Abstract
Carbon tetrachloride (CCl4)-induced hepatic fibrosis has been considered to be linked to oxidative stress and mediated by aldehydic lipid peroxidation products. In the present study, we investigated whether collagen synthesis is induced by F2-isoprostanes, the most proximal products of lipid peroxidation and known mediators of important biological effects. By contrast with aldehydes, F2-isoprostanes act through receptors able to elicit definite signal transduction pathways. In a rat model of CCl4-induced hepatic fibrosis, plasma F2-isoprostanes were markedly elevated for the entire experimental period; hepatic collagen content also increased. When hepatic stellate cells (HSCs) from normal liver were cultured with F2-isoprostanes in the concentration range found in the in vivo studies (10−9–10−8 M), a striking increase in DNA synthesis (reversed by the thromboxane A2 antagonist SQ 29 548), in cell proliferation and in collagen synthesis was observed. Total collagen content was similarly increased. Moreover, F2-isoprostanes markedly increased the production of transforming growth factor-β1 by U937 cells, considered a model of liver macrophages. The data provide evidence for the possibility that F2-isoprostanes generated by lipid peroxidation in hepatocytes mediate HSC proliferation and collagen production seen in hepatic fibrosis.
Similar content being viewed by others
Main
Increased deposition of collagen and other extracellular matrix-proteins is a feature of many chronic diseases affecting the liver, lung, arteries and nervous systems. In carbon tetrachloride (CCl4) experimentally-induced hepatotoxicity, besides the classical steatonecrosis, fibrosis also develops and evolves into cirrhosis in the chronic intoxication. CCl4 hepatotoxicity is now considered a model of oxidative stress in vivo. The relation between oxidative stress and collagen hyperproduction was first proposed by Chojker et al,1 who observed that the addition of ascorbic acid and iron to cultured fibroblasts strongly stimulates lipid peroxidation and, at the same time, the production of collagen and procollagen alpha 1 (I) mRNA; the effects are reproduced by the addition to the same fibroblasts of malonaldehyde, one of the end products of lipid peroxidation.
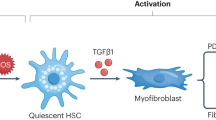
One of the most effective fibrogenic mediators is transforming growth factor (TGF)-β, which strongly stimulates the production of matrix proteins (particularly collagen) in various cellular types.2, 3, 4, 5, 6 In chronic CCl4 intoxication,7 an increase in TGF-β mRNA occurs in nonparenchymal cells. Among liver nonparenchymal cells, hepatic stellate cells (HSC) represent a very important source of production of matrix proteins. The activation of HSC, which occurs quickly even in culture, is accompanied by an increased production of matrix proteins, by cellular proliferation and by the typical change from the resting to the myofibroblast-like phenotype (expression of smooth muscle-alpha actin; α-SMA).
It has been reported8 that lipid peroxidation induced in vitro in human HSC or the treatment of the latter with 4-hydroxynonenal (the most cytotoxic aldehyde originating from lipid peroxidation9) stimulates the expression of procollagen α1 (I) gene. Also, the treatment of various lineages of macrophages10 with 4-hydroxynonenal induces mRNA production and synthesis of TGF-β1. Finally, 4-hydroxynonenal added to cultured HSC upregulates the synthesis of procollagen α1 (I).11
Despite the many studies carried out on oxidative stress, there has not been a reliable and noninvasive method to monitor lipid peroxidation in vivo, as with the use of blood and urine. Some years ago, however, the group of Morrow et al12 demonstrated the production of a series of prostaglandin F2-like compounds, named F2-isoprostanes, which are formed in vivo and in vitro by free radical-catalyzed peroxidation of phospholipid-bound arachidonic acid,13 a pathway that is independent of the cyclooxygenase pathway. F2-isoprostanes might therefore be considered a reliable marker of oxidative stress (lipid peroxidation)14 and thus be used to evaluate the oxidative status in a number of human pathologies, such as alcoholic liver disease and diabetes.15
Since aldehydic lipid peroxidation products, like 4-hydroxynonenal, have been reported8, 9, 10, 11 to induce collagen expression and synthesis, we have investigated whether analogous effects can be obtained with F2-isoprostanes, the most proximal derivatives of peroxidizing arachidonic acid. One potential advantage of isoprostanes over aldehydes is that while aldehydes can interact with cellular macromolecules by addition processes only, isoprostanes could possess receptors able to induce specific signal transduction pathways. Indeed, F2-isoprostanes, besides being markers of oxidative stress, are also known to mediate important biological effects16 such as vasoconstriction of renal glomerular arterioles,17 an effect that appears to be mainly mediated through thromboxane A2 receptor (TxA2r) activation.
