Abstract
We evaluated p186Her2 overexpression and HER2 oncogene amplification in recurrent vulvar Paget's disease. We identified six patients with recurrent vulvar Paget's disease in our archives. The number of recurrences ranged from 1 to 11 over a time period of 1–168 months. Recurrences were evaluated immunohistochemically for p185Her2 overexpression with the HercepTestR and for HER2 oncogene amplification with fluorescence in situ hybridization. p185Her2 overexpression was scored as 3 in two patients, as 2 in two patients, and as 1 in two patients. All patients with scores 2 and 3 showed HER2 oncogene amplification. Overexpression of p185Her2 and HER2 oncogene amplification appears to be common in recurrent vulvar Paget's disease.
Similar content being viewed by others
Main
Paget's disease of the vulva is an uncommon carcinoma of apocrine gland origin. Most cases are limited to the epidermis and mucosa, but some invade the dermis. Definitive treatment of vulvar Paget's disease is often difficult because of the anatomical and surgical limitations in this region. Surgical excision is associated with recurrence rates of up to 60%.1, 2, 3 This is often due to positive resection margins, but it has also been suggested that Paget's disease arises multifocally from pluripotent stem cells.
Human epidermal growth factor receptor 2 (HER2) oncogene, encoding for the 185-kDa transmembrane glycoprotein receptor (p185Her2), is involved in transformation and progression of many human cancers, including Paget's disease.4, 5, 6, 7, 8, 9 Tanskanen et al5 reported HER2 oncogene amplification and p185Her2 overexpression in seven of 13 primary vulvar Paget's disease, but p185Her2 expression in recurrent Paget's disease has not been studied. We analyzed p185Her2 overexpression and HER2 oncogene amplification in six patients with recurrent vulvar Paget's disease.
Materials and methods
Among the 11 patients with primary Paget's disease identified in the archives of our institutions, six had recurrent disease. All vulvar tissues of the primary and recurrent vulvar Paget's disease were analyzed immunohistochemically with HercepTestR (DAKO, Denmark), an FDA-approved assay of p185Her2 overexpression. Staining results were scored as score 0 (negative; no staining is observed, or membrane staining in less than 10% of the tumor cells); score 1 (negative; faint/barely perceptible focal membrane staining in more than 10% of tumor cells); score 2 (positive; weak to moderate staining of the complete cell membrane in more than 10% of the tumor cells) and score 3 (positive; strong staining of the complete membrane in more than 10% of the tumor cells). Positive and negative controls were used to validate each run of the assay. All cases with a score 2 and 3 were further analyzed with fluorescence in situ hybridization (FISH, PathVysion HER2-DNA probe kit; Vysis, Downers Grove, IL, USA).
Results
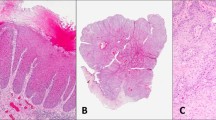
All six patients with recurrences had positive surgical margins in the initial and recurrent excisions (Figure 1). Recurrences were seen as early as 1 month after primary surgery. Two patients had one recurrence, two patients had two, and one patient had three recurrences. One patient had 11 resections of recurrent disease over a period of 168 months after primary vulvectomy (Table 1 ). Three patients developed invasive disease in their recurrences after 9, 58 and 168 months, respectively (pt. 1, 4 and 5). p185Her2 overexpression was scored as 3 in two patients, as score 2 in two patients, and as score 1 in 2 patients (Figure 2a–d). All patients with score 2 and score 3 showed HER2 oncogene amplification (Figure 3). The recurrences showed the same scores as the corresponding primary tumors (Table 1). Analysis of the five tumors that did not recur showed a score 3 and HER2 oncogene amplification in three patients, while two patients were negative for p185Her2 overexpression.
Hematoxylin and eosin stains of the recurrent Paget's disease with intraepidermal proliferation of large pale cells (a) and focal invasion (b) (pt. #4); (c) p185Her2 overexpression in intraepidermal Paget's cells with a strong membranous staining of the entire cell membranes, score 3 (pt. #1, see clinical picture in Figure 1); (d) p185Her2 positivity (score 2) in an invasive area of recurrent Paget's disease (pt. #4).
