powder under liquid nitrogen using small mortar and pestle. Then the total proteins were extracted in lysis buffer containing 20 mM Tris–HCl (pH 7.2), 10 mM EDTA, 0.3 M NaCl, 0.1% Triton X-100, 0.05% Tween 20 and protease inhibitor cocktail overnight at 4°C. Equal amounts lysate proteins were subjected to western blotting analysis, as described above.
Observation of Actin-Ring Formation
Rat bone marrow cells were cultured in Lab-Teck chamber slides for 4 days. Cells were starved in α-MEM containing 0.1% FBS and pretreated with or without recombinant galectin-3 for 60 min. These cells were stimulated with 100 ng/ml RANKL and further incubated for 30 min. Cells were fixed in 4% paraformaldehyde for 20 min at room temperature and permeabilized with 0.1% Triton X-100 for 4 min. After rinsing in PBS, cells were stained for actin with phalloidin-TRITC diluted with PBS for 20 min at room temperature. Actin ring was observed under fluorescence microscopy.
Dentine Resorption Assay
Rat bone marrow cells were cultured in 24-well plates (1 × 106 cells per well) in α-MEM containing 15% FBS in the presence of 10−8 M 1α,25(OH)2D3 and 10% (v/v) htROSCM. After 4 days of culture, the cells were detached from the culture plates with 0.05% trypsin and 0.02% EDTA in PBS. The osteoclasts were replated on dentine slices (diameter 4.4 mm) that had been placed in 96-well plates, which were then further incubated in the α-MEM containing 15% FBS for 3 days in the presence or absence of galectin-3 in the absence of any osteoclastogenic factors. After incubation, attached cells were completely removed from the dentine slices by ultrasonification. Resorption pits on the slices were observed using a scanning electron microscopy (JEOL JSM-5400LV, Tokyo, Japan) as described previously.28, 30 The total areas of resorption pits and the total number of resorption pits per dentine slice were analyzed by use of Scion Image version Beta 4.02 software.
RT-PCR
RAW-D cells were cultured in six-well plates (1.5 × 105 cells per well) with RANKL and TNF-α for the indicated time. Total cellular RNA was extracted by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and subjected to PCR using RT-PCR kit (Takara Bio, Otsu, Japan). The primers used for PCR were as follows: galectin-3 forward, GTTGCCTTCCACTTTAACCC, galectin-3 reverse, CCGGAGGTTCTTCATCCGAT; TRAP forward, CAGCTGTCCTGGCTCAAAA, TRAP reverse, ACATAGCCCACACCGTTCTC; GAPDH forward, AAACCCATCACCATCTTCCA, GAPDP reverse, GTGGTTCACACCCATCACAA. PCR reactions at 94°C for 30 s, at 55–60°C for 30 s, and at 72°C for 1 min were carried out for 25–30 cycles. The PCR products were separated by electrophoresis on 1.5% agarose gels and visualized by ethidium bromide staining with UV light illumination.
siRNA Transfection
RAW-D cells were plated in 6-well plates (1.5 × 105 cells per well) or 96-well plates (6.8 × 103 cells per well) before 24 h of transfection. The cells were transfected transiently with siRNA for galectin-3, using siPORT lipid transfection reagent (Ambion, Woodward St Austin, TX, USA) as described previously.31 After 4 h of transfection, the cells were incubated with 20 ng/ml of RANKL and 1 ng/ml of TNF-α for the indicated time.
RESULTS
Extremely High Expression of Galectin-3 in Distal Tibia of AA Rats with Severe Bone Destruction
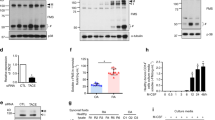
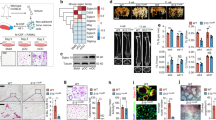
To understand the involvement of galectin-3 in the regulation of inflammatory bone destruction, we investigated its expression in distal tibia of AA rats with severe bone destruction around the ankle joints. In contrast to control rats injected with mineral oil alone (Figure 1a), which have no histological abnormality, the distal tibia (Figure 1b) and tarsal bones of rats with severe arthritis showed marked bone destruction. Aggregates of numerous multinucleated osteoclast-like cells were observed in the bone marrow cavity of distal tibia and tarsal bones in AA rats (Figure 1d). In control rats, only a limited number of cells were positive for galectin-3 (Figure 1e, g and i); however, in AA rats, numerous galectin-3-positive cells were detected in the distal tibia (Figure 1f, h and j). In line with these data, the protein level of galectin-3 was dramatically augmented in protein extracts obtained from the distal tibia of AA rats (Figure 1m). A low level of galectin-3 expression was observed in osteoclasts in AA rats (Figure 1h). In contrast, an extremely high expression of galectin-3 was detected in numerous mononuclear cells observed in the bone marrow cavity of distal tibia in AA rats. A high-magnification view of galectin-3-positive mononuclear cells (Figure 1j) showed that these cells were likely macrophages and granulocytes. Control rabbit IgG showed no staining signal both in control rats (Figure 1k) and AA rats (Figure 1l). To confirm the cell type of galectin-3-positive cells abundant in AA rats, we performed double-immunofluorescence staining using anti-macrophage antibody (anti-ED1 antigen antibody) or anti-granulocyte antibody (anti-neutrophil elastase antibody), respectively. As shown in Figure 1n and o, the ED1-positive cells and neutrophil elastase-positive cells expressed galectin-3, demonstrating that galectin-3-positive mononuclear cells observed in AA rats are indeed macrophages and granulocytes. Isotype control Abs showed no staining in these sections (data not shown). These data suggest that an extremely high level of galectin-3 is secreted by inflammatory cells that are infiltrated in sites showing severe bone destruction in AA rats. Supplementary Figure 1 shows the time–course expression of galectin-3 proteins in AA rats. Expression of galectin-3 was detected from 2 weeks after adjuvant –injection, however, the expression level is rather suppressed. The expression of galectin-3 protein was significantly augmented from 3 to 4 weeks. In contrast, osteoclast number reached to the maximum level at 3 weeks followed by a gradual decrease in the number in the area of bone destruction. These data suggest a role of galectin-3 as the negative regulator of osteoclastogenesis in vivo.
