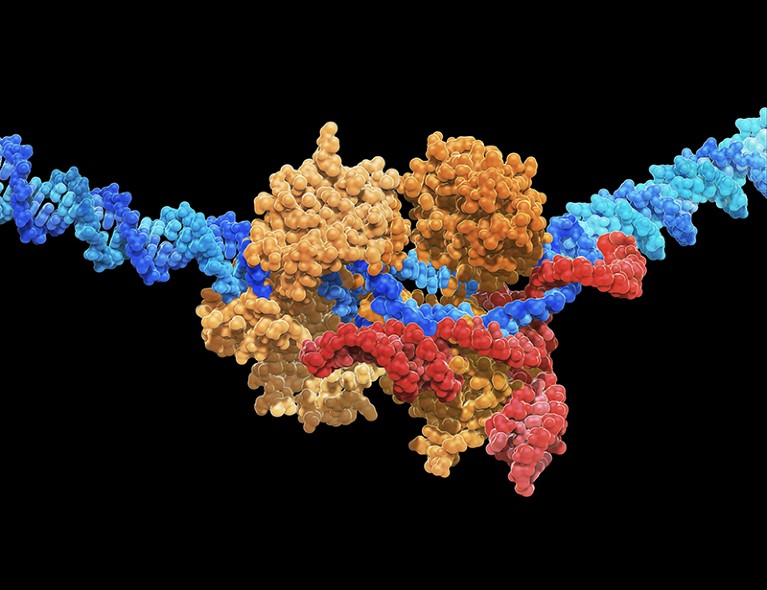
Gene-editing systems, such as CRISPR-Cas9, can be used to give stem cells immune-evasive properties.CARLOS CLARIVAN/SPL
After decades of development, the dream of regenerative medicine has become a clinical reality — in part. Researchers can now cultivate stem cells in a laboratory, transform them into specialized cell types and then transplant them into people to alleviate disease.
In theory, this strategy promises an endless supply of replacement parts for ailing and ageing bodies: neurons to combat Parkinson’s disease, insulin-producing pancreatic cells to reverse type 1 diabetes, heart muscle cells to enhance cardiac function, and more.
But there’s a catch: therapies derived from stem cells must be customized to the patient — a process that is both slow and expensive. Or they can be made using donor cells. But, because the immune system tends to reject foreign cells, these ‘allogeneic’ off-the-shelf treatments require the concurrent administration of immune-dampening medicines — a strategy that raises the risk of complications such as infection and cancer.
Now, researchers are exploring a third approach — one that could fully realize the vision of mass-produced cell therapies for everyone, without the need for immune suppression.
By harnessing the power of gene-editing techniques, particularly CRISPR–Cas systems, to endow stem cells with immune-evasive properties, researchers can fashion stem cells that circumvent the immune system’s recognition mechanisms. They can also incorporate fail-safe features to ensure that the cells can be eliminated in the event of unforeseen complications. Such ‘stealth’ cells could, in principle, underpin a wide range of cell-replacement therapies, and billions of dollars have been invested in this work over the past five years.
The idea still requires validation. Only a small number of people have so far received any form of cell-replacement therapy derived from immune-edited stem cells, and no clinical results have yet been publicly disclosed. But with more products of this kind slated to enter human testing later this year, researchers are optimistic.
“We know in theory that it will work,” says Torsten Meissner, an immunologist at Beth Israel Deaconess Medical Center in Boston, Massachusetts, who points to the natural precedent of immune evasion to underscore his conviction: “Tumours have figured it out. Viruses have figured it out. Pregnancy is the other example.” Now, he says, biotechnology companies just need to work out how to emulate the same tactics for therapeutic gain.
Incognito mode
Strategies differ, but there are some gene edits that all researchers agree must underpin any universal stem-cell-derived therapy. There is also widespread consensus that the optimal product should incorporate as few edits as possible, both to minimize the potential for unintended genetic consequences and to streamline manufacturing and regulatory approval.
Beyond that, the scientific community is divided. The complexities of the immune system have fuelled spirited debates over the exact genetic manipulations necessary to create a cell therapy that is both capable of bypassing immune defences and delivering meaningful health benefits.
“The immune system is pervasive and persistent,” says Charles Murry, a cardiovascular pathologist at the University of Washington in Seattle and chief executive of StemCardia in Seattle, one of a growing number of biotechnology companies developing gene-editing strategies to overcome immune barriers in regenerative cell treatments.
It might take the immune system a while to find donor cells, Murry notes, “but find them it does. It’s ancient, smart and has lots of tricks up its sleeves.” Researchers must, therefore, be equally crafty when designing cells to evade it.
Could CAR-T-cell therapy offer hope to children with cancer?
In most cases, the process starts by disrupting at least one part of the cell’s major histocompatibility complex (MHC), a cluster of proteins that functions like a molecular identity card, displaying unique pieces of cellular information that tell the immune system’s foot soldiers — a group of cells known as T lymphocytes — whether the cell is friend or foe.