An elevated level of plasma F2-isoprostanes has been reported18, 19 to be associated with acute CCl4 intoxication. We therefore investigated whether elevated isoprostane levels are maintained throughout the experimental period. In parallel studies, isolated HSC were cultured and treated with F2-isoprostanes in the range of concentrations found in the in vivo studies in order to evaluate the effects of these prostanoids on cell proliferation and collagen synthesis. Since it is generally believed5, 6, 20, 21 that activation of HSC follows the release of soluble factors (cytokines, mainly TGF-β) by cells of macrophage lineages such as Kupffer cells or liver macrophages, the effects of F2-isoprostanes on TGF-β release by the human promonocyte cell line U937 was also studied. The results support the idea that F2-isoprostanes are among the active agonists inducing increase in collagen deposition, at least in CCl4-induced liver fibrosis.
Materials and methods
In Vivo Studies
The experimentation was carried out by following guidelines prescribed by Italian D.L. 27 January 1992, no. 116, with the approval of Siena University Ethic-Deontological Committee.
Male Sprague–Dawley rats (Harlan-Nossan, Correzzana, Italy), initial weight 80 g, were maintained in a temperature- and light-controlled environment, and fed Harlan Global diet and water ad libitum.
In all, 20 rats were injected intraperitoneally two times weekly with a CCl4-mineral oil solution (1:1, v/v) at the dose of 125 μl/100 g body weight. Eight saline-treated animals, age and weight matched, served as controls. The increase in body weight of the treated animals was slower as compared to the respective controls (−13, −24, −20, −26 and −31% at the 2nd, 3rd, 5th, 6th and 7th week, respectively), while the liver weight to body weight ratio significantly increased. No mortality occurred during the experimental period of time. Groups of three animals were killed after CCl4 treatment for 1, 2, 3, 5, 6 and 7 weeks, respectively. Killing was performed 24 h after the last injection with the exception of a group killed 4 h after the first injection and a group killed 48 h after the third injection. Blood and livers were processed for biochemical and morphological analysis.
Preparation procedure and gas-mass analysis of plasma F2-isoprostanes
For F2-isoprostane determination, platelet poor plasma was obtained from heparinized blood by centrifugation at 2400 g and butylated hydroxytoluene (90 μM) was added to plasma as an antioxidant. Aliquots of plasma were stored under nitrogen at −70°C until analysis (within 2 months). Immediately after thawing, plasma (1 ml) was spiked with tetradeuterated PGF2α (500 pg in 50 μl of ethanol) as an internal standard.
The procedure of Nourooz-Zadeh et al22 was used for the preparation of plasma F2-isoprostanes (free isoprostanes) prior to gas-mass analysis. This procedure is similar to that classically described by the group of Morrow and Roberts,23 which involves solid-phase extraction on an octadecylsilane (C18) and silica cartridge, followed by thin-layer chromatography. In the procedure of Nourooz-Zadeh, on the other hand, the silica cartridge and the thin-layer chromatography steps were replaced with an aminopropyl (NH2) cartridge solid-phase extraction. Such aminopropyl solid-phase extraction was shown22, 24 to be highly efficient in retaining tritium-labelled 9α, 11α-PGF2α, with which recovery studies were performed and allowed a higher (nearly 70%) recovery.