Expression of galectin-3 in distal tibia of AA rats with severe bone destruction. Microscopic observations of distal tibia of control rats (a, c, e, g, i, k) and AA rats (b, d, f, h, j, l) at 21 days after adjuvant injection. Representative sections were stained with hematoxylin and eosin (H&E) (a–d) or rabbit antigalectin-3 Ab (e–j), and control rabbit IgG (k, l). (c, d) High-magnification views of (a) (*) and (b) (**), respectively. (g, i) High-magnification views of (e) (*) and (e) (#), respectively. (h, j) High-magnification views of (f) (*) and (f) (#), respectively. Arrows indicate the galectin-3-positive cells in AA rat. A low level of galectin-3 expression was detected on osteoclasts in AA rats (h). In contrast, an extremely high level of galectin-3 expression was detected in infiltrated mononuclear cells in AA rats (j). (m) Marked induction of galectin-3 proteins in the protein extracts of distal tibia of AA rats detected by western blot analysis. Equal amounts of protein extracts (control: control rats; AA: AA rats) were subjected to SDS–PAGE and immunoblotting, followed by detection with rabbit antigalectin-3 Ab. (n, o) Expression of galectin-3 in ED-1-positive macrophages and neutrophil elastase-positive cells. Photographs show double-immunofluoresence staining of distal tibia of AA rats at 21 days after the adjuvant injection. (n) Sections were stained with murine antirat ED-1 Ab (red, left panel) and rabbit anti-rat galectin-3 Ab (green, middle panel). The merged image of ED-1-positive cells and galectin-3-positive cells are shown at right. Arrows indicate cells expressing both of ED-1 antigen and galectin-3 (yellow). (o) Sections were stained with goat anti-neutrophil elastase Ab (red, left panel) and rabbit anti-galectin-3 Ab (green, middle panel). The merged image of neutrophil elastase-positive cells and galectin-3-positive cells are shown at right. Arrows indicate cells expressing both of neutrophil elastase and galectin-3 (yellow; original magnifications a, b, e, f: × 10; c, d, g, h, i, j, k, l, n, o: × 100).
Inhibition of Osteoclast Formation by Galectin-3
To estimate the role of galectin-3 observed in the area of severe bone destruction in AA rats, the recombinant galectin-3 was added an in vitro culture system for evaluating osteoclast differentiation. Galectin-3 significantly inhibited formation of TRAP-positive multinucleated osteoclast-like cells in RAW-D cell cultures in a dose-dependent manner (Figure 2a and b). Galectin-3 also significantly inhibited osteoclast formation in a rat bone marrow culture system depleted of stromal cells (Figure 2c). To determine that the inhibitory effect of galectin-3 on osteoclastogenesis was not because of cytotoxicity or reduced cell growth, MTT assay was performed on RAW-D cells and stromal cell-depleted rat bone marrow cells treated with galectin-3 (1–10 μg/ml) for 72 h. Galectin-3 did not show any cytotoxicity (Figure 2d). These data suggest that galectin-3 is an effective and potent inhibitor of osteoclastogenesis in vitro.
Inhibition of osteoclast formation by galectin-3. (a, b) RAW-D cell culture. RAW-D cells were cultured in 96-well plates with RANKL (20 ng/ml) and TNF-α (1 ng/ml) for 3 days in the presence or absence of the indicated concentrations of galectin-3. Cells were fixed and stained for TRAP, and TRAP-positive multinuclear cells with three or more nuclei were counted. (c) Bone marrow culture. Stromal cell-depleted nonadherent rat bone marrow cells (NABMC) were cultured in 96-well plates with 1α,25(OH)2D3 (10−8 M), RANKL (20 ng/ml), and 10% (v/v) htROSCM for 4 days in the presence of various concentrations of galectin-3. Cells were fixed and stained for TRAP, and TRAP-positive multinuclear cells were counted. (d) MTT assay. RAW-D cells and NABMC were cultured for forming osteoclasts as described above in the presence or absence of the indicated concentrations of galectin-3. MTT assay was performed, and the absorbance was read at 595 nm. Data present mean±s.d. from triplicate cultures in one representative experiment. Similar results were obtained in three independent experiments. Data were analyzed by Student’s t-test. *P<0.05, **P<0.01, and ***P<0.001 compared with the culture without galectin-3.
