Abstract
LX are tetraene-containing eicosanoids generated by lipoxygenase (LO) transformation of arachidonic acid (Serhan and Romano, 1995). LX possess potent anti-inflammatory activity in vivo, and temporal biosynthesis of LX, concurrent with spontaneous resolution, has been observed during exudate formation (Levy et al, 2001). Limited results are currently available on the involvement of LX in clinical settings. Recently, a rabbit anti-LXA4 antiserum has been raised to produce an enzyme-linked immunosorbent assay (ELISA) kit for LXA4 (Levy et al, 1993). Although specific and accurate with isolated cells, this kit has not been tested with complex biological matrix such as urine. Initial attempts to determine urinary excretion of LXA4 using the LXA4 ELISA kit were unsuccessful because of high unspecific absorbance readings. In this report, we show that the LXA4 extraction procedure indicated in the ELISA kit is inadequate for urinary measurements of immunoreactive (i)LXA4. We present the development of a new extraction technique, more selective for LX, that abolishes background contamination and minimizes the unspecific readings. Using this method, we show for the first time that urine from healthy subjects contain (i)LXA4 material and identify a urinary tetraene with the physical properties of a LXA4 metabolite. Although reliable methods have been previously established to quantitate LXA4 from whole blood (Brezinski et al, 1992), the present extraction technique, which optimizes for LXA4 recovery from human urine, represents a substantial achievement for LX investigation and may open a new avenue of clinical studies on LXA4.
Similar content being viewed by others
Main
To assess the suitability of the recently developed LXA4 enzyme-linked immunosorbent assay (ELISA) (Neogen Corporation, Lexington, Kentucky) for urinary measurements, we spiked urine aliquots with increasing amounts of synthetic LXA4. As illustrated in Figure 1A, urine extracted with the method illustrated in the ELISA kit did not show the expected linear response, indicating that urine may contain material, not removed by routine extraction, that heavily interfered with the execution of the ELISA. A new extraction technique was therefore set up with the objective of a higher selectivity for LX.
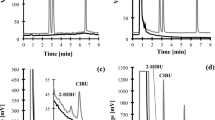
Validation of a new LX extraction method and description of a novel urinary tetraene. A, Urine aliquots (3 ml) were spiked with increasing concentrations of authentic LXA4 (from 0–2 ng/ml) and extracted using either the protocol described in the instructions to the LXA4 enzyme-linked immunosorbent assay (ELISA) kit (▪) or the new technique (□). In parallel, the same concentrations of LXA4 were used to construct a standard curve. Samples and standards were subjected to ELISA measurements. iLXA4 levels in standard and in urine were plotted for regression analysis. Results, corrected for PGB2 recovery, are from a single experiment representative of two with duplicates. B, Urine (5 ml) was extracted and suspended with 100 μl of methanol. 5 μl of this suspension was injected into a dual pump reversed phase high-performance liquid chromatography (RP-HPLC) gradient system equipped with a photodiode array detector. The column waters symmetry C18, 3 μm, 2.1 × 150 mm was eluted with methanol/water/acetic acid (65/35/0.01%; v:v:v). Retention times of authentic LXA4, LXB4, and their all-trans isomers are indicated by arrows. The ultraviolet (UV) spectrum of urinary material eluting beneath the peak at 4.3 minutes is shown in the inset.