The determinations were carried out by gas chromatography/negative ion chemical ionization tandem mass spectrometry analysis. An ion trap was used as mass analyser (ThermoFinnigan Instrument) as reported previously.25 Samples (1 μl) were injected into the gas chromatograph in undecane containing 10% N,O-bis (trimethylsilyl) trifluoroacetamide. The carrier gas was ultrapure helium and methane was used as reagent gas (flow 1.2 ml/min). The collision energy used was 1.3 eV. The measured ions were m/z 299 and m/z 303 derived from the typical ions (m/z 569 and m/z 573) produced from 8-epi-PGF2α also referred to as 15-F2t-IsoP (the most represented and the generally evaluated isomer for F2-isoprostane measurement) and the tetradeuterated derivative of PGF2α, respectively.
Hydroxyproline content
Total collagen was determined by hydrolyzing liver samples in 6 N HCl and by measuring hydroxyproline according to the method of Kivirikko et al.26 Collagen content was estimated by multiplying the amount of hydroxyproline by a factor of 7.69. Results are expressed as milligram of collagen per gram of liver (wet weight).
In Vitro Studies
HSC isolation and culture
HSC were prepared by means of sequential pronase/collagenase digestion method as reported27 and purified by density-gradient centrifugation, using Nycodenz 18% (Nycomed, Oslo, Norway). HSC were harvested from the top of gradient and seeded (106 cells/ml) in 25 cm2 flasks in Dulbecco's modified Eagle's medium (DMEM) plus 20% fetal bovine serum and antibiotics. The viability and purity of preparation was tested by trypan blue exclusion and autofluorescence of vitamin A (Figure 1a), respectively. Cells were incubated in 95% air–5% CO2 at 37°C and allowed to adhere. After 1 day, the cells appeared quiescent and negative for the marker α-SMA (Figure 1b), detected by means of Smooth muscle-alpha actin Immunoistology Kit (IMMH-2, Sigma, St Louis, USA). Experiments were performed at the second serial passage when the cells exhibited the α-SMA marker.
(a) Autofluorescence of freshly isolated HSC from rat liver. Cells examined under ultraviolet illumination at 328 nm revealed yellow fluorescence indicating the presence of vitamin A; expression of α-SMA. (b) Control: after 1 day, cells appear quiescent and negative for the marker; (c) by 7 days in culture, all the cells are activated. (original magnification × 120).
Culture treatment with F2-isoprostanes
HSC were seeded at density of 6 × 104 cells/ml in DMEM supplemented with 10% fetal bovine serum and allowed to grow to confluence. At 2 h before treatment, the medium was changed with serum-free DMEM. HSC were treated for 48 h with 8-epi-PGF2α (BIOMOL Research Laboratories Inc., USA) in the range of concentrations seen in the in vivo experiments (10−7–10−10 M). Stock solution of 8-epi-PGF2α (1 mg/ml in ethanol) was diluted to a 10−5 M concentration and then further diluted with DMEM to obtain final concentrations ranging from 10−7 to 10−10 M. Ethanol diluted with serum-free DMEM (1%) was used as vehicle.
DNA synthesis and cell proliferation
DNA synthesis was evaluated by measuring 3H-thymidine incorporation in HSC, according to Boscoboinik et al.28 Subconfluent cells were incubated with 8-epi-PGF2α as mentioned above. At 6 h before assay, 10 μCi/ml of 3H-thymidine (ICN; specific activity 6.7 Ci/mmol) was added. Radioactivity was counted in a Packard 2100 TriCarb liquid scintillation analyser. DNA content was measured in HSC according to Taylor et al.29 Cell proliferation was evaluated by cell counting and trypan blue exclusion, after 24 h of treatment with 8-epi-PGF2α. Data are expressed as the number of cells per well.
Collagen synthesis assay
HSC were seeded onto 12-well plates and grown to visual confluence. At 2 h before treatment, the medium was changed to serum-free DMEM to allow the cells to become relatively quiescent. Collagen synthesis was assessed as reported.30 Briefly, 16 h before the end of the 48 h treatment with 8-epi-PGF2α, 10 μCi/ml of 3H-proline (Amersham International; specific activity 23 Ci/mmol) were added to each well. Media were harvested for determination of 3H-proline incorporation into collagen and noncollagen proteins following the collagenase digestion method, by using highly purified bacterial collagenase (Calbiochem Cod. 234134, 250 IU). Incorporation of radioactivity into collagen and noncollagen proteins was determined following precipitation with trichloroacetic acid. Collagen-incorporated radioactivity was recovered in the trichloroacetic acid-soluble fraction, while noncollagen radioactivity was recovered from trichloroacetic acid precipitate. Data were expressed as tritiated proline incorporation (dpm) per microgram of DNA. Percentage collagen synthesis was estimated using the formula of Diegelman and Peterkofsky.31
Collagen content assay
The collagen content was evaluated in the media of HSC cultures treated with 8-epi-PGF2α for 48 h, using the Kit Sircol Collagen Assay (Biocolor, Tebu-Bio, Milan, Italy).
Inhibition experiments
DNA synthesis was estimated as mentioned above under ‘DNA synthesis and cell proliferation’ paragraph. Subconfluent cells were made quiescent by incubation in serum-free DMEM medium for 2 h and then treated for 24 h with the following agents: 10−10–10−8 M 8-epi-PGF2α; 10−9–10−7 M SQ 29 548; and 8-epi-PGF2α plus SQ 29 548 in a molar ratio 1:10. The 3H-thymidine incorporation was determined as above.
Evaluation of TGF-β1 in macrophage cell line
The human promonocyte cell line U937 (ATCC, Rockville, MD, USA) was used. The cells were seeded at 106/ml and cultured in RPMI-1640 plus 10% fetal bovine serum in the presence of 150 nM phorbol 12-myristate 13-acetate for 24 h to induce the macrophage-like phenotype of adherence. At 2 h before treatment, the medium was removed and replaced with serum-free medium. Cells were treated for 24 h with 8-epi-PGF2α in the range of concentrations of 10−6–10−10 M. The concentration of TGF-β1 in the media was measured with an ELISA method using a Quantikine Kit from R&D System.
Statistical analysis
Results were reported as means±s.e.m. Comparisons between groups were carried out by Student's t-tests. Correlation coefficients were determined by Pearson's test. All tests were two-tailed. The value of P<0.05 was considered statistically significant.
Results
Plasma Isoprostanes in the CCl4 Treatment
In the model of chronic CCl4 intoxication, the levels of plasma F2-isoprostanes, even if lower than in the acute intoxication (in which concentrations of over 4000 pg/ml have been found18, 19), were maintained elevated (Figure 2) for the entire experimental period (with a sharp peak at 4 h and decrease at 48 h, Figure 2 inset, with respect to the usual time of measurement, that is, 24 h after the last injection). In particular, very high levels of F2-isoprostanes were found at 7 weeks (the end of the experiment, Figure 3a) and this was accompanied by a marked increase of hepatic collagen content (measured as hydroxyproline, Figure 3b). Plasma alanine aminotransferase (ALT) activity was also increased (Figure 3c), although with a pattern different from that of plasma isoprostanes.
Plasma F2-isoprostanes in rats chronically intoxicated with CCl4. Measurement were carried out 4 h after the first injection and 24 h after the third, sixth, ninth, 15th, 18th and 21st injection (I, II, III, V, VI and VII week of treatment, respectively). In the inset, the values for 4 h after the first injection, 24 and 48 h after the third injection and 24 h after the sixth injection are reported. h, hours; a.i., after injection; a.1°i., after the first injection; a.3°i., after the third injection; and a.6°i., after the sixth injection.
Plasma F2-isoprostanes, hepatic collagen content and plasma ALT in rats chronically treated with CCl4. (a) Plasma F2-isoprostanes (pg/ml) (see Figure 2). (b) Hepatic collagen content (evaluated as hydroxyproline) after I, II, III and VII weeks of CCl4 treatment. (c) Serum ALT activity (Sigma Alanine aminotransferase Test). Means±s.e.m. *P<0.05 vs control.
The histological aspects of the livers at 1, 2, 3 and 7 weeks of treatment showed a progressive fibrosis and finally a clear cirrhosis (Figure 4).
Hepatic histology in rats after chronic CCl4 treatment. Control rat livers show a normal architecture. In the treated animals, a progressive injury with hepatocellular necrosis and marked collagen deposition was seen. In particular, at the VII week, a frank cirrhosis was evident. Liver sections were stained with haematoxylin–eosin (a) and Mallory trichrome for collagen staining (b). (Original magnification × 100).
Effect of F2-Isoprostanes on HSC
The addition of F2-isoprostanes (10−10–10−8 M) to cultured activated HSC induced a two- to four-fold increase in DNA synthesis (Figure 5), as measured by tritiated thymidine incorporation. Also, the cell number was increased (231±26 and 197±42% with 10−9 and 10−8 M isoprostanes, respectively, as compared to vehicle control).
Effect of F2-isoprostanes on DNA synthesis of HSC. Cells were treated with 8-epi-PGF2α for 48 h. (a) Tritiated thymidine was added to the incubation medium 6 h before the end of the treatment. Results are expressed as 3H-thymidine incorporation (cpm) per well. Each sample was run in quadruplicate. Data are the means±s.e.m. of three experiments. *P<0.05 vs control. C, control; V, vehicle.
A similar 2.5-fold increase was also seen in collagen synthesis, as measured by tritiated proline incorporation (Figure 6a). The relative collagen production, that is, the percentage of collagen production over the total protein production (collagenic plus noncollagenic proteins), was increased by 3.0- to 3.5-fold (Figure 6b). Total collagen content (Figure 6c) was similarly increased. The lack of effect at 10−7 M is unclear, but most likely associated to toxic effects.
Effect of F2-isoprostanes on synthesis and content of collagen in HSC treated with 8-epi-PGF2α for 48 h. (a) Data are expressed as 3H-proline incorporation (dpm) per microgram of DNA. (b) Percentage collagen synthesis determined by calculating collagen production as percentage of total protein production (means±s.e.m.), using the formula of Diegelmann and Peterkofsky.31 (c) Collagen content as evaluated in the media of HSC using the Kit Sircol Collagen Assay. Each sample was run in quadruplicate. Data are the means±s.e.m. of three experiments. *P<0.05 vs control. C, control; V, vehicle; CP, collagen protein; NCP, noncollagen protein.
The most active concentrations were between 10−8 and 10−9 M (=10 and 1 nM), exactly as those found in the in vivo intoxication (3000–500 pg/ml=9.0–1.5 pmol/ml=9.0–1.5 nM). No changes at all in HSC viability were seen (not shown).
On the other hand, 4-hydroxynonenal similarly added to cultured HSC at much higher (0.1–5 μM) concentrations stimulated DNA synthesis (Figure 7) to a much lower extent (nearly 1.6-fold at 1 μM concentration) as compared to isoprostanes. No effect was seen on collagen synthesis (not shown).
Effect of 4-hydroxynonenal on HSC proliferation. Cells were incubated for 48 h with 4-hydroxynonenal. Tritiated thymidine was added to the incubation medium 6 h before the end of the treatment. Results are expressed as 3H-thymidine incorporation (cpm) per well. Each sample was run in quadruplicate. Data are the means±s.e.m. of three experiments. *P<0.05 vs control. C, control.