The Inhibitory Effect of Galectin-3 on Osteoclast Formation was not Mediated in a Carbohydrate-Dependent Manner
The biological activities of extracellular galectin-3 are mainly modulated through its interactions with various β-galactose-containing glycans by its CRD.32 In general, the regulatory effects of galectin-3 on cell signaling are reversed by addition of β-lactose.33, 34 To know whether the inhibitory effect of recombinant galectin-3 is dependent on the moiety of CRD, the galectin-3 was added to the stromal cell-depleted rat bone marrow culture system in the presence or absence of β-lactose. Its inhibitory effect on osteoclast differentiation was not reversed by the addition of β-lactose (Figure 3a), suggesting that recombinant galectin-3 may inhibit osteoclastogenesis without binding to the β-galactoside moiety of glycoproteins expressed on cell surface of osteoclast precursors.
Inhibition of osteoclastogenesis by galectin-3. (a) The inhibitory effect of galectin-3 on osteoclast formation was not mediated in a carbohydrate-dependent manner. Stromal cell-depleted rat bone marrow cells were cultured in 96-well plates with 1α,25(OH)2D3 (10−8 M), RANKL (20 ng/ml), and 10% (v/v) htROSCM for 4 days in the presence or absence of 10 μg/ml galectin-3. Various concentrations of β-lactose were added to these cultures. Cells were fixed and stained for TRAP, and TRAP-positive multinuclear cells were counted. (b, c) Inhibitory effect of galectin-3 is intrinsic to recombinant galectin-3. RAW-D cells were cultured in 96-well plates with RANKL (20 ng/ml) and TNF-α (1 ng/ml) for 3 days in the presence or absence of the 10 μg/ml of galectin-3 and heat-inactivated galectin-3 preparations (b) or galectin-7 (c). Cells were fixed and stained for TRAP, and TRAP-positive multinuclear cells with three or more nuclei were counted. Results are presented as mean±s.d. from triplicate cultures in one representative experiment. Similar results were obtained in three independent experiments. Data were analyzed by Student’s t-test. ***P<0.001 compared with the culture without galectin-3 (control).
To find out whether this inhibitory effect is intrinsic to galectin-3, heat-inactivated galectin-3 preparations and galectin-7, a prototype galectin bearing only CRD,35 were also assessed in vitro culture system. Neither heat-inactivated galectin-3 nor galectin-7 inhibited osteoclastogenesis (Figure 3b and c), strongly suggesting that the observed inhibitory effects on osteoclastogenesis are an intrinsic property of galectin-3.
Inhibition of NFATc1 Induction Induced by RANKL by Galectin-3
It is well known that MAPK, NF-κB, and NFATc1 pathways are important in RANKL-induced osteoclast differentiation. To know the molecular mechanisms of galectin-3-mediated inhibition of osteoclastogenesis, we investigated the effect of galectin-3 on these pathways with western blot analysis. Although addition of recombinant galectin-3-stimulated phosphorylation of ERK, p38 MAPK, and JNK in the absence of RANKL (Figure 4a), costimulation with RANKL did not affect the level of galectin-3-induced phosphorylation of ERK, p38, or JNK. IκB degradation associated with NF-κB activation induced by RANKL was not affected by galectin-3 (Figure 4a). In contrast, recombinant galectin-3 markedly inhibited RANKL-induced NFATc1 expression (Figure 4b). During osteoclastogenesis in RAW-D cell culture system, in which RANKL and TNF-α were added, the peak induction of NFATc1 came around 16 h after adding these cytokines (Figure 4c). The NFATc1 level was markedly suppressed by the addition of galectin-3 at 16 h after cytokine treatment (Figure 4d). Taken together, these results strongly suggest that recombinant galectin-3 regulates osteoclast differentiation by suppressing the level of NFATc1 in the protein level.
Marked inhibition of NFATc1 expression by galectin-3. RAW-D cells were starved with α-MEM containing 0.1% FBS for 5 h and incubated with or without 10 μg/ml of galectin-3 for 60 min. RANKL (100 ng/ml) was added, and the cells were further incubated for 20 min. Total cell lysates were prepared, and equal amounts of proteins were subjected to western blot analysis. (a) Galectin-3 does not affect most RANKL signaling. The cell lysates were subjected to western blot analysis using anti-P-p38, p38, P-JNK, JNK, P-ERK, ERK, IκB, and anti-actin Abs. (b) Marked inhibition of NFATc1. Cell lysates were also analyzed by western blotting using anti-NFATc1 or anti-actin Ab. (c) Time–course expression of NFATc1 in osteoclastogenesis. RAW-D cells were stimulated with RANKL (20 ng/ml) and TNF-α (1 ng/ml) for the indicated period of time. Total cell lysates were prepared, and equal amounts of proteins were subjected to western blot analysis using anti-NFATc1 or anti-actin Ab. (d) Marked suppression of NFATc1 by galectin-3. RAW-D cells were stimulated with or without RANKL (20 ng/ml) and TNF-α (1 ng/ml) for 16 h in the presence or absence of 10 μg/ml of galectin-3. Total cell lysates were prepared, and equal amounts of proteins were subjected to western blot analysis using anti-NFATc1 or anti-actin Ab.
