Abstract
Integrin αvβ3 is expressed by newly formed blood vessels in diseased and neoplastic tissue and can therefore be used as a marker for angiogenesis. We investigated its expression on the vasculature of 40 colon carcinomas using the anti-αvβ3-specific monoclonal antibody LM609. The average relapse-free interval and overall survival in patients suffering from colon carcinomas with high vascular expression of αvβ3 integrin was significantly reduced compared with that in patients with low αvβ3 integrin expressing tumor vasculature. Moreover, the expression level of αvβ3 integrin correlated with the presence of liver metastases. In conclusion, we propose vascular expression of αvβ3 integrin as a prognostic indicator for colon carcinoma.
Similar content being viewed by others
INTRODUCTION
Angiogenesis, the sprouting of new vessels from preexisting capillaries and postcapillary venules, is a crucial step in normal physiological processes such as embryonic development, the menstrual cycle, wound healing, and inflammation. It also contributes to pathological conditions like diabetic retinopathy, macular degeneration, psoriasis, rheumatoid arthritis, and childhood hemangiomas. Moreover, solid tumors are highly dependent on the formation of new blood vessels to grow beyond the critical size of a few millimeters (1).
The angiogenic process consists of three distinct steps. First, it is initiated by the release of cytokines, such as VEGF, bFGF, IL-8, and TNFα. In a second step, one or more of these substances stimulate the proliferation and migration of endothelial cells and smooth muscle cells. This step is accompanied by the secretion of proteolytic enzymes by endothelial cells, facilitating the vascular invasion process by degradation of the extracellular matrix. Eventually, the invasive vascular sprout reaches the hypoxic source (e.g., a tumor or an ischemic region in wounded or diseased tissue), where it differentiates. In the case of tumoral angiogenesis, the newly formed vessels not only serve for nutrition and waste removal but also as a conduit for tumor cell dissemination (2, 3).
In recent years, interest has been focused on αvβ3, a member of the integrin family that was identified as a marker of angiogenic vascular tissue. The αvβ3 is also expressed by malignant melanoma cells (4), by certain activated leukocytes, by macrophages, osteoclasts, and platelets, and at low levels by some vascular, intestinal, and uterine smooth muscle cells (5, 6).
During neovascularization, integrin αvβ3 is actively involved in the second step of angiogenesis, which includes the maturation of blood vessels during embryogenesis, and injection of an anti-αvβ3 antibody (LM609) into embryos generated a disruption of the normal pattern of vascular development by perturbing vessel lumen formation (7). The same anti-integrin antibody was also reported to inhibit human breast cancer growth and angiogenesis in human skin (8).
The aim of this study was to investigate a possible correlation between vascular αvβ3 integrin expression in colon carcinomas and clinical parameters such as survival and relapse-free interval.
MATERIAL AND METHODS
Cases
Frozen tissue samples of both normal mucosa and tumor were obtained from 40 patients having undergone surgery for colon cancer between 1990 and 1995 at the Tiefenauspital and the Sonnenhofklinik in Bern. Tumors were classified at the Institute of Pathology, University of Bern, according to the TNM classification of the International Union Against Cancer. Tumor sizes were pT1 (10 cases), pT2 (6 cases), pT3 (32 cases), and pT4 (1 case). 18 patients had lymph node involvement; the remaining 22 patients were node negative. In 4 cases, distant organ metastases were reported. There were 5 Dukes A adenocarcinomas, 16 Dukes B, 17 Dukes C, and 2 Dukes D. Carcinomas of 33 patients were located at the left hemicolon, 6 at the right hemicolon, and 1 at the colon transversum. The median age of the patients was 65.5 years. There were 19 female and 21 male patients. The median follow-up time of the patients was almost 48 months (range, 4–82 months).