Discussion
This is the first report of p185Her2 overexpression and HER2 oncogene amplification in recurrent Paget's disease of the vulva. In a large retrospective series of 100 women with Paget's disease treated in eight institutions, 34% of patients developed recurrences in the median time of 3 years.1 This is partly due to the apparently multifocal nature of the disease and partly to the difficulties in recognizing the extent of the lesions clinically. Recurrences have also been described in skin grafts from other parts of the body.10 The mechanism by which Paget's cells spread intraepidermally and infiltrate the dermis seems to be induced by a mobility factor (heregulin-alpha) that acts through the p185Her2 receptor.11 The major problem with the recurrences was the extension of the Paget's disease to surrounding nongenital skin such as the inner side of the thigh, perianal skin and mons pubis, rendering surgical revision excision difficult.
Trastuzumab is a recombinant DNA-derived humanized human monoclonal immunoglobulin G (IgG) antibody that binds to the antigen of p185Her2. Immunotherapy with trastuzumab is a well-tolerated option for first-line treatment of women with metastatic breast cancer and presently under investigation for several other cancers.12 Possible antiproliferative mechanisms of trastuzumab include downregulation of HER2 receptors, activation of immune effector cells (antibody-dependent cell-mediated cytotoxicity), reduction of S-phase cell-cycle progression, and reduction of vascular endothelial growth factor for angiogenesis.13 However, trastuzumab is generally confined only to patients whose tumors stain at score 3 level or with gene amplification detected by FISH.
In conclusion, overexpression of p185Her2 and HER2 oncogene amplification appears to be common in primary and recurrent vulvar Paget's disease including the invasive components. These results suggest that a clinical trial of trastuzumab for women with high-risk Paget's disease of the vulva is justified.
References
Fanning J, Lambert L, Hale M, et al. Paget's disease of the vulva-prevalence of associated vulvar adenocarcinoma, invasive Paget's disease and recurrence after surgical excision. Am J Obstet Gynecol 1999;180:24–27.
Kodama S, Kaneko T, Saito M, et al. A clinicopathologic study of 30 patients with Paget's disease of the vulva. Gynecol Oncol 1995;56:63–70.
Sarmiento JM, Wolff BG, Burgart LJ, et al. Paget's disease of the perianal region—an aggressive disease? Dis Colon Rectum 1997;40:1187–1194.
Green MC, Murray JL, Hortobagyi GN . Monoclonal antibody therapy for solid tumors. Cancer Treat Rev 2000;26:269–286.
Tanskanen M, Jahkola T, Asko-Seljavaara S, et al. HER2 oncogene amplification in extramammary Paget's disease. Histopathology 2003;42:575–579.
Lammie GA, Barnes DM, Millid W, et al. An immunohistochemical study of the presence of c-erbB-2 protein in Paget's disease. Histopathology 1989;15:505–514.
Keatings L, Sinclair J, Wright C, et al. c-erbB-2 oncoprotein expression in mammary and extramammary Paget's disease: an immunohistochemical study. Histopathology 1990;17:243–247.
Meissner K, Riviere A, Haupt G, et al. Study of HERneu-protein expression in mammary Paget's disease with and without underlying breast carcinoma and in extramammary Paget's disease. Am J Pathol 1990;137:1305–1309.
Wolber RA, Dypuis BA, Wick MR . Expression of c-erbB-2 oncoprotein in mammary and extramammary Paget's disease. Am J Clin Pathol 1991;96:243–247.
Misas JE, Larson JE, Podczaski E, et al. Recurrent Paget's disease of the vulva in an split-thickness graft. Obstet Gynecol 1990;76:543–544.
Schelfhout VR, Coene ED, Delaey B, et al. Pathogenesis of Paget's disease: epidermal heregulin-alpha factor, and the HER receptor family. J Natl Cancer Inst 2000;92:622–628.
Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002;20:719–726.
Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185 HER antibody for human cancer therapy. Proc Natl Acad Sci USA 1992;89:4285–4289.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reich, O., Liegl, B., Tamussino, K. et al. p185HER2 overexpression and HER2 oncogene amplification in recurrent vulvar Paget's disease. Mod Pathol 18, 354–357 (2005). https://doi.org/10.1038/modpathol.3800243
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800243
Keywords
This article is cited by
-
COX-2 and Her-2/neu are overexpressed in Paget’s disease of the vulva and the breast: results of a preliminary study
Archives of Gynecology and Obstetrics (2008)
-
Extramammary Paget’s Disease in Chinese Males: A 21‐year Experience
World Journal of Surgery (2007)
-
Malignant adnexal neoplasms
Modern Pathology (2006)
-
Androgen receptors are frequently expressed in mammary and extramammary Paget's disease
Modern Pathology (2005)