Inhibition of Bone-Resorbing Activity of Osteoclasts by Galectin-3
We further investigated the effect of recombinant galectin-3 on the function of mature osteoclasts. We replated osteoclasts formed in the rat whole bone marrow culture system on dentine slices, culturing them in the presence or absence of galectin-3 for 72 h. Galectin-3 dramatically reduced both the total area of resorption pits and the total number of pits (Figure 5a–c). In spite of the marked inhibition of osteoclast function, galectin-3 did not affect adhesion of osteoclasts on dentine surface (Figure 5a lower panels). These results demonstrate that galectin-3 inhibits the bone-resorbing activity of mature osteoclasts without affecting adhesiveness of osteoclasts. Interestingly galectin-3 suppressed the formation of actin ring, a cytological structure highly associated with osteoclasts in function (Supplementary Figure 4).
Marked inhibition of osteoclast function by galectin-3. Mature osteoclasts obtained from rat whole bone marrow cultures were replated on dentine slices and cultured for 72 h in the presence of the indicated concentrations of galectin-3. Resorption pits on the dentine slices were visualized by scanning electron microscopy (a, upper panels). Demonstration of TRAP-positive osteoclasts observed on dentine slices (a, lower panels). Total area of resorption pits (b) and number of pits (c) per dentine slice were analyzed with Scion Image version Beta 4.02 software. Results are presented as mean±s.d. from triplicate cultures in one representative experiment. Similar results were obtained in three independent experiments. Data were analyzed by Student’s t-test. *P<0.05, **P<0.01 and ***P<0.001 compared with the culture without galectin-3 (bars indicate 200 μm in a).
Successful Inhibition of Bone Destruction by Intrajoint Injection of Galectin-3 in AA Rats
To investigate the effects of galectin-3 on osteoclastic bone destruction, rats with AA were given injections in the ankle-joint cavity with recombinant galectin-3 or PBS for 2 weeks. Galectin-3 was injected into the left ankle joint, as an internal control, PBS (vehicle) was injected into right ankle joint. At the end of the experiment, we examined radiographs of hind paws. Although the inflammation level was not significantly affected, bone destruction was clearly affected. As shown in Figure 6a, the right ankle joints treated with PBS showed severe bone destruction in the distal tibia and metatarsal bones (Figure 6a, control), whereas in the left ankle joints treated with recombinant galectin-3, bone destruction was significantly inhibited, especially in metatarsal bones (Figure 6a, galectin-3). To further determine the inhibitory effect of bone destruction, histological analysis was performed. In the control sections that injected with PBS only, marked accumulation of osteoclasts identified as TRAP-positive multinucleated cells were observed in the area of metatarsal bones (Figure 6c, e and f). In contrast, the number of osteoclasts was significantly decreased in the area of metatarsal bones by injection with galectin-3 (Figure 6b, d and f). These results strongly suggest that recombinant galectin-3 suppresses bone destruction accompanying arthritis through inhibition of osteoclast formation.
Suppression of bone destruction by intra-articularly injected galectin-3. Rats with adjuvant-induced arthritis received injections in the cavity of their ankle joints with recombinant galectin-3 (5 μg) or PBS. Recombinant galectin-3 was injected into the left ankle joint (panel: left) and the PBS was injected into right ankle joint (panel: right) as an internal control. The injections were performed twice a week for 2 weeks beginning on day 6 after the adjuvant injection. (a) X-ray photograph showing a marked suppression of bone destruction associated with adjuvant-induced arthritis by intra-articularly injected galectin-3. The arrows indicate the portions of increased bone mass compared with control. (b–f) Metatarsal area of control (c, e) and galectin-3-injected paw (b, d) were histologically analyzed. Representative sections stained with TRAP (b–e). (d, e) High-magnification views of (b) (#) and (c) (##), respectively. The injection of galectin-3 inhibited formation of TRAP-positive osteoclasts compared with control in the metatarsal area (d, e). (f) Histological quantification of galectin-3-mediated inhibition of osteoclastogenesis. Each value represents the mean number of osteoclasts±s.d. in randomly selected 10 unit areas taken from the typical tissue sections of the metatarsal bone. Data were analyzed by Student’s t-test. ***P<0.001 compared with the control sections that injected only PBS. These data represent the typical results from four independent experiments. Mt: metatarsal bone, Ta: tarsal bone (original magnifications b, c: × 10; d, e: × 50).
