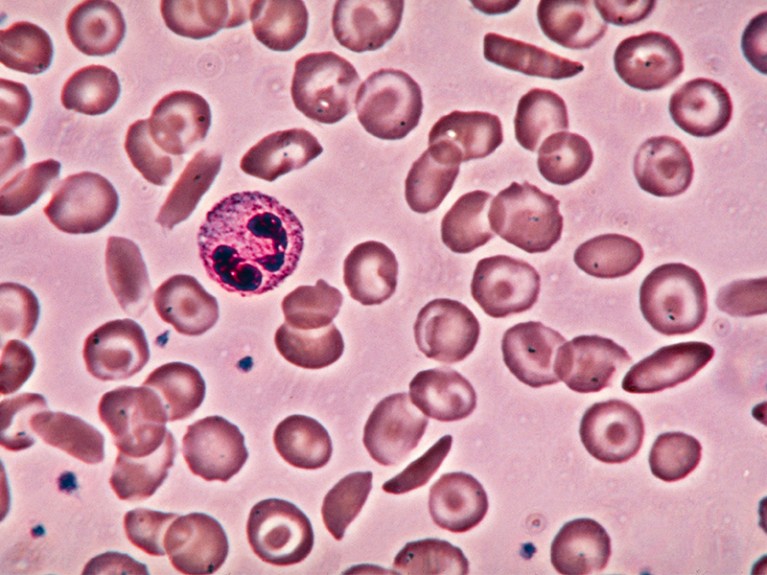
A gene-editing therapy to correct deformed red blood cells in sickle-cell disease is in the works — but at what cost?Credit: Eric Grave/SPL
“We wish to suggest a structure for the salt of deoxyribose nucleic acid (D.N.A.),” wrote James Watson and Francis Crick in this journal in 1953 (J. D. Watson and F. H. C. Crick Nature 171, 737–738; 1953). “This structure has novel features which are of considerable biological interest.”
In the 70 years since those famous words were published, researchers have poured huge effort into unravelling those features and harnessing them for medicine. The result is a flourishing understanding of the genetic causes of diseases — and a host of therapies designed to treat them.
Seventy years from now, the world might look back on 2023 as a landmark, as well. This year could see the first authorization of a therapy based on CRISPR–Cas9 gene editing, that involves tweaking the DNA in the body’s non-reproductive (somatic) cells. Gene editing allows scientists — and could soon permit clinicians — to make changes to targeted regions in the genome, potentially ‘correcting’ genes that cause disease. Regulators in the United States, the European Union and the United Kingdom are evaluating a therapy that uses this approach to treat sickle-cell disease, and a decision could be made in the next few months.
CRISPR gene therapy shows promise against blood diseases
But even as such advances accrue, researchers are worrying about the future role of gene editing — as well as other, more established forms of gene therapy — in treating disease. Gene therapies currently carry eye-watering price tags, putting them out of the reach of many who need them. High prices could diminish the willingness of government funders to pay for gene-therapy research. And that, in turn, would make it harder for research institutions to continue to attract top talent to the field. Researchers, especially health economists, must work urgently with industry and governments to find a more affordable funding model.
Million-dollar treatments
CRISPR–Cas9’s speedy path to the clinic was paved by years of steady advances in forms of gene therapy that use a virus to shuttle genes into cells. Over the past decade, regulators have approved several such gene therapies, for example CAR-T-cell therapies, which engineer immune cells to treat cancer. Hundreds more are in clinical trials.
These therapies typically cost something like US$1 million for a single treatment, and more once the costs of administering them, such as hospital stays and procedures required to isolate and manipulate cells, are factored in. Last year, the US Food and Drug Administration approved the first gene therapy to treat haemophilia B, a genetic disease that impairs blood clotting. The price is $3.5 million per treatment, making the therapy, called Hemgenix, the most expensive drug in the world.