Tissue Processing
From each specimen, several samples of tumor and of noninvolved adjacent mucosa were embedded in OCT compound medium (Tissue Tek, Bayer, Switzerland), snap-frozen, and kept at −80° C. For each case, one sample of neoplastic and normal tissue was selected, and serial cryostat sections, 4–5 μm thick, were cut, air-dried for 1 hour, and kept in airtight boxes at −20° C until use.
Immunohistochemistry
Tissue sections were immunostained using a biotin/streptavidin technique with alkaline phosphatase. All incubations were done at room temperature. Staining with anti-αvβ3 integrin mAb LM609 was done as follows: the sections were dried at room temperature for 30 minutes, fixed in ethanol for 1 minute, rinsed in distilled water, and immersed in PBS for 5 minutes. After blocking for 15 minutes with 10% normal rabbit serum, the sections were incubated for 1 hour with the anti-αvβ3 monoclonal antibody LM609, washed with PBS, and incubated for 30 minutes with biotinylated F(ab)2 fragments of rabbit anti-mouse immunoglobulins. After rinsing with PBS, the sections were incubated for 30 minutes with a streptavidin-alkaline phosphatase complex, washed with PBS, and reactive sites revealed by incubating the sections for 30 minutes with a compound of naphthol phosphate, fast red, and levamisole in Tris-HCl buffer 0.1 m. Counterstaining was performed by hematoxylin staining for 3 minutes. Control sections were not incubated with the mAb LM609 but included in all subsequent staining steps.
Antibodies and Other Reagents
Monoclonal mouse anti-human integrin αvβ3 (LM609) were from Chemicon (Temecula, CA), normal rabbit serum and biotinylated F(ab′)2 fragment rabbit anti-mouse immunoglobulins were from DAKO A/S (Glostrup, Denmark). Streptavidin-conjugated alkaline phosphatase were from Biogenex (San Ramon, CA) and Fast Red, from DAKO Corporation (Carpinteria, CA).
Evaluation
LM609 staining intensity was as follows: tumor sections and 6 sections of normal mucosa were assessed by three independent observers blinded to patient data. To quantify the immunohistochemical results, four categories ranging from 0 (no expression of αvβ3 integrin) to III (high expression) were used. The staining intensity of vessels was evaluated in five distinct fields (200 ×) for each section, and a mean value was calculated.
Statistical Analysis
The statistical analyses have been performed using the computer package Stata (9). Survival curves have been estimated by the Kaplan-Meier method (10) and compared with the log rank test (11). The Cox regression (12) has been used to model the effect of various factors on survival. Each continuous variable has been recoded in three categories, using as cut-offs the values corresponding to the 25th and 75th centiles of the corresponding distribution. This was done to single out observations in the tails of the distributions. For αvβ3 integrin, such cut-off values were 0.9 and 2 (values corresponding to the expression level as defined in Fig. 3); for VCAM-1, these values were 0.16 and 1.25; for age, they were 58 and 73 years; and for infiltration they were 36 and 78. For tumor grading, five patients were Grade 1 and only two were Grade 3; therefore, Categories 2 and 3 were fused. All variables were then coded into binary indicators. An exploratory model including all available variables was fitted to the data. All P values reported are for two-sided tests.
RESULTS
The expression of integrin αvβ3 was analyzed in 40 human colon carcinoma and 6 normal tissues by immunohistochemistry on frozen sections. The integrin was detected by the monoclonal anti-αvβ3 antibody LM609. Placenta expressing high levels of αvβ3 was used as a positive control (Fig. 1).
Detection of αvβ3 integrin on frozen sections, placenta and melanoma metastases. Expression of αvβ3 integrin in third-trimester placenta predominates on the surface of the syncytiotrophoblast, whereas it is only weakly expressed on endothelial cells of fetal blood vessels. In melanoma metastases, melanoma cells and endothelia of the tumor vasculature exhibited intense αvβ3 integrin reactivity. Control sections were not incubated with the primary antibody but were included in all following staining steps.
Angiogenesis is used as a measure of aggressive tumor growth and is accompanied by elevated αvβ3 expression by angiogenic endothelial cells. In sections of colon carcinoma, αvβ3 expression was restricted to the tumor vasculature (Fig. 2). Tumor cells as well as blood vessels in normal colon tissue were negative. For statistical analysis, we therefore considered only staining intensities of clearly identifiable vessels.
Detection of αvβ3 integrin expression on frozen sections of colon carcinoma and normal colon tissue. αvβ3 integrin was detected on endothelial cells of the tumor vasculature (arrow). In larger vessels, we observed, in addition, labeling within the vessel wall indicating expression of αvβ3 integrin by smooth muscle cells. Control sections were not incubated with the primary antibody but were included in all following staining steps (bar = 50 μm).
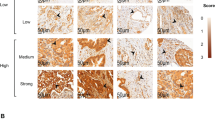
We next examined whether vascular αvβ3 integrin expression on sections of colon carcinomas correlated with the aggressiveness of the tumor. Therefore, we graded the expression level of vascular αvβ3 from 0 (no expression), I (very low expression), II (intermediate expression), or III (high expression; Fig. 3). The photographs shown in Figure 3 were then used as a standard for the evaluation by 3 independent observers. All the six control colon tissues fall into αvβ3 Category 0; no tumor tissue was found with this grading. The expression level of αvβ3 correlated with overall survival (Fig. 4). For statistical analysis of overall and disease-free survival, Cox regression (12) has been used to model the effect of various factors. Those included as a measure of inflammation the expression of VCAM-1 by the tumor vasculature and also leukocyte infiltration. We also determined the degree of tumor invasiveness (T stage and nodal status) as well as the grade of the tumor. Each of these variables has been recoded in three categories using as cut-offs the values corresponding to the 25th and 75th percentiles of the corresponding distribution (Table 1). For αvβ3 these values corresponded to less than 0.9 (≤25%), 0.9–2 (26–75%) and more than 2 (>75%). The median survival time for these categories were 72.5, 51.5, and 24 months, respectively (P = .0528) and the median disease-free survival was 60, 48, and 24 months (P = .014). When fitting a multivariate model, the only parameters that achieved significance for overall survival are the variables identifying the patients with αvβ3 levels of 0.9–2, >2, and of N stage 3. All other parameters did not reach significance and were therefore of no or little prognostic value in this group of patients. Although the confidence intervals are rather wide because of the small sample size, the hazard ratios suggest a trend of increased rate of failure with increasing αvβ3 levels. Similar results held for disease-free survival; however, the associations with αvβ3 integrin expression appeared slightly weaker. At best, the risk of death was 10 times higher for patients expressing αvβ3 integrin above the 75th percentile (expression level more than 2) when compared with patients falling into the 25 lower percentiles (expression level less than 0.9). The expression level of αvβ3 integrin was also correlated with the presence of liver metastases. Tumors that had developed metastases in the liver generally showed a higher grading for αvβ3 integrin expression than did tumors without metastases (Fig. 5).
Kaplan-Meier analysis of survival of colon carcinoma patients correlated with vascular αvβ3 integrin expression. We grouped them into categories defined in Figure 3 according to the vascular expression of αvβ3 integrin expression. From the 40 patients, 10 are in Category I, 20 are in Category II, and 10 are in Category III.
DISCUSSION
In this study, we revealed expression of αvβ3 integrin on the vasculature of colon adenocarcinomas with the established monoclonal antibody LM609. The expression level of αvβ3 was graded and correlated with survival data of the patients and metastatic potential of the tumors. Our data indicate that expression level of αvβ3 integrin may thus be used as a prognostic factor in colon carcinoma.
Expression of integrin αvβ3 was identified on tumoral vessels and was absent in vessels of normal tissue. Integrin αvβ3 was not only found on capillaries but also in the walls of larger vessels and in tumor stroma. These findings are consistent with αvβ3 expression on vascular and intestinal smooth muscle cells (13).
The statistical analysis showed that mean relapse-free interval proved significantly lower in patients suffering from colon carcinomas with high vascular expression of αvβ3. These findings are in accordance with recent observations that indicate a shortened relapse-free interval in breast cancer with high vascular expression of αvβ3 integrin (14). A more dramatic tendency was observed for the overall survival time of the patients. We found that αvβ3 integrin expression represents the best prognostic factor when compared with the tumor grading, node status, depth of invasion, and inflammatory response.
Colorectal cancer often gives rise to metastases in the liver. We therefore sought to correlate vascular αvβ3 expression in colorectal cancer with liver metastases. Tumors that had spread to the liver showed an αvβ3 integrin expression that was nearly double the level of nonmetastatic colon cancers. This finding underlines the fact that tumors with a strong angiogenic activity form metastases more easily, although it remains elusive whether this is caused by an increased dissemination of neoplastic cells through newly formed blood vessels or improved growth conditions at distant sites.
In conclusion, we show that the degree of αvβ3 integrin expression on the vasculature of colon carcinomas is of high prognostic value.
References
Carmeliet C . Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000; 6: 389–395.
Folkman J . The role of angiogenesis in tumor growth. Semin Cancer Biol 1992; 3: 65–71.
Blood CH, Zetter BR . Tumor interactions with the vasculature: angiogenesis and tumor metastasis. Biochim Biophys Acta 1990; 1032: 89–118.
Cheresh DA . Structure, function and biological properties of integrin αvβ3 on human melanoma cells. Cancer Metastasis Rev 1991; 10: 3–10.
Weerasinghe D, McHugh KP, Ross FP, Brown EJ, Gisler RH, Imhof BA . A role for the αvβ3 integrin in the transmigration of monocytes. J Cell Biol 1998; 142: 595–607.
Jones JI, Prevette T, Gockerman A, Clemmons DR . Ligand occupancy of the αvβ3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor I. Proc Natl Acad Sci U S A 1996; 93: 2482–2487.
Drake CJ, Cheresh DA, Little CD . An antagonist of integrin αvβ3 prevents maturation of blood vessels during embryonic neovascularization. J Cell Sci 1995; 108: 2655–2661.
Brooks PC, Strömblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA . Antiintegrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest 1995; 96: 1815–1822.
StataCortp. Stata statistical software. Release 5.0. College Station, TX: Stata Corporation; 1997.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SW, Mantel N, McPherson K, Peto J, Smith PG . Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analyses and examples. Br J Cancer 1977; 35: 1–39.
Cox DR . Regression models and life tables. J R Stat Soc B 1972; 34: 187–220.
Eliceiri BP, Cheresh DA . The role of αv integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest 1999; 103: 1227–1230.
Gasparini G, Brooks PC, Biganzoli E, Vermeulen PB, Bonoldi E, Dirix LY, Ranieri G, Miceli R, Cheresh DA . Vascular integrin αvβ3: A new prognostic indicator in breast cancer. Clin Cancer Res 1998; 4: 2625–2634.
Acknowledgements
This work was supported by the Ligue Suisse contre le Cancer, Grant No. KFS 981–02–2000, the Swiss National Science Foundation, Grant No. 3100–059173.99, and the Gabriella Giorgi-Cavaglieri Foundation.
We thank Claude Magnin for expert technical assistance, Mireille Redard for technical advice in the immunohistochemical assays, Joan Stalder for help in producing the frozen sections, and Dr. Jan Lübbe for providing the tissue blocks of metastatic melanoma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vonlaufen, A., Wiedle, G., Borisch, B. et al. Integrin αvβ3 Expression in Colon Carcinoma Correlates with Survival. Mod Pathol 14, 1126–1132 (2001). https://doi.org/10.1038/modpathol.3880447
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880447