Expression Blockage of Endogenous Galectin-3 in Osteoclast had no Significant Effect on Osteoclastogenesis
As a low level of galectin-3 was detected in osteoclasts in the bone destruction sites (Figure 1h), we investigated the involvement of endogenous galectin-3 in the regulatory process of osteoclast differentiation. We examined the gene expression pattern of galectin-3 gene during osteoclastogenesis in RAW-D cells by RT-PCR and western blot analysis. In 3 days, RAW-D cells differentiate into TRAP-positive osteoclasts in the presence of RANKL and TNF-α. The expression of galectin-3 mRNA increased within 48 h after RANKL and TNF-α treatment and was further upregulated over a period of 72 h (Supplementary Figure 2A). In line with the expression pattern of galectin-3 mRNA, the protein level of galectin-3 increased at 48 h and at 72 h after RANKL and TNF-α treatment (Supplementary Figure 2B). The protein level of galectin-3 also increased at 48 h and at 72 h after treatment with RANKL or TNF-α alone (Supplementary Figure 2C and D). However, in the absence of RANKL and TNF-α, the level of galectin-3 protein was augmented even when cells were just cultured on plastic plates (Supplementary Figure 2E). These data suggest that the increase in galectin-3 in RAW-D cell cultures was not associated with osteoclastogenesis induced by osteoclastogenic factors.
We performed a loss-of-function experiment using the specific siRNA for galectin-3. Galectin-3 knockdown had no effect on the osteoclast differentiation in RAW-D cells (Supplementary Figure 3A). Supplementary Figure 3B shows a successful knockdown of galectin-3 expression in protein level by treatment with galectin-3-specific siRNA. These results suggest that endogenous galectin-3 expressed in osteoclasts had no regulatory function in osteoclastogenesis.
DISCUSSION
In this study, we showed that marked expression of galectin-3 in numerous macrophages and granulocytes penetrated to the sites of severe bone destruction in AA rats, whereas normal rats expressed only a suppressed level of galectin-3 in the corresponding joint tissues. Several reports have shown that galectin-3 is expressed in synovial fibroblasts of patients with RA.36, 37 When arthritic bone destruction occurs, it is believed that osteoclast precursors as well as inflammatory cells accumulated in the inflamed synovial membrane migrate to the sites of future bone destruction.38, 39 Shou et al40 recently showed that galectin-3 expression was upregulated in a rat collagen-induced arthritis model. It is now clear that galectin-3 expression is associated with the incidence of arthritis. However, its actual role in inflammatory bone destruction has been ambiguous. Our current study suggests an involvement of galectin-3 in the negative regulation of bone destruction in rats with AA.
Ortega et al22 reported that galectin-3 has a regulatory role in the recruitment of osteoclasts from the site of calcified cartilages to form a new bone marrow cavity in the long-bone development in matrix metalloproteinase-1 (MMP-1) gene-deficient mice. Galectin-3, a substrate for MMP-1, was abnormally accumulated in the chondro-osseus junction and prohibited normal penetration of osteoclasts from the periosteum into the central region of the future bone column. However, they do not mention any role for galectin-3 in osteoclastogenesis. In this study, using an in vitro culture system, we have successfully shown a clear regulatory role of galectin-3 in osteoclastogenesis. We also have successfully shown a marked inhibition of the osteoclastic-resorbing function using an in vitro assay system. In galectin-mediated inhibition of osteoclastogenesis, galectin-3 markedly inhibited induction of NFATc1 protein expression, affecting neither the phosphorylation of ERK, JNK, or p38 nor degradation of IkB induced by RANKL. Our findings suggest that inhibitory effect of galectin-3 on osteoclastogenesis was not through a simple blockage of RANKL signaling. The possibility was ruled out that galectin-3 treatment induces osteoprotegerin, a decoy receptor for RANKL,41 to block RANKL activity, as galectin-3 treatment affected neither the phosphorylation of ERK, JNK, or p38 nor degradation of IkB induced by RANKL. Our current study demonstrates that inhibition mediated by galectin-3 was not blocked by the addition of β-lactose, which is expected to block biological activities of proteins of the galectin family. This suggests that a site independent of CRD on galectin-3 interacts with receptor-like molecules on cell surface of osteoclast precursors to inhibit osteoclast formation. Similarly, it has been reported that the osteoclast inhibitory lectin, a C-type lectin, inhibits osteoclast formation42, 43 without acting through their carbohydrate recognition moieties.44 As the N-terminal portions bearing no glycan-binding sites have unique properties to bind nonglycan ligands,45 this portion of galectin-3 is likely to contribute to the suppression of osteoclastogenesis. Elucidating the receptors for galectin-3 expressed on the cell surface of osteoclasts and their precursors will be challenging. We could not detected any inhibitory effect on osteoclastogenesis by the other galectin family member galectin-7, a prototype galectin composed of only CRD, which acts mainly on epithelial cells.35 Furthermore, heat-inactivated galectin-3 preparation showed no inhibitory effect on osteoclastogenesis. Therefore, an inhibitory effect of galectin-3 on osteoclastogenesis is thought to be an intrinsic activity of galectin-3 protein itself, possibly through non-CRD of galectin-3. Recently, Janelle-Montcalm et al46 reported that extracellular localization of galectin-3 shows a deleterious effect in joint tissues. They observed joint swelling in normal mice by intra-articular injection of human recombinant galectin-3. The most important difference between their work and ours is that they injected the joints of normal mice, not mice with arthritis. In our study, intra-articularly injected galectin-3 only suppressed bone destruction but not inflammation itself. As our study clearly showed the suppressive effect of galectin-3 on osteoclast formation and function not only in vitro but also in vivo, it is assumed that galectin-3 expresses the opposite function in response to inflammatory or normal conditions in joint tissues.
Galectin-3 has been shown to function through both intracellular and extracellular actions. Intracellular galectin-3 is implicated in several basic cellular processes such as pre-mRNA splicing, control of cell cycle, and apoptosis,1, 47 whereas extracellular galectin-3 regulates cell adhesion and chemoattraction and is also involved in signal transduction events on cell surface.33, 34, 48 Although osteoclasts express endogenous galectin-3, its function is not clear.24 In this study, the targeted inhibition of endogenous galectin-3 expression by the specific siRNA did not affect the formation of osteoclasts. As we have successfully blocked galectin-3 expression in the protein level by a galectin-3-specific siRNA, the possibility of insufficient blockage of gene expression can be ruled out. Our results suggest that the endogenous galectin-3 expressed by osteoclasts does not have a regulatory role in osteoclastogenesis.
It has been reported that the level of galectin-3 was elevated in sera and synovial fluid in RA patients.21 However, the meaning of this increase has not been well understood. In this study, the immunohistochemical data clearly showed that galectin-3 expression was dramatically augmented in numerous macrophages and granulocytes penetrating the area of distal tibia with severe bone destruction in AA rats. In vitro studies showed that recombinant galectin-3 potently inhibits osteoclast differentiation and bone-resorption activity without causing any cytotoxicity. Galectin-3 inhibits osteoclastic function without affecting adhesiveness of osteoclasts to dentine surface. It would be interesting to assess the signaling mechanism in the suppression of osteoclastic function. The NFATc1 signaling could similarly be suppressed in the inhibition of osteoclast function as demonstrated in osteoclastogenesis. We successfully demonstrated an inhibition of actin-ring formation in mature osteoclasts by treatment with galectin-3, suggesting an involvement of cytoskeletal regulation in galectin-3-mediated suppression of bone resorption. As the actin-ring formation is considered to be prerequisite for bone resorption, suppression of actin-ring formation by galectin-3 would affect formation of ruffled border, the ultrastructure required for bone resorption. Furthermore, we successfully ameliorated the severity of bone destruction by administering recombinant galectin-3 into the joint cavities of AA rats. Here we would like to propose the hypothesis that an extremely high level of galectin-3 in the area of severe bone destruction in AA rats acts as a negative regulator for osteoclastogenesis and osteoclastic function to prevent excessive bone destruction.
Several reports have described the essential role of IFN-β in the negative regulation of RANKL-dependent osteoclast differentiation.49, 50, 51 The continuous production of IFN-β by injection of IFN-β-expressing fibroblast or daily subcutaneous treatment with recombinant IFN-β inhibited inflammation as well as bone destruction in a system of collagen-induced arthritis in mice.52, 53 In addition, the expression of IFN-β was increased in synovial tissue of patients with RA. Its increased production in RA may be a reactive attempt to inhibit inflammation and bone destruction.54 The increased galectin-3 in the area of severe bone destruction in AA rats could serve as a potent negative regulator for osteoclastogenesis, like IFN-β. Our results suggest the utility of exogenously administrated galectin-3 as one of the effective therapies for preventing pathological bone resorption observed in various bone diseases such as RA.
References
Hsu DK, Liu FT . Regulation of cellular homeostasis by galectins. Glycoconj J 2004;19:507–515.
Liu FT . Molecular biology of IgE-binding protein, IgE-binding factors, and IgE receptors. Crit Rev Immunol 1990;10:289–306.
Liu FT, Hsu DK, Zuberi RI, et al. Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol 1995;147:1016–1028.
Truong MJ, Gruart V, Kusnierz JP, et al. Human neutrophils express immunoglobulin E (IgE)-binding proteins (Mac-2/epsilon BP) of the S-type lectin family: role in IgE-dependent activation. J Exp Med 1993;177:243–248.
Truong MJ, Gruart V, Liu FT, et al. IgE-binding molecules (Mac-2/epsilon BP) expressed by human eosinophils. Implication in IgE-dependent eosinophil cytotoxicity. Eur J Immunol 1993;23:3230–3235.
Hrdlicková-Cela E, Plzák J, Smetana Jr K, et al. Detection of galectin-3 in tear fluid at disease states and immunohistochemical and lectin histochemical analysis in human corneal and conjunctival epithelium. Br J Ophthalmol 2001;85:1336–1340.
Nachtigal M, Al-Assaad Z, Mayer EP, et al. Galectin-3 expression in human atherosclerotic lesions. Am J Pathol 1998;152:1199–1208.