Gene therapies are more costly to develop and produce than are more well-established treatments based on small-molecule drugs. But gene therapies can also carry the hope of a cure, freeing recipients from both long-term reliance on expensive medicines and the risk of hospitalizations. Some have argued that this justifies the high cost: if a therapy can save millions in downstream treatments, the initial outlay would still save money overall. Over time, after all, the costs of more-conventional treatments add up: one study, for example, found that in the United States, the cost of treating a person with sickle-cell anaemia until the age of 64 is $1.7 million (K. M. Johnson et al. Blood Adv. 7, 365–374; 2023).
Researchers welcome $3.5-million haemophilia gene therapy — but questions remain
Even in wealthy countries, health-care systems are ill-equipped to shoulder the high initial costs associated with gene therapies. In 2021, therapeutics developer Bluebird Bio in Somerville, Massachusetts, withdrew plans to market a gene therapy for β-thalassaemia — another blood disorder — in Europe, after failing to reach an agreement with European authorities over the price. It said it would focus its sales efforts on the United States, where there has been comparatively little regulation of drug costs.
But even in the United States, costs matter. US health insurance is often subsidized by employers, and some are already saying that they will probably restrict their coverage of gene therapies in the next year, says Steven Pearson, president of the Institute for Clinical and Economic Review, a health-economics think tank in Boston, Massachusetts.
Low- and middle-income countries, meanwhile, are left entirely in the lurch. This is especially painful given that some of the diseases under consideration, such as β-thalassaemia and sickle-cell disease, are more common in poorer parts of the world than in wealthy nations. In some sub-Saharan regions, for example, it is estimated that about 2% of children are born with sickle-cell disease. This is likely to be an underestimate, given how little screening is taking place.
Improving access
It is too soon to know how much the CRISPR–Cas9 treatment for sickle-cell disease would cost; neither of its developers, Vertex Pharmaceuticals in Boston, Massachusetts, or CRISPR Therapeutics in Cambridge, Massachusetts, have disclosed what they will charge. But researchers are bracing themselves for the price tag to come.
At the Third International Summit on Human Genome Editing, held in London in March, much of the discussion centred on making gene-editing therapies accessible, particularly to low- and middle-income countries. The focus was on technological approaches to streamline the production and testing of such treatments. The sickle-cell treatment, for example, requires clinicians to isolate and edit blood-forming stem cells, destroy those that remain in the body, and then reinfuse the edited cells. Converting this to a genome-editing procedure that could be performed directly in the body rather than in isolated cells could make the treatment cheaper and more accessible.
Expensive treatments for genetic disorders are arriving. But who should foot the bill?
Another appealing approach is to develop gene-therapy platforms that have already been confirmed to be safe and effective. Gene-therapy developers could then just swap in a gene that targets the chosen disease, without the gamut of tests of safety and efficacy that are required when starting from scratch.
But technological solutions such as these will go only so far. US drug pricing has little to do with how much it costs to produce a therapy, says Pearson, because companies can charge as much as the market will bear. How much that price will drop in other countries could be limited by intellectual property rights and hindered by the complexities of making generic copies of biological drugs such as gene therapies. Some academic centres are trying to develop and deploy gene therapies without relying on pharmaceutical companies, but it is unclear how far such efforts can stretch without the financial resources and regulatory expertise found in industry.
In addition to pricing, gene-therapy technologies are mired in debates around regulation and intellectual property. How each of these plays out will determine how far researchers can go in capitalizing on Watson and Crick’s initial discovery. It’s important that scientists have an active role in these debates, and that they push such discussions to the fore sooner rather than later.

 CRISPR gene therapy shows promise against blood diseases
CRISPR gene therapy shows promise against blood diseases
 Researchers welcome $3.5-million haemophilia gene therapy — but questions remain
Researchers welcome $3.5-million haemophilia gene therapy — but questions remain
 Expensive treatments for genetic disorders are arriving. But who should foot the bill?
Expensive treatments for genetic disorders are arriving. But who should foot the bill?
 License CRISPR patents for free to share gene editing globally
License CRISPR patents for free to share gene editing globally
 The paper: Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid
The paper: Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid