Abstract
A subset of breast cancer patients benefits from preoperative bevacizumab and chemotherapy, but validated predictive biomarkers are lacking. Here, we aimed to evaluate tissue-based angiogenesis markers for potential predictive value regarding response to neoadjuvant bevacizumab treatment in breast cancer. In this randomized 1:1 phase II clinical trial, 132 patients with large or locally advanced HER2-negative tumors received chemotherapy ± bevacizumab. Dual Factor VIII/Ki-67 immunohistochemical staining was performed on core needle biopsies at baseline and week 12. Microvessel density (MVD), proliferative microvessel density (pMVD; Factor VIII/Ki-67 co-expression), glomeruloid microvascular proliferation (GMP), and a gene expression angiogenesis signature score, were studied in relation to pathologic complete response (pCR), clinico-pathologic features and intrinsic molecular subtype. We found that high baseline MVD (by median) significantly predicted pCR in the bevacizumab-arm (odds ratio 4.9, P = 0.012). High pMVD, presence of GMP, and the angiogenesis signature score did not predict pCR, but were associated with basal-like (P ≤ 0.009) and triple negative phenotypes (P ≤ 0.041). pMVD and GMP did also associate with high-grade tumors (P ≤ 0.048). To conclude, high baseline MVD significantly predicted response to bevacizumab treatment. In contrast, pMVD, GMP, and the angiogenesis signature score, did not predict response, but associated with aggressive tumor features, including basal-like and triple-negative phenotypes.
Similar content being viewed by others
Introduction
Angiogenesis, new blood vessel growth from the existing vasculature1,2, is a hallmark of cancer and promotes tumor progression and metastasis1,3. Bevacizumab is a humanized monoclonal antibody against vascular endothelial growth factor A (VEGF-A), a key factor in this process4. It was approved by the FDA in 20084 for use in metastatic HER2-negative breast cancer in combination with paclitaxel5, although later studies reported only small improvements in progression-free survival6,7 and no effect on overall survival5,6,7. Consequently, the FDA-approval was withdrawn in 20114. In the preoperative setting, however, bevacizumab in combination with chemotherapy has shown improved pathologic complete response (pCR) in several clinical trials in HER2-negative breast cancer8,9,10,11,12, with improved overall survival in one study13. Currently, although bevacizumab is not routinely used in breast cancer, it has an important clinical role in several other solid cancer types14. Also, new combinations including bevacizumab and immune check point inhibitors show promising results15,16. A major challenge concerning bevacizumab treatment is the lack of biomarkers for patient stratification17.
In tumor tissue, microvessel density (MVD) has shown prognostic value in breast cancer18,19. As a novel marker of activated angiogenesis, proliferation of microvessels, pMVD (proliferative microvessel density) has shown even stronger association with prognosis compared with MVD20. Other tissue markers of angiogenesis are the “vascular nest” phenotype of glomeruloid microvascular proliferation (GMP)21, assumed to be VEGF-A associated22, as well as a microarray-based 32-gene angiogenesis signature23,24. Presence of GMP has been associated with reduced breast cancer survival and lack of response to chemotherapy25,26.
Here, in a phase II clinical trial, patients with locally advanced (HER2-negative) or large breast cancers (≥ 2.5 cm) were randomized to receive chemotherapy ± bevacizumab. The purpose of the study was to evaluate whether tissue-based markers of angiogenesis (MVD, pMVD, GMP, 32-gene signature) could predict response to bevacizumab treatment and provide a basis for patient stratification.
Results
High microvessel density predicts pCR in bevacizumab treated patients
The NeoAva study design is presented in Fig. 1. As previously reported27, pCR in breast and axillary lymph nodes was seen in 15 patients (23%) in the bevacizumab-arm, and in 8 patients (12%) in the chemotherapy only-arm (Fisher’s exact test, P = 0.17). Among ER positive tumors, 11 patients (20%) responded in the bevacizumab-arm, compared with only 3 (5%) in the chemotherapy only-arm (Fisher’s exact test, P = 0.02). No significant difference in response was seen between the two treatment arms for ER negative patients.
Microvessels were evaluated in 128 patients at baseline (64 in each treatment arm, four excluded) (Fig. 2). When selecting the three HPFs (high power fields) with the highest number of vessels, median MVD was 112.1 vessels/mm2 (mean 120.8, range 52.4–267.9), and median pMVD was 5.8 vessels/mm2 (mean 6.4, range 0.0–20.4). MVD and pMVD were dichotomized by median values, and no significant differences were seen between the two treatment arms at baseline (Pearson’s chi-square test).
Factor VIII/Ki-67 dual immunohistochemical staining. Combined immunohistochemical staining of Factor VIII (red) and Ki-67 (blue), 400×. (A) Factor VIII positive, non-proliferative microvessels (MVD), (B) Proliferating microvessels (pMVD) by Factor VIII/Ki-67 co-expression, indicated by arrows, C Glomeruloid Microvascular Proliferation (GMP).
High MVD (≥ median) was significantly associated with pCR in the bevacizumab arm (OR, odds ratio, 4.9, P = 0.012), but not in the chemotherapy only-arm (Table 1 and Fig. 3). In the subgroup of patients treated with paclitaxel and bi-weekly bevacizumab, 14 of 45 patients responded28. High MVD was seen in 10 of the 14 responders in the paclitaxel subgroup (OR of 5.0; P = 0.018; 1 case with missing MVD-status). The subgroup receiving docetaxel and tri-weekly bevacizumab was too small for a similar comparison (1 of 21 patients responded). When analysing the subgroup of patients receiving paclitaxel in the chemotherapy only-arm, no association between MVD and pCR was found (n = 47, 6 responders, 1 case with missing MVD status). In contrast, pMVD was not associated with response in any of the treatment arms; this was also found for the paclitaxel subgroup (P = 0.7). At baseline, GMP was present in 14 of 130 patients evaluated (11%), seven in each arm, with no association with treatment response (Table 1 and Fig. 3). The 32-gene angiogenesis signature score was not significantly associated with response in any of the treatment arms (Fig. 3).
pCR according to MVD, pMVD, GMP and the 32-gene angiogenesis signature score. Each bar represents number of patients by high and low biomarker for all patients, the bevacizumab-arm (Bev + Chemo), and the chemotherapy only-arm (Chemo). pCR (pathologic complete response) rates are presented by %-estimates and indicated by dark blue color. P values by Pearson’s chi-square (χ2) test or Fisher’s exact test. aFisher’s exact test. MVD microvessel density, pMVD proliferative microvessel density, A.S. angiogenesis signature score. Median as cut-off value.
Stratified by ER (estrogen receptor) status, high MVD tended to associate with response in the bevacizumab-arm in the ER positive subgroup. High MVD was seen in 7 of the 10 responders (70%) in ER-positive bevacizumab-treated patients, compared with high MVD seen in 15 of 42 (38%) non-responders (OR 4.2, P = 0.075, Fisher’s exact test; 2 cases with missing MVD-status). No significant associations were seen among ER negative cases, or in the chemotherapy only-arm. With the low number of patients included in these groups, and the few responders observed, these latter analyses should be interpreted with care. pMVD and GMP did not predict response in either arms when stratifying for ER status (data not shown).
Angiogenesis markers pMVD, GMP and a 32-gene signature associate with aggressive tumor features
Increased vascular proliferation (pMVD) was significantly associated with high histologic grade (OR 2.6, P = 0.048), negative ER (OR 5.9, P = 0.003) and PR (progesterone receptor) status (OR 2.2, P = 0.032), and strongly associated with the triple negative phenotype (OR 9.0, P = 0.001) (Table 2). Further, pMVD differed between the intrinsic molecular subtypes (Kruskal–Wallis test, P < 0.0005) (Fig. 4), with high values seen significantly more often in the basal-like subgroup compared with other categories (OR 20.1, P = 0.001) (Table 2). Similar findings were seen for GMP, with a significant and strong association with high histologic grade (OR 6.2, P = 0.003), the triple negative phenotype (OR 3.7, P = 0.041), and a tendency to associate with ER negative tumors (OR 3.5, P = 0.051). Most GMP positive tumors (9 of 14 cases) had HER2-enriched or basal-like phenotypes, and the association with the basal-like group was highly significant when compared with the other subgroups (OR 5.4, P = 0.009).
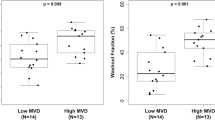
MVD, pMVD and the 32-gene angiogenesis signature score across intrinsic molecular breast cancer subtypes. MVD: microvessel density; pMVD: proliferative microvessel density, and the 32-gene angiogenesis signature score across intrinsic molecular breast cancer subtypes. Lum: luminal; HER2: HER2-enriched, Basal: basal-like; Norm: normal breast-like. P-values by the Kruskal–Wallis test, and error bars with 95% confidence interval of the mean. Number of patients; MVD and pMVD: N = 127 (Basal: N = 20, HER2: N = 9, Lum A: N = 53, Lum B: N = 40, Norm: N = 5), angiogenesis signature score: N = 131 (Basal: N = 21, HER2: N = 9, Lum A: N = 55, Lum B: N = 40, Norm: N = 6).
Whereas high MVD was associated with larger tumors (OR 2.4, P = 0.026, for T3 vs T2 tumors), and also significant for T3 and T4 tumors combined versus T2 (data not shown), there were no associations with any other clinico-pathologic features, including histologic grade, hormone receptor status (ER/PR), or the triple negative phenotype (Table 2). MVD was not associated with intrinsic molecular subtypes by PAM50 (Kruskal–Wallis, continuous; Fig. 4; or Pearson’s chi-square tests, dichotomous), although there was a trend for higher MVD in the basal-like group (OR 2.7, P = 0.056, Table 2).
The 32-gene angiogenesis signature score differed across molecular subtypes (P < 0.0005) (Fig. 4), with highest values in the basal-like subtype, and also high expression in the HER2-enriched category. The pattern was similar to the pMVD distribution (by immunohistochemistry) across intrinsic molecular subtypes, and the signature score was significantly correlated with pMVD values (Spearman’s ρ = 0.30, P = 0.001). Also, high signature score (by median) significantly associated with ER negative (OR 5.3, P = 0.002), triple negative (OR 4.9, P = 0.004) and basal-like tumors (OR 27.8, P < 0.0005), but not with PR status, tumor size or histologic grade (data not shown).
Lower values of angiogenesis markers MVD and pMVD at week 12 but with no association to treatment response
Median MVD and pMVD were 53.9 and 1.5 vessels/mm2 in the bevacizumab-group at week 12, and 87.4 and 4.4 vessels/mm2 in the chemotherapy only-group. Using matched samples, MVD and pMVD showed significantly lower vessel numbers at week 12 compared with the values at baseline (in both arms) (P < 0.0005, Wilcoxon signed rank test). The average reduction in the bevacizumab-arm for MVD and pMVD was 51% and 61%, respectively, compared with 32% and 45% in the chemotherapy only-arm.
When dichotomizing MVD and pMVD vessel numbers at week 12, by baseline median values, only 1 of 38 patients in the bevacizumab-arm, and 6 of 39 patients in the chemotherapy only-arm, had high MVD, although this did not reach statistical significance (OR 6.7, CI 0.8–58.8, P = 0.11, Fisher’s exact test). For pMVD, 4 of 38 patients had pMVD above baseline median in the bevacizumab arm, and 18 of 39 in the chemotherapy only-arm (OR 7.3, CI 2.2–24.5, P = 0.001, Pearson’s chi-square test). At week 12, neither high MVD nor high pMVD (by baseline median values) were associated with response to treatment. pMVD at 12 weeks, but not MVD, still correlated with the 32-gene angiogenesis signature score estimated at 12 weeks (Spearman’s ρ = 0.34, P = 0.004); the angiogenesis score was not significantly different between the treatment arms at week 12 (data not shown).
The absolute difference in MVD and pMVD values from baseline to week 12 was calculated, and there was a trend towards patients in the bevacizumab-arm having a larger reduction in MVD (by median), than in the chemotherapy only-group (OR 2.3, CI 0.9–5.7, P = 0.082). For pMVD, this difference between baseline and week 12 was not significantly different between the treatment arms. Notably, the difference in MVD and pMVD values between baseline and week 12 were not significantly associated with response.
Neither MVD or pMVD at week 12, nor the difference from baseline to week 12 for MVD or pMVD, were associated with any clinico-pathologic variables or intrinsic breast cancer subtypes (Pearson’s chi-square or Fisher’s exact tests; data not shown).
Discussion
In the neoadjuvant setting, improved pCR rates have been reported with the addition of bevacizumab to anthracycline and taxane based chemotherapy8,9,10,11,12. In the present study, we analysed whether tissue-based evaluation of angiogenesis markers MVD, pMVD, GMP and a 32-gene signature score could predict response to this treatment. Notably, high baseline MVD was the only marker that showed an association to increased pCR rates in the bevacizumab-arm. High MVD was still significantly associated with response when selecting for patients receiving bi-weekly bevacizumab in combination with paclitaxel (excluding patients receiving tri-weekly bevacizumab in combination with docetaxel). Also, in ER-positive bevacizumab-treated patients, there was a trend towards association with high MVD. Similar findings, with high MVD predicting response in breast cancer, were reported by Tolaney et al.29, but was not found in the smaller study by Yang et al.30. However, in these two studies, the chemotherapy regimens differed from ours, and subgroup analyses stratifying by ER status were not performed. High MVD and prediction of response to bevacizumab treatment was also reported in a recent study of ovarian cancer31.
We found that significantly more patients had lower pMVD values at week 12 (by baseline median) in the bevacizumab-arm compared with the chemotherapy only-arm. There was also a trend towards more patients in the bevacizumab-arm having a larger reduction in MVD from baseline to week 12. These findings are in line with results from preclinical models, were both pMVD and MVD were reduced after bevacizumab treatment32.
We initially hypothesized that patients with increased numbers of proliferating microvessels would respond better to anti-angiogenesis treatment. However, although pMVD was significantly lower in the bevacizumab-arm compared with the chemotherapy only-arm after 12 weeks of treatment, we found no association between pMVD values and response (pCR) to bevacizumab treatment in this study, neither using baseline values, week 12 values, nor the difference from baseline to week 12. However, baseline pMVD was significantly related to several markers of aggressive disease, including high histologic grade as well as the triple negative and basal-like phenotypes, whereas this was not seen for MVD. Further, the 32-gene angiogenesis signature score based on high pMVD levels in our previous study24, was not associated with response, but was correlated with tissue-based pMVD expression and showed similar associations as pMVD across intrinsic molecular breast cancer subtypes.
A limitation regarding analyses of tissue-based markers in the neoadjuvant setting is the small amount of tissue available. Core needle biopsies and not surgical specimens were examined, in contrast to previous studies in this field18,20,24,25, possibly influencing hot-spot selection for MVD and pMVD assessment18,20,24. Also, the frequency of GMP positive tumors was lower than previously reported25. However, despite the limited amount of tissue available, similar associations concerning clinico-pathologic variables and intrinsic molecular subtypes were found for pMVD and GMP as previously published20,25,26,33.
In summary, in this randomized phase II clinical trial of neoadjuvant chemotherapy ± bevacizumab in large or locally advanced HER2-negative primary breast cancer, high baseline MVD, reflecting the overall vascular density, was the only significant predictor of bevacizumab treatment response. Evaluation of MVD requires immunohistochemical staining of vessels on formalin-fixed paraffin-embedded tissues, a well-established method in pathology departments. Although there might be issues regarding reproducibility of vessel assessment, our experience is that high levels of agreement can be reached after initial training in the research setting, as supported by high inter- and intra-observer agreement in our study. However, whether MVD is a useful and robust clinical biomarker in relation to bevacizumab used as neoadjuvant therapy should be further validated in the routine setting as a potential basis for patient stratification. Other markers for activated angiogenesis, including pMVD, GMP, and a 32-gene signature score, did not predict response, but were all associated with aggressive breast cancer features including high histologic grade, basal-like and triple negative tumors.
Methods
Patients and study design
As described27, patients with large primary breast cancer (tumor diameter ≥ 2.5 cm) and locally advanced disease, previously untreated HER2-negative breast carcinomas were eligible. Other key inclusion criteria were WHO performance status 2, adequate hematologic and biochemical parameters, and no sign of metastatic disease. Additional prerequisites were normal organ function in general and normal left ventricular ejection fraction. Concomitant medications with anticoagulants, other than low dose acetylsalicylic acid (160 mg or lower) were not allowed. 138 patients were included during 2008–2012 (Oslo University Hospital, Oslo; St. Olav’s Hospital, Trondheim, Norway). Patients were stratified based on tumor size (2.5 ≤ T ≤ 5 cm, T > 5 cm) and hormone receptor status [positive for estrogen receptor (ER), progesterone receptor (PR), or both], and randomized 1:1 to receive chemotherapy ± bevacizumab. A block randomization procedure was used, and randomization was performed by the centralized research support facility at Oslo University Hospital (Oslo, Norway). The randomization list was not known to the personnel responsible for providing information or treatment to the patients. Six patients were excluded, leaving 66 patients in each arm for further analyses (Fig. 1). Clinical characteristics of included patients and adverse effects have been published27. Analyses performed in this study, focusing on angiogenesis markers and treatment response, were done as part of exploratory analyses and did therefore not form the basis for power calculations. Written informed consent was obtained from all patients prior to inclusion. The study was approved by the protocol review board at Clinic for Cancer Therapy at Oslo University Hospital, the regional ethics committee (REK SørØst) and the Norwegian Medicines Agency, and carried out in accordance with the Declaration of Helsinki, International Conference on Harmony/Good Clinical practice. Trial Registration: http://www.clinicaltrials.gov/; identifier NCT00773695. Registered on October 16, 2008.
Treatment and response evaluation
The chemotherapy regimen consisted of four cycles of FEC100 (5FU 600 mg/m2, epirubicine 100 mg/m2, and cyclophosphamide 600 mg/m2) given every third week, followed by four cycles with docetaxel (100 mg/m2) every third week or 12 weekly infusions with paclitaxel 80 mg/m2. Bevacizumab was administered intravenously at a dose of 15 mg/kg every third week, or 10 mg/kg every other week in patients receiving docetaxel (n = 21) or paclitaxel (n = 45), respectively28.
The primary end-point was pathologic complete response (pCR), defined as the absence of invasive carcinoma in the breast and in the axillary lymph nodes.
Immunohistochemistry
Formalin-fixed paraffin-embedded routine core needle biopsies at time of diagnosis, in addition to a biopsy collected before treatment start and at week 12, were available. Of the 132 patients in the cohort, 131 and 94 patients had available tissue at time of diagnosis, and at week 12, respectively.
Staining was done on 4–5 µm formalin-fixed paraffin-embedded standard tissue sections. Pre-treatment of the sections was done, i.e. pressure cooker antigen retrieval in Target Retrieval Solution pH 6.0 (Dako S1699, Glostrup, Denmark), and 8 min incubation with Dual Endogenous Enzyme-Blocking Reagent (Dako 2003). The sections were incubated for 60 min at room temperature with polyclonal rabbit anti-human Factor VIII related antibody (Dako A0082) diluted 1:1600 and monoclonal mouse Ki-67 antibody (Dako M7240) diluted 1:200 in Antibody diluent (Dako S3022). Alkaline phosphatase goat anti-mouse (H + L) IgG (Southern Biotech, Birmingham, AL, USA) diluted 1:100 in horseradish peroxidase goat anti-rabbit EnVision (Dako K4011) was applied for 30 min at room temperature. Visualization of Ki-67 with Ferangi Blue™ Chromogen System (Biocare Medical, Concord, CA, USA, FB812S) for 15 min and of Factor VIII with AEC + Substrate-Chromogen (Dako K3469) for 15 min at room temperature. Counterstaining was not done. Positive and negative controls were included in each staining round.
Evaluation of tissue-based angiogenesis markers
Microvessel density (MVD)
As described by Weidner et al.18, the tissue sections were first examined at low magnifications (25× and 100×) to identify the most vascular areas of the invasive tumor (“hot-spots”) within the core needle biopsies. Then, vessels were counted in 3–10 consecutive fields, depending on amount of available tissue, at high magnification (400x, field size 0.23 mm2)34.
Vessels were recognized by their Factor VIII positive endothelial cells combined with their morphology. Any red-stained endothelial cell or cluster of endothelial cells that was clearly separate from adjacent microvessels, tumor cells, and other connective tissue elements, was considered a single, countable vessel. Notably, vessel lumens were not necessary for a structure to be defined as a vessel, but in cases with GMP or long, winding and branching vessels, each lumen was regarded as a single vascular unit and counted. The fields used to count vessels should contain at least 50% invasive tumor tissue (with exceptions for the week 12 biopsies, as discussed below), and vessels associated with necrotic areas or fibrotic scars were excluded34.
Proliferative microvessel density (pMVD)
pMVD is a measure of the number of microvessels containing proliferating endothelial cells within the evaluated tumor area. Proliferating endothelial cells were recognized by their co-expression of Factor VIII and Ki-67. The same microscopic fields and vessel criteria were used for MVD and pMVD assessment. Ki-67 positive nuclei outside the endothelial cell layer or within the vessel lumen were not counted. Microvessels were regarded as proliferating when they contained one or more proliferating endothelial cells34.
Evaluation of tissue biopsies at week 12
Biopsies from 94 patients were available at week 12. Of these, 46 patients had biopsies with ≥ 50% tumor tissue in at least three high power fields (HPFs) and 12 patients had < 50% tumor cells in at least three HPFs in their biopsy. In biopsies from 19 patients, no tumor cells were seen, but there were changes in the connective tissue consistent with chemotherapy induced tissue reaction. Vessels were counted in these biopsies. However, if the biopsy did not include tumor cells or tissue changes suggesting chemotherapy treatment reactions, patients were excluded because of uncertainty regarding the representativeness of the sample (N = 12). Also, five cases had less than three HPFs for evaluation and were excluded, leaving 77 patients for analyses (38 in the bevacizumab-arm and 39 in the chemotherapy only-arm).
Glomeruloid microvascular proliferation (GMP)
Focal glomerulus-like aggregates of closely associated and multilayered Factor VIII positive endothelial cells, GMPs, were evaluated (by O.S.), as either present or absent25. Lumen formation was not necessary for the glomerulus-like structures to be registered as GMP. At baseline, 130 patients had enough tumor tissue for GMP evaluation.
Variables and cut-off values
For MVD and pMVD, the three HPFs with highest number of vessels were selected for further analysis. At baseline, four patients had less than three HPFs for evaluation of MVD and pMVD, and were therefore excluded, leaving 128 patients, 64 in each treatment arm, for further analyses. Baseline MVD and pMVD were dichotomized by the median value. Cut-off by the upper quartile was also explored, but gave somewhat weaker results (data not shown). For MVD and pMVD at week 12, the baseline median values were used as cut-off values. Vascular proliferation Index (VPI), the ratio between the number of microvessels containing proliferating endothelial cells and the total number of Factor-VIII positive microvessels (pMVD/MVD), given as a percentage, was also analysed but gave somewhat weaker results (data not shown). The average value of all HPFs, and the one HPF with the highest vessel count for MVD, pMVD, and VPI, were also examined, but the results were similar or weaker as for the maximum of three HPFs (data not shown).
Inter- and intra-observer agreement
Before evaluating MVD, pMVD and VPI in the research series, training was done on a test set of 24 tissue sections (endometrial carcinoma). These were scored separately by an experienced pathologist (I.M.S.). Difficulties concerning evaluation of vessels were discussed. Inter- and intra-observer agreements were estimated by Kappa (к) and Spearman’s rho (ρ), dichotomized by median value, and the training was not ended until both inter- and intra-observer agreements reached a satisfactory and stable value. The final inter- and intra-observer agreement reached Kappa values (κ) ≥ 0.7 and Spearman’s rho (ρ) > 0.8. Intra-observer agreement for the main study, for both MVD and pMVD showed Kappa values, κ = 0.7 and Spearman’s ρ = 0.934.
Gene expression analysis
Gene expression profiling was performed using 40 ng total RNA and one color Sureprint G3 Human GE 8 × 60 k Microarrays (Agilent Technologies), as previously described27. Molecular subtype determination was performed using the PAM50 algoritm35. A 32-gene expression signature based on differentially expressed genes in pMVD high vs low cases (by median value), originally determined in an endometrial cancer cohort24, was applied in the present data set. The angiogenesis signature score was generated by subtracting the sum of the expression values for the down-regulated genes from the sum of expression values for the up-regulated genes. The microarray data are available in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-4439.
Statistical analysis
Data were analysed using SPSS (version 22.0, IBM corp., Armonk, NY, USA). Associations between categorical variables were evaluated by Pearson’s chi-square (χ2) test or Fisher’s exact test when appropriate. Odds ratios (OR) and 95% confidence intervals (CI) are presented. For paired data (baseline and week 12 biopsies), Wilcoxon signed rank test was used. Spearman’s rank correlation test was applied when comparing bivariate continuous variables, and the Kruskal–Wallis test for analysing differences across intrinsic breast cancer subtypes.
All statistical tests were two-sided, and statistical significance was assessed at 5% level, and P values between 5 and 10% were regarded as borderline significant.
Ethical approval and informed consent
The study was approved by the protocol review board at Clinic for Cancer Therapy at Oslo University Hospital, the regional ethics committee (REK SørØst) and the Norwegian Medicines Agency, and carried out in accordance with the Declaration of Helsinki, International Conference on Harmony/Good Clinical practice. Written informed consents were obtained from all patients prior to inclusion.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request. The microarray dataset supporting the conclusions of this article is available in the ArrayExpress database, accession number E-MTAB-4439 (http://www.ebi.ac.uk/arrayexpress). The trial is registered in the http://www.clinicaltrials.gov/ website; identifier NCT00773695. Registered on October 16, 2008.
References
Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186. https://doi.org/10.1056/NEJM197111182852108 (1971).
Pecorino, L. Molecular Biology of Cancer 2nd edn. (Oxford University Press, Oxford, 2005).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674. https://doi.org/10.1016/j.cell.2011.02.013 (2011).
Ferrara, N. & Ten Adamis, A. P. years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 15, 385–403. https://doi.org/10.1038/nrd.2015.17 (2016).
Miller, K. et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 357, 2666–2676. https://doi.org/10.1056/NEJMoa072113 (2007).
Robert, N. J. et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J. Clin. Oncol. 29, 1252–1260. https://doi.org/10.1200/JCO.2010.28.0982 (2011).
Miles, D. W. et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 28, 3239–3247. https://doi.org/10.1200/JCO.2008.21.6457 (2010).
von Minckwitz, G. et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N. Engl. J. Med. 366, 299–309. https://doi.org/10.1056/NEJMoa1111065 (2012).
Bear, H. D. et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N. Engl. J. Med. 366, 310–320. https://doi.org/10.1056/NEJMoa1111097 (2012).
Sikov, W. M. et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 33, 13–21. https://doi.org/10.1200/JCO.2014.57.0572 (2015).
Earl, H. M. et al. Efficacy of neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin, and cyclophosphamide, for women with HER2-negative early breast cancer (ARTemis): an open-label, randomised, phase 3 trial. Lancet Oncol. 16, 656–666. https://doi.org/10.1016/S1470-2045(15)70137-3 (2015).
Nahleh, Z. A. et al. SWOG S0800 (NCI CDR0000636131): addition of bevacizumab to neoadjuvant nab-paclitaxel with dose-dense doxorubicin and cyclophosphamide improves pathologic complete response (pCR) rates in inflammatory or locally advanced breast cancer. Breast Cancer Res. Treat. 158, 485–495. https://doi.org/10.1007/s10549-016-3889-6 (2016).
Bear, H. D. et al. Neoadjuvant plus adjuvant bevacizumab in early breast cancer (NSABP B-40 [NRG Oncology]): secondary outcomes of a phase 3, randomised controlled trial. Lancet Oncol. 16, 1037–1048. https://doi.org/10.1016/S1470-2045(15)00041-8 (2015).
Roviello, G. et al. The role of bevacizumab in solid tumours: a literature based meta-analysis of randomised trials. Eur. J. Cancer 75, 245–258. https://doi.org/10.1016/j.ejca.2017.01.026 (2017).
Dhillon, S. & Syed, Y. Y. Atezolizumab first-line combination therapy: a review in metastatic nonsquamous NSCLC. Target Oncol. 14, 759–768. https://doi.org/10.1007/s11523-019-00686-w (2019).
McDermott, D. F. et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 24, 749–757. https://doi.org/10.1038/s41591-018-0053-3 (2018).
Lambrechts, D., Lenz, H. J., de Haas, S., Carmeliet, P. & Scherer, S. J. Markers of response for the antiangiogenic agent bevacizumab. J. Clin. Oncol. 31, 1219–1230. https://doi.org/10.1200/JCO.2012.46.2762 (2013).
Weidner, N., Semple, J. P., Welch, W. R. & Folkman, J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N. Engl. J. Med. 324, 1–8. https://doi.org/10.1056/NEJM199101033240101 (1991).
Uzzan, B., Nicolas, P., Cucherat, M. & Perret, G. Y. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 64, 2941–2955 (2004).
Arnes, J. B. et al. Vascular proliferation is a prognostic factor in breast cancer. Breast Cancer Res. Treat. 133, 501–510. https://doi.org/10.1007/s10549-011-1785-7 (2012).
Nagy, J. A. & Dvorak, H. F. Heterogeneity of the tumor vasculature: the need for new tumor blood vessel type-specific targets. Clin. Exp. Metastasis 29, 657–662. https://doi.org/10.1007/s10585-012-9500-6 (2012).
Sitohy, B. et al. Early actions of anti-vascular endothelial growth factor/vascular endothelial growth factor receptor drugs on angiogenic blood vessels. Am. J. Pathol. 187, 2337–2347. https://doi.org/10.1016/j.ajpath.2017.06.010 (2017).
Stefansson, I. M., Salvesen, H. B. & Akslen, L. A. Vascular proliferation is important for clinical progress of endometrial cancer. Cancer Res. 66, 3303–3309. https://doi.org/10.1158/0008-5472.CAN-05-1163 (2006).
Stefansson, I. M. et al. Increased angiogenesis is associated with a 32-gene expression signature and 6p21 amplification in aggressive endometrial cancer. Oncotarget 6, 10634–10645 (2015).
Straume, O. et al. Prognostic importance of glomeruloid microvascular proliferation indicates an aggressive angiogenic phenotype in human cancers. Cancer Res. 62, 6808–6811 (2002).
Akslen, L. A. et al. Glomeruloid microvascular proliferation is associated with lack of response to chemotherapy in breast cancer. Br. J. Cancer 105, 9–12. https://doi.org/10.1038/bjc.2011.203 (2011).
Silwal-Pandit, L. et al. The longitudinal transcriptional response to neoadjuvant chemotherapy with and without bevacizumab in breast cancer. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-17-0160 (2017).
von der Lippe Gythfeldt, H. et al. Immune phenotype of tumor microenvironment predicts response to bevacizumab in neoadjuvant treatment of ER-positive breast cancer. Int. J. Cancer https://doi.org/10.1002/ijc.33108 (2020).
Tolaney, S. M. et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc. Natl. Acad. Sci. U.S.A. 112, 14325–14330. https://doi.org/10.1073/pnas.1518808112 (2015).
Yang, S. X. et al. Gene expression profile and angiogenic marker correlates with response to neoadjuvant bevacizumab followed by bevacizumab plus chemotherapy in breast cancer. Clin. Cancer Res. 14, 5893–5899. https://doi.org/10.1158/1078-0432.CCR-07-4762 (2008).
Bais, C. et al. Tumor Microvessel Density as a Potential Predictive Marker for Bevacizumab Benefit: GOG-0218 Biomarker Analyses. J. Natl. Cancer Inst. https://doi.org/10.1093/jnci/djx066 (2017).
Lindholm, E. M. et al. Effect of antiangiogenic therapy on tumor growth, vasculature and kinase activity in basal- and luminal-like breast cancer xenografts. Mol. Oncol. 6, 418–427. https://doi.org/10.1016/j.molonc.2012.03.006 (2012).
Nalwoga, H. et al. Vascular proliferation is increased in basal-like breast cancer. Breast Cancer Res. Treat. 130, 1063–1071. https://doi.org/10.1007/s10549-011-1740-7 (2011).
Kruger, K. et al. Microvessel proliferation by co-expression of endothelial nestin and Ki-67 is associated with a basal-like phenotype and aggressive features in breast cancer. Breast 22, 282–288. https://doi.org/10.1016/j.breast.2012.07.008 (2013).
Parker, J. S. et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 27, 1160–1167. https://doi.org/10.1200/JCO.2008.18.1370 (2009).
Acknowledgements
We thank Gerd Lillian Hallseth, Randi Hope Lavik, and Bendik Nordanger for excellent technical assistance.
Funding
This work was supported by grants from the Pink Ribbon Movement and Norwegian Breast Cancer Society (Grant Number 11003001), and the Norwegian Research Council (Grant Number 191436/V50). In addition, the following supported the project: the K. G. Jebsen Center for Breast Cancer Research, the South-Eastern Norway Regional Health Authority, the University of Bergen, the Research Council of Norway through its Centers of Excellence funding scheme (Grant Number 223250), and the Norwegian Cancer Society and Helse Vest Research Fund to L.A.A. The study was co-sponsored by Roche Norway and Sanofi Aventis Norway. The funders did not influence the design, data collection or the reporting of the results of the study.
Author information
Authors and Affiliations
Contributions
K.K. and L.A.A. designed this particular study, conducted data collection, statistical analyses and writing of the manuscript, with critical input from the other co-authors. O.E. and B.N. contributed to design of the clinical trial and data collection. E.B. and Ø.G. contributed to data collection. K.K., O.S., and L.A.A. evaluated immunochistochemical staining of tissue slides. L.S-P., I.M.S., E.W., L.A.A., and K.K. contributed to the microarray analyses. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The main sponsor for the study was Oslo University Hospital. The study was co-sponsored by Roche Norway and Sanofi-Aventis, contributing to funding of the study. All authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krüger, K., Silwal-Pandit, L., Wik, E. et al. Baseline microvessel density predicts response to neoadjuvant bevacizumab treatment of locally advanced breast cancer. Sci Rep 11, 3388 (2021). https://doi.org/10.1038/s41598-021-81914-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81914-0
This article is cited by
-
Digital image analysis and machine learning-assisted prediction of neoadjuvant chemotherapy response in triple-negative breast cancer
Breast Cancer Research (2024)
-
Spatiotemporal multi-scale modeling of radiopharmaceutical distributions in vascularized solid tumors
Scientific Reports (2022)
-
Molecular perspective on targeted therapy in breast cancer: a review of current status
Medical Oncology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.