Zuberi RI, Hsu DK, Kalayci O, et al. Critical role for galectin-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. Am J Pathol 2004;165:2045–2053.
Feldmann M, Brennan FM, Maini RN . Rheumatoid arthritis. Cell 1996;85:307–310.
Gough A, Sambrook P, Devlin J, et al. Osteoclastic activation is the principal mechanism leading to secondary osteoporosis in rheumatoid arthritis. J Rheumatol 1998;25:1282–1289.
Gravallese EM, Harada Y, Wang JT, et al. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol 1998;152:943–951.
Boyle WJ, Simonet WS, Lacey DL . Osteoclast differentiation and activation. Nature 2003;423:337–342.
Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998;93:165–176.
Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999;402:304–309.
Takayanagi H, Iizuka H, Juji T, et al. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum 2000;43:259–269.
Toh K, Kukita T, Wu Z, et al. Possible involvement of MIP-1alpha in the recruitment of osteoclast progenitors to the distal tibia in rats with adjuvant-induced arthritis. Lab Invest 2004;84:1092–1102.
Urushibara M, Takayanagi H, Koga T, et al. The antirheumatic drug leflunomide inhibits osteoclastogenesis by interfering with receptor activator of NF-kappa B ligand-stimulated induction of nuclear factor of activated T cells c1. Arthritis Rheum 2004;50:794–804.
Miyazaki M, Fujikawa Y, Takita C, et al. Tacrolimus and cyclosporine A inhibit human osteoclast formation via targeting the calcineurin-dependent NFAT pathway and an activation pathway for c-Jun or MITF in rheumatoid arthritis. Clin Rheumatol 2007;26:231–239.
del Pozo V, Rojo M, Rubio ML, et al. Gene therapy with galectin-3 inhibits bronchial obstruction and inflammation in antigen challenged rats through interleukin-5 gene downregulation. Am J Respir Crit Care Med 2002;166:732–737.
López E, del Pozo V, Miguel T, et al. Inhibition of chronic airway inflammation and remodeling by galectin-3 gene therapy in a murine model. J Immunol 2006;176:1943–1950.
Ohshima S, Kuchen S, Seemayer CA, et al. Galectin-3 and its binding protein in rheumatoid arthritis. Arthritis Rheum 2003;48:2788–2795.
Ortega N, Behonick DJ, Colnot C, et al. Galectin-3 is a downstream regulator of matrix metalloproteinase-9 function during endochondral bone formation. Mol Biol Cell 2005;16:3028–3039.
Ho MK, Springer TA . Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol 1982;128:1221–1228.
Niida S, Amizuka N, Hara F, et al. Expression of Mac-2 antigen in the preosteoclast and osteoclast identified in the op/op mouse injected with macrophage colony-stimulating factor. J Bone Miner Res 1994;9:873–881.
Kuratani T, Nagata K, Kukita T, et al. Induction of abundant osteoclast-like multinucleated giant cells in adjuvant arthritic rats with accompanying disordered high bone turnover. Histol Histopathol 1998;13:751–759.
Wu Z, Nagata K, Iijima T . Immunohistochemical study of NGF and its receptors in the synovial membrane of the ankle joint of adjuvant-induced arthritic rats. Histochem Cell Biol 2000;114:453–459.
Kukita A, Kukita T, Shin JH, et al. Induction of mononuclear precursor cells with osteoclastic phenotypes in a rat bone marrow culture system depleted of stromal cells. Biochem Biophys Res Commun 1993;196:1383–1389.
Kukita A, Kukita T, Hata K, et al. Heat-treated osteoblastic cell (ROS17/2.8)-conditioned medium induces the formation of osteoclast-like cells. Bone Miner 1993;23:113–127.
Watanabe T, Kukita T, Kukita A, et al. Direct stimulation of osteoclastogenesis by MIP-1alpha: evidence obtained from studies using RAW264 cell clone highly responsive to RANKL. J Endocrinol 2004;180:192–201.
Kukita T, Kukita A, Hata K, et al. 12-O-Tetradecanoylphorbol-13-acetate inhibits osteoclast-like cell differentiation in rat bone marrow cultures by inducing macrophage polykaryons. Endocrinology 1992;130:577–584.
Kukita T, Wada N, Kukita A, et al. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med 2004;200:941–946.
Ochieng J, Furtak V, Lukyanov P . Extracellular functions of glaectin 3. Glycoconj J 2004;19:527–535.
Ochieng J, Leite-Browning ML, Warfield P . Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem Biophys Res Commun 1998;246:788–791.
Stillman BN, Hsu DK, Pang M, et al. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol 2006;176:778–789.
Magnaldo T, Fowlis D, Darmon M . Galectin-7, a marker of all types of stratified epithelia. Differentiation 1998;63:159–168.
Neidhart M, Zaucke F, von Knoch R, et al. Galectin-3 is induced in rheumatoid arthritis synovial fibroblasts after adhesion to cartilage oligomeric matrix protein. Ann Rheum Dis 2005;64:419–424.
Dasuri K, Antonovici M, Chen K, et al. The synovial proteome: analysis of fibroblast-like synoviocytes. Arthritis Res Ther 2004;6:R161–R168.
Pearson CM, Wood FD . Studies of arthritis and other lesions induced in rats by the injection of mycobacterial adjuvant. VII. Pathologic details of the arthritis and spondylitis. Am J Pathol 1963;42:73–95.
Terrier F, Hricak H, Revel D, et al. Magnetic resonance imaging and spectroscopy of the periarticular inflammatory soft-tissue changes in experimental arthritis of the rat. Invest Radiol 1985;20:813–823.
Shou J, Bull CM, Li L, et al. Identification of blood biomarkers of rheumatoid arthritis by transcript profiling of peripheral blood mononuclear cells from the rat collagen-induced arthritis model. Arthritis Res Ther 2006;8:R28.
Yasuda H, Shima N, Nakagawa N, et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 1998;139:1329–1337.
Zhou H, Kartsogiannis V, Hu YS, et al. A novel osteoblast-derived C-type lectin that inhibits osteoclast formation. J Biol Chem 2001;276:14916–14923.
Zhou H, Kartsogiannis V, Quinn JM, et al. Osteoclast inhibitory lectin, a family of new osteoclast inhibitors. J Biol Chem 2002;277:48808–48815.
Gange CT, Quinn JM, Zhou H, et al. Characterization of sugar binding by osteoclast inhibitory lectin. J Biol Chem 2004;279:29043–29049.
Mey A, Leffler H, Hmama Z, et al. The animal lectin galectin-3 interacts with bacterial lipopolysaccharides via two independent sites. J Immunol 1996;156:1572–1577.
Janelle-Montcalm A, Boileau C, Poirier F, et al. Extracellular localization of galectin-3 has a deleterious role in joint tissues. Arthritis Res Ther 2007;9:R20.
Dumic J, Dabelic S, Flögel M . Galectin-3: an open-ended story. Biochim Biophys Acta 2006;1760:616–635.
Sano H, Hsu DK, Yu L, et al. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol 2000;165:2156–2164.
Takayanagi H, Kim S, Matsuo K, et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature 2002;416:744–749.
Hayashi T, Kaneda T, Toyama Y, et al. Regulation of receptor activator of NF-kappa B ligand-induced osteoclastogenesis by endogenous interferon-beta (INF-beta) and suppressors of cytokine signaling (SOCS). The possible counteracting role of SOCSs- in IFN-beta-inhibited osteoclast formation. J Biol Chem 2002;277:27880–27886.
Nakamura T, Kukita T, Shobuike T, et al. Inhibition of histone deacetylase suppresses osteoclastogenesis and bone destruction by inducing IFN-beta production. J Immunol 2005;175:5809–5816.
Triantaphyllopoulos KA, Williams RO, Tailor H, et al. Amelioration of collagen-induced arthritis and suppression of interferon-gamma, interleukin-12, and tumor necrosis factor alpha production by interferon-beta gene therapy. Arthritis Rheum 1999;42:90–99.
van Holten J, Reedquist K, Sattonet-Roche P, et al. Treatment with recombinant interferon beta reduces inflammation and slows cartilage destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Rheum Ther 2004;6:R239–R249.
van Holten J, Smeets TJ, Blankert P, et al. Expression of interferon beta in synovial tissue from patients with rheumatoid arthritis: comparison with patients with osteoarthritis and reactive arthritis. Ann Rheum Dis 2005;64:1780–1782.
Acknowledgements
We thank Dr Y Aida (Kyushu University, Faculty of Dental Science) for helpful discussions. We also thank Dr T Iijima (Emeritus Professor, Kyushu University) for encouragement. We appreciate Dr Dovie R Wylie for copyediting our article. This work was supported in part by a grant for Scientific Research from the Japanese Ministry of Education Science and Culture (project 16390528).
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE
The authors state no conflict of interest.
Supplementary Information accompanies the paper on the Laboratory Investigation website (http://www.laboratoryinvestigation.org)
Rights and permissions
About this article
Cite this article
Li, YJ., Kukita, A., Teramachi, J. et al. A possible suppressive role of galectin-3 in upregulated osteoclastogenesis accompanying adjuvant-induced arthritis in rats. Lab Invest 89, 26–37 (2009). https://doi.org/10.1038/labinvest.2008.111
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2008.111
Keywords
This article is cited by
-
Modulation of osteoclastogenesis through adrenomedullin receptors on osteoclast precursors: initiation of differentiation by asymmetric cell division
Laboratory Investigation (2021)
-
Differential regulation and correlation between galectin-9 and anti-CCP antibody (ACPA) in rheumatoid arthritis patients
Arthritis Research & Therapy (2020)
-
Loss of Lgals3 Protects Against Gonadectomy-Induced Cortical Bone Loss in Mice
Calcified Tissue International (2020)
-
Enhanced cortical bone expansion in Lgals3-deficient mice during aging
Bone Research (2018)
-
Galectin-8 induces functional disease markers in human osteoarthritis and cooperates with galectins-1 and -3
Cellular and Molecular Life Sciences (2018)