“That’s the ‘universal’ element of the universal donor cell,” Murry explains. This edit strips the transplanted cell of its enemy identity, allowing it to seamlessly blend into its new environment and evade T-cell detection.
But the lack of MHC expression also presents a problem. Without the usual distinguishing markers of either ally or adversary, the edited cell becomes susceptible to attack by a different set of immune actors — natural killer (NK) cells, which have evolved to target and eliminate abnormal cells, including those without the proper MHC signatures.
To counteract this vulnerability, some researchers reintroduce genes that encode specific MHC antigens — ones that allow the cell to temper NK cells without inciting T-cell responses. Others are putting in genes that express ‘checkpoint’ proteins, molecules designed to directly curb the activity of NK cells.
Sana Biotechnology in Seattle, which favours the latter approach, reported last year that just three edits — two to eliminate MHC expression and one to boost expression of a checkpoint protein called CD47 — were sufficient to shield cells of rhesus monkeys (Macaca mulatta) from the animals’ immune systems1. It also showed that human cells, modified in the same manner, could ameliorate diabetes when transplanted into a mouse model2.
In November, Sana announced that it had the go-ahead to begin testing, in people, of donated human pancreatic cells that had been edited in this way. Trials of a stem-cell-derived product are likely to follow.
But not everyone has managed to replicate the findings around CD47. And with conflicting reports about how best to restrain NK-cell activity, stem-cell biologist Audrey Parent at the University of California, San Francisco (UCSF), sees that piece of the immune-evasion puzzle as the primary bottleneck in the field. “The NK cell part is not resolved yet,” she says.
Covert agents
Disagreement around NK-cell inhibition arises, at least in part, from the various methods laboratories use to assess the modified cells’ ability to evade immune detection. Although most research groups evaluate their edited stem cells in engineered mice with human-like immune systems, these ‘humanized’ models cannot faithfully replicate the complete immune response that cell products will face in people’s bodies.
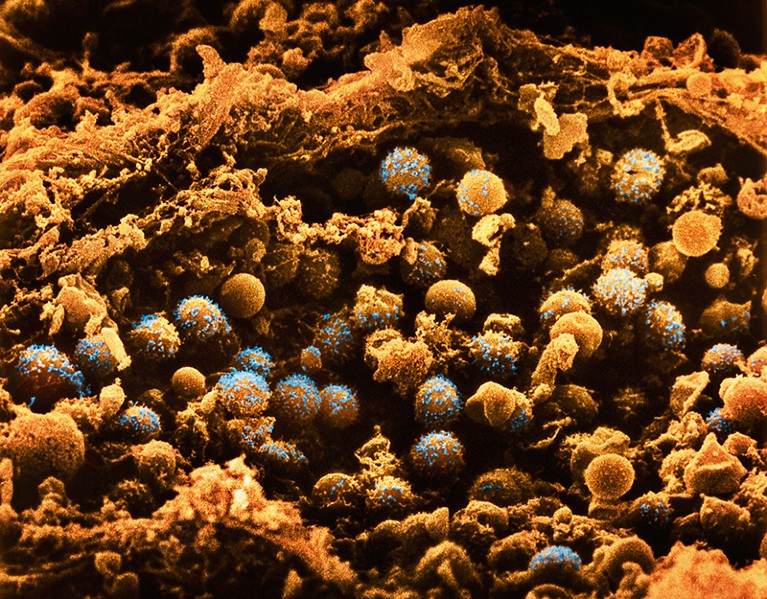
Pancreatic cells could potentially be edited to treat diabetes.Credit: LENNART NILSSON, BOEHRINGER INGELHEIM INTERNATIONAL GmbH/SPL
Conversely, others generate gene-edited stem cells from monkeys and transplant them into other monkeys, mirroring the clinical scenario with humans. But this strategy is constrained by ethical concerns and the expense of experimentation with primates. Moreover, monkeys, although genetically similar to people, have distinct immune systems that might not faithfully reflect human responses.
Deepta Bhattacharya, an immunologist at the University of Arizona in Tucson, favours a different approach. When it comes to pushing the boundaries of immune evasion, he advocates evaluating universal gene-edited products that are intended for human use in mice with fully intact, natural immune systems. If cell therapies can pass this cross-species test, he reasons, they should be well-suited for transplantation into any human recipient.
Early this year, Bhattacharya and his colleagues reported that human stem cells containing a battery of 12 gene edits could survive in mice for months, with no signs of immune recognition or rejection3.
“A few of [the edits] we don’t think we actually need,” Bhattacharya says. But some edits that he considers crucial for thwarting rejection target a branch of the body’s natural defence mechanism known as the complement system. This system acts as a first line of defence against potential invaders by preparing antibodies to mark and eliminate foreign cells.
“Antibodies are tricky,” says Chad Cowan, co-founder and chief executive of Clade Therapeutics, a Boston-based biotech firm that is developing stem-cell-derived therapies for cancer and autoimmune conditions. (Bhattacharya is also a scientific co-founder.) “I think we’ve solved the cellular side of the equation,” Cowan says. “But antibodies actually turn out to be a bigger barrier than we thought.”
Clade’s solution, currently unpublished, involves engineering cells to secrete an enzyme that degrades and incapacitates nearby antibodies, thereby neutralizing the complement system. Another approach comes from Sonja Schrepfer, head of the hypoimmune platform at Sana who, together with UCSF heart surgeon Tobias Deuse and their colleagues, reported last year that overexpression of a protein that binds and disables antibodies can achieve the same result4.
Neither approach has been vetted in people — and, as molecular endocrinologist Timothy Kieffer at the University of British Columbia in Vancouver points out: “Strategies to thwart the highly evolved immune system are numerous, but are only hypothetical until proven otherwise.”
“The true test can only come in clinical trials,” he says.
Kieffer is also chief scientific officer of Fractyl Health, a metabolic therapeutics company in Lexington, Massachusetts. But two years ago, while serving as chief scientific officer for ViaCyte in San Diego, California, Kieffer played a pivotal part in launching the first clinical study of a stem-cell-derived product that incorporated immune-cloaking edits.
This pioneering product, developed in collaboration with biotech firm CRISPR Therapeutics in Boston, was named VCTX210. Designed to help people with type 1 diabetes to produce their own insulin, the product incorporated a suite of four gene edits collectively intended to enhance immune evasion and bolster cell survival. A subsequent version of this therapy, termed VCTX211, included an additional two edits, each aimed at further enhancing the robustness and functionality of the cells.
Invisibility shield
How effective these therapies were at sidestepping immune detection and improving the control of type 1 diabetes remains unclear. As Nature went to press, no results had been publicly disclosed. And both Vertex (which acquired ViaCyte in 2022, but is now working on separate stem-cell-derived therapies, using gene-editing technologies from CRISPR Therapeutics) and CRISPR Therapeutics (which now wholly owns the VCTX210 and VCTX211 assets) declined to comment on their immune-evasive cell-therapy programmes.
Also unclear is whether any safety concerns emerged in these trials. This matter is of utmost importance to researchers such as Kieffer because, as he explains, “concerns arise with manipulating the genome of cells for therapy, particularly when the goal is to endow them with an invisibility cloak that could be problematic should the cells become dangerous to the recipient”.
In the ViaCyte-CRISPR-Therapeutics trials, the companies took the precautionary step of encapsulating their immune-evasive cells in small, sticking-plaster-sized pouches, which are then implanted beneath the person’s skin. These devices contain pores that allow blood vessels to enter, providing oxygen and nutrients to the metabolically active cells inside, but prevent any therapeutic cells from escaping. If any unanticipated issues arise, they can be swiftly retrieved before rogue cells cause widespread damage.
Turbocharged CAR-T cells melt tumours in mice — using a trick from cancer cells
Another safety measure involves the integration of genetic fail-safe features into the edited cells themselves. These features include drug-inducible suicide genes that can be activated by administration of a relatively benign medication. Researchers are also adorning modified cells with surface proteins that can be targeted with clinically approved antibody drugs, thereby achieving the same goal of cell destruction should any transplants turn cancerous or problematic in other ways.
In the end, the optimal safety strategy — not to mention the ideal amount of gene editing necessary to tamp down immune responses — can vary with the disease. A ready-to-use cell therapy for cancer does not necessarily need to incorporate the same design features as one tailored for diabetes, for instance, given the differences in the immune system that these cell products will confront and the distinct risk–benefit consideration in each disease. “There is no one catch-all solution,” Meissner says.
Certain parts of the body, including the eye and the brain, also enjoy an ‘immune privileged’ status, meaning that only a limited set of immune cells can enter them. This has led companies such as BlueRock Therapeutics in Cambridge, Massachusetts, which is developing off-the-shelf stem-cell-derived therapies for Parkinson’s disease, to tailor their immune-editing strategies accordingly. “There are some unique opportunities when you’re in the brain,” says BlueRock’s head of immunology, Greg Motz.
Those opportunities won’t be the last word on universal cell therapies, of course. Rather, Murry expects to see incremental advancement in the field, with short-term wins and losses informing long-term editing strategies.
“I would love it to be perfect out of the gates, but that’s not realistic,” Murry says. “This is going to be like peeling an onion.”

 Turbocharged CAR-T cells melt tumours in mice — using a trick from cancer cells
Turbocharged CAR-T cells melt tumours in mice — using a trick from cancer cells
 Could CAR-T-cell therapy offer hope to children with cancer?
Could CAR-T-cell therapy offer hope to children with cancer?
 CRISPR cancer trial success paves the way for personalized treatments
CRISPR cancer trial success paves the way for personalized treatments