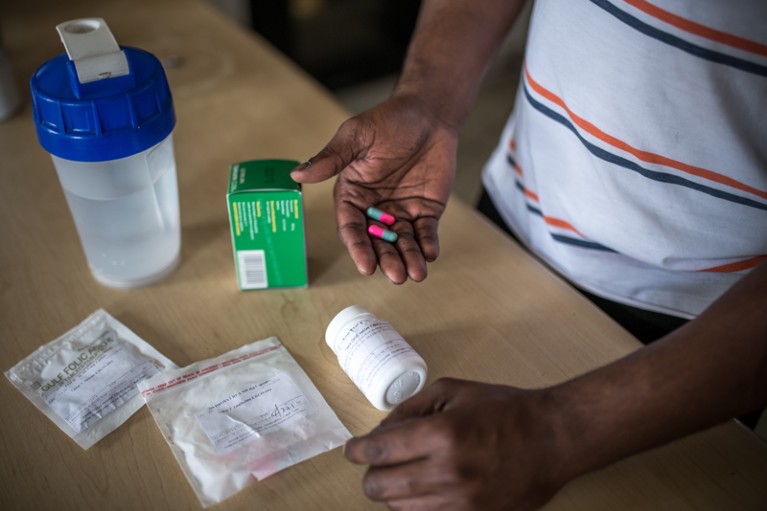
In the absence of targeted therapies, people with sickle-cell disease are taking combinations of drugs, such as folic acid, penicillin and hydroxyurea.Credit: Aurélie Marrier d'Unienville for Nature
There was a time when Olu Akinyanju felt that no one was listening.
In 1994, the physician founded Sickle Cell Foundation Nigeria, with a mission to provide support for people with sickle-cell disease — a hereditary blood disorder that affects 20 million individual worldwide. The condition is most common in tropical regions of sub-Saharan Africa, but is also found in many other parts of the globe. It can cause strokes, organ failure and harrowing episodes of excruciating pain. Between 50% and 90% of children in sub-Saharan Africa and India with the disease will die before their fifth birthday.
For years, Akinyanju tried and failed to get traction with the World Health Organization (WHO). And leading health policymakers in African countries also had other health and development priorities.
Now the landscape is changing. As we describe in a News Feature this week, sickle-cell disease is finally catching the attention of funders, governments and pharmaceutical companies. But as they work on innovative ways to tackle the disease, one challenge stands out: how to get treatments to those in need.
Gene therapy is facing its biggest challenge yet
Most patients come from communities that have long faced discrimination and economic hardship. They can be stigmatized, and discussions about the condition tend to be rare. That’s partly why, although scientists have known the disease’s root molecular cause for 70 years, research has produced few new treatments.
But in the past decade, more support groups have started to spring up in Nigeria. Internationally, organizations ranging from the WHO to the American Society of Hematology have made treatments for sickle-cell disease a priority. Newborn-screening programmes have been expanding, and efforts are being made to deploy an old chemotherapy drug called hydroxyurea in Africa to help ease symptoms.
Last week, the US Food and Drug Administration (FDA) approved the first drug, voxelotor, to target the cause of the disease. Made by Global Blood Therapeutics in South San Francisco, California, it reduces the interactions between mutated haemoglobin proteins that lead to the sickled blood cells characteristic of the condition. That came hot on the heels of the FDA approving a drug called crizanlizumab, made by Novartis in Basel, Switzerland, which helps to stop the sickled cells from sticking together.
In October, the US National Institutes of Health (NIH) and the Bill & Melinda Gates Foundation in Seattle, Washington, announced a landmark programme to develop gene-based technologies to treat sickle-cell disease and HIV in Africa. Both will contribute US$100 million over the next 4 years, and the ambition is to fund treatments into clinical trials within 10 years.
These developments are promising, but they don’t address one stark reality. Most people with the disease struggle to access even basic health care, and the new treatments have a hefty price tag.
In 2017, the FDA approved a treatment called Endari, made by Emmaus Medical in Torrance, California. Endari is a formulation of the amino acid glutamine, and costs $13,000 a year. Unsurprisingly, US physicians are struggling to get insurance companies to foot the bill — meaning that many people are unable to access the treatment.
Targeted stem-cell attack could make transplants safer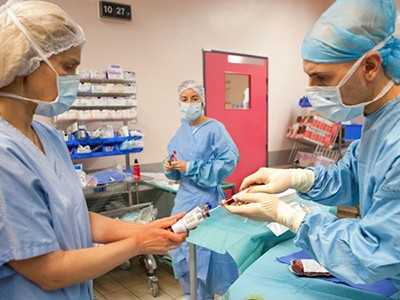
The first gene therapies for the disease, which involve an elaborate procedure much like a stem-cell transplant, are likely to cost upwards of $1 million per patient. And transplant procedures and hospital stays will push costs higher. The excitement even of voxelotor’s landmark approval needs to be tempered by the fact that the treatment costs $125,000 per year per patient.
This means that advocates such as Akinyanju cannot yet slow down. They have made impressive gains. But alongside the growing sums being invested in research and development, foundations, advocates and patients will continue to need support — especially for the costs of treatments.
Researchers can help — not only through their work, but also by continuing to pressure the government officials, donors and health-care providers with whom they interact to consider the issue of who will foot the bill.
The payment question isn’t confined to sickle-cell disease. It bedevils many of the bespoke drugs emerging from biomedical research. What is clear is that the current health-care models won’t work: insurance companies baulk at the costs, and public systems often can’t afford them. An answer will require the combined efforts of biomedical scientists, health-care economists, public-health experts and others.
The NIH and the Gates foundation want a future in which the disease can be treated with a one-time therapy in an outpatient setting — and that is potentially achievable. But companies, funders and governments must find ways to ensure that the costs are not shouldered by communities that have already suffered for too long.

 Gene therapy is facing its biggest challenge yet
Gene therapy is facing its biggest challenge yet
 Targeted stem-cell attack could make transplants safer
Targeted stem-cell attack could make transplants safer
 Scientists use gene-edited stem cells to treat HIV — with mixed success
Scientists use gene-edited stem cells to treat HIV — with mixed success
 Super-precise new CRISPR tool could tackle a plethora of genetic diseases
Super-precise new CRISPR tool could tackle a plethora of genetic diseases
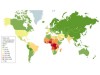 Sickle cell disease
Sickle cell disease
 Therapeutic strategies for sickle cell disease: towards a multi-agent approach
Therapeutic strategies for sickle cell disease: towards a multi-agent approach