In this protocol, 1 to 10 ml of urine were added of 4 volumes of ice-cold methanol and placed at −80° C for at least 3 hours. Samples were centrifuged (1500 g, 4° C, 15 minutes), 50 ng of prostaglandin (PG) B2 added to supernatants to calculate extraction recovery, and materials taken to dryness in round bottom flasks, using a rotavapor apparatus (BÜCHI Labortechnik AG, Flawil, Switzerland). Dry residues were suspended with 200 μl of methanol followed by 5 + 5 ml of deionized (d)H2O, acidified to pH 3.5 using HCl, and applied to a C18 cartridge (Sep-Pak Classic, Waters, Milan, Italy), preactivated with 10 ml of methanol and 10 ml of dH2O. The cartridge was neutralized with 10 to 20 ml of dH2O and washed with 10 ml of hexane. Materials were eluted with 5 ml of hexane/ethyl acetate (40/60; v:v), then dried in a Savant apparatus (Savant, Rome, Italy) equipped with a refrigerated vapor trap (model RVT 104) and a speed vacuum centrifuge (model SC 110). Dry residues were suspended with 200 μl of benzene/ethyl acetate/methanol (60/40/20; v:v:v), followed by 800 μl of benzene/ethyl acetate (60/40; v:v). Samples were applied to 500 mg silica cartridges (Bond Elut LRC, Varian, Turin, Italy) pre-activated with 5 ml of benzene/ethyl acetate/methanol (60/40/30; v:v:v) followed by 5 ml of benzene/ethyl acetate (60/40; v:v). Columns were washed with 5 ml of benzene/ethyl acetate (60/40; v:v) and materials eluted with 5 ml of benzene/ethyl acetate/methanol (60/40/20; v:v:v). Elutes were taken to dryness, finally suspended with 100 μl of methanol and, stored at −80° C until use. Aliquots were used for ELISA measurements. As shown in Figure 1A, this technique abolished the unspecific readings, giving a PGB2 extraction recovery determined by reversed phase high-performance liquid chromatography (RP-HPLC), of 79.9 ± 1.5% (n = 50).
With the new technique, 0.056 ± 0.01 ng/mg creatinine of iLXA4 levels was detected in urine from a population of 20 healthy subjects (10 men and 10 women, aged 32 ± 9.3 years). Moreover, using RP-HPLC and online ultraviolet (UV) spectroscopy, we individuated a peak bearing a typical tetraene profile with UV absorbing bands at 288.7–300.5–315 nm (Fig. 1B). Materials collected beneath this peak gave a concentration-dependent LXA4 ELISA reading, this confirming its LXA4-related nature. The physical characteristics of this material are consistent with those of a LXA4 β-or ω-oxidation product.
In conclusion, the new LX extraction method provides significant technical advances for LXA4 measurements in clinical settings.
Note Added in Proof
Comparable results were obtained when the extraction method described in this paper was tested with the newly developed 15-epi-LXA4 ELISA kit.
References
Brezinski DA, Nesto RW, and Serhan CN (1992). Angioplasty triggers intracoronary leukotrienes and lipoxin A4: Impact of aspirin therapy. Circulation 86: 56–63.
Levy BD, Bertram S, Tai HH, Israel E, Fischer A, Drazen JM, and Serhan CN (1993). Agonist-induced lipoxin A4 generation: Detection by a novel lipoxin A4-ELISA. Lipids 28: 1047–1053.
Levy BD, Clish CB, Schmidt B, Gronert K, and Serhan CN (2001). Lipid mediator class switching during acute inflammation: Signals in resolution. Nat Immunol 7: 612–619.
Serhan CN and Romano M (1995). Lipoxin biosynthesis and actions: Role of the human platelet LX-synthase. J Lipid Mediat Cell Signal 12: 293–306.
Acknowledgements
This work was supported in part by grants from the Italian Ministero dell'Università e della Ricerca Scientifica (60%) to MR and GD and from Rhone-Poulenc to SG.
We thank Neogen Corporation for providing the LXA4 ELISA kits used in this study and Dr. Clary Clish from the Center for Experimental Therapeutics and Reperfusion Injury, Brigham and Women's Hospital, Boston, Massachusetts for helpful discussion and technical advise.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romano, M., Luciotti, G., Gangemi, S. et al. Urinary Excretion of Lipoxin A4 and Related Compounds: Development of New Extraction Techniques for Lipoxins. Lab Invest 82, 1253–1254 (2002). https://doi.org/10.1097/01.LAB.0000028823.53486.4A
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.LAB.0000028823.53486.4A
This article is cited by
-
Inflammatory bowel disease as a disorder of an imbalance between pro- and anti-inflammatory molecules and deficiency of resolution bioactive lipids
Lipids in Health and Disease (2016)
-
A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution
Histochemistry and Cell Biology (2004)