Inhibition Experiments
To investigate whether TxA2r are involved in the effects of F2-isoprostanes on HSC, activated HSC were incubated in the presence of both 8-epi-PGF2α and the specific TxA2r antagonist SQ 29 548, in a molar ratio of 1:10. After the addition of tritiated thymidine, the incorporation of the latter in HSC was measured. As shown in Figure 8, the stimulatory effect of 8-epi-PGF2α was almost completely abolished, with only a small increase when 10−9 M concentration of the isoprostane was used. This could suggest that much of the effect of 8-epi-PGF2α is mediated by TxA2r.
Effect of SQ 29 548 on 8-epi-PGF2α-stimulated DNA synthesis by HSC. Tritiated thymidine was added to the incubation medium 6 h before the end of the treatment. Cells were treated with 1 × 10−10 to 1 × 10−8 M 8-epi-PGF2α; 1 × 10−9 to 1 × 10−7 M SQ 29 548; and 8-epi-PGF2α plus SQ 29 548 in a molar ratio 1:10. Results are expressed as 3H-thymidine incorporation (cpm) per well. Data are the means±s.e.m. of three experiments. *P<0.05 vs vehicle, **P<0.05 vs respective 8-epi concentration. C, control; V, vehicle.
Effect of F2-Isoprostanes on TGF-β1 Production by U937 Cells
F2-isoprostanes also increased the production of TGF-β1 by U937 cells (Figure 9). The increase, although statistically significant (10−9–10−7), was however lower (∼2-fold) than that seen for other parameters in HSC and was maximal at higher concentrations (10−7 M). No effect was seen on TGF-β1 production by HSC (not shown).
Effect of F2-isoprostanes (8-epi-PGF2α) on TGF-β1 production by U937 human promonocytic cells. The cells were incubated for 24 h with 8-epi-PGF2α. TGF-β1 was assayed in the media with an ELISA method. Each sample was run in quadruplicate. Data are the means±s.e.m. of three experiments. *P<0.05 vs control. C, control; V, vehicle.
Discussion
Besides being markers of oxidative stress, F2-isoprostanes appear to be mediators of important biological effects. The first one of these to be revealed17, 32, 33 was the vasoconstriction of renal glomerular arterioles, as demonstrated by the direct infusion of 8-epi-PGF2α into the renal artery. It appears to act through the activation of receptors analogous to those for TXA2r,17 an effect believed to be important in explaining the Hepato-Renal Syndrome.34
Other biological effects of 8-epi-PGF2α are those on muscle vascular cells and on endothelial cells in which DNA synthesis and cell proliferation is stimulated.33, 35 These effects also are probably due to activation of receptors related to TXA2r. Finally, 8-epi-PGF2α seems to mediate the increased production of TGF-β1 in kidney mesangial and glomerular cells exposed to high ambient glucose such as that produced by streptozotocin-induced diabetes.36
Therefore, it seemed likely that HSC, which have been reported to respond to lipid peroxidation products, could be activated by F2-isoprostanes, the direct derivatives of arachidonic acid. The present study demonstrates that 8-epi-PGF2α, added to cultured activated HSC in the range of concentrations found in chronic CCl4 intoxication, stimulates cell proliferation and DNA and collagen synthesis (as measured by 3H-thymidine and 3H-proline incorporation, respectively) and markedly increases the percentage of collagen production over total protein production (collagenic plus noncollagenic proteins). Total collagen content is also increased. The most effective concentrations were between 10−8 and 10−9 M, exactly as those found in plasma in the in vivo intoxication (9.0–1.5 nM). Therefore, we suggest that F2-isoprostanes generated by lipid peroxidation in hepatocytes stimulate one of the main functions of HSC that is collagen production.
The assumption that F2-isoprostanes are generated in hepatocytes is supported by a 40-year long-lasting literature,37, 38, 39, 40, 41 demonstrating (in vivo and in vitro studies) that CCl4 induces lipid peroxidation in the liver and in particular in the endoplasmic reticulum of hepatocytes. Lipid peroxidation is induced in both acute and chronic42 CCl4 intoxication and there is no indication that the process occurs in mesenchymal (nonparenchymal) cells. Moreover, negligible amounts of F2-isoprostanes are released by cultured Kupffer cells when challenged with CCl4.43 Also, it has been shown18, 19 that both free and total (sum of free plus esterified) isoprostanes are dramatically increased in the liver after CCl4 intoxication.
The results of inhibition studies would suggest that F2-isoprostanes stimulate collagen production through activation of HSC receptors related to those for TxA2. In addition, F2-isoprostanes stimulate the production of TGF-β1 by U937 cells, assumed as a model for Kupffer cells or liver macrophages. This would suggest an additional paracrine pathway for stimulation of HSC and consequent synthesis of collagen. The receptor for 8-epi-PGF2α has been extensively investigated and whether such a receptor is identical or analogous to17 or distinct from33, 44, 45, 46 that for TxA2 on HSC is not definitively clarified.
The 8-epi-PGF2α potently contracts retinal vessels, elicits endothelin 1 release from retinal preparation, increases thromboxane production in the retina and cultured endothelial cells and also increases Ca2+ transients in retinal endothelial cells.47 All these effects may play a role in the retinopathy of prematurity, since it has been suggested that oxidative stress such as reoxygenation after an asphyxic episode is frequently encountered in premature newborns48, 49 and the isoprostane-induced generation of thromboxane47 may produce vasoconstriction with ischemia of the retina. As ischemia and tissue hypoxia precede angiogenesis,48, 49 the overall pathway may be relevant in the revascularization of the retinopathy of prematurity and, with the obvious changes, in the revascularization of the retinopathy of diabetes; in both cases, increased levels of plasma isoprostanes have been reported.50, 51, 52
In the liver, a sequence of similar events can be even more easily guessed: the liver is endowed with the appropriate cells (HSC) to produce extracellular matrix proteins, particularly collagen and it is endowed with Kupffer cells and macrophages able to synthesize cytokines (TGF-β, mainly), which in turn are able to stimulate HSC. Other cytokines (FGF) may contribute to the proliferation of fibroblasts, thus perpetuating collagen production.53 The activation of HSC and the consequent collagen hyperproduction appears to be an important step in liver fibrosis. The production of isoprostanes may be the initial step of the puzzle, at least when an oxidative stress can be recognized (CCl4 is able to generate isoprostanes even in minimal doses (1–10 μl/rat)). The isoprostanes so generated will stimulate HSC through receptors related to TXA2r. HSC will thus produce excess of collagen; alternatively or in addition, HSC can be stimulated by the increased production of TGF-β by Kupffer cells or macrophages. In any case, the cytokine-mediated recruitment of inflammatory cells is needed for the full feature of fibrosis.
Although other liver cell types are also able to produce collagen,54, 55, 56, 57, 58, 59 the difficulty and the complexity in isolating them precludes a definitive allocation to HSC alone of the data seen in the in vivo experiments. However, the in vitro stimulation of collagen synthesis by HSC under the same conditions (isoprostane concentrations) found in the in vivo experiments seems to warrant that at least a consistent part of collagen production is due to the HSC.
Whether or not such a cascade of events might also induce human fibrosis is unclear. However, the fact that plasma isoprostanes are elevated not only in experimental ethanol-induced liver damage (not shown) generally recognized as an oxidative stress injury60 but also in alcoholic patients61, 62, 63, 64 lends some support to such a possibility. Finally, thiacetamide, which also induces an increased level of plasma isoprostanes,18 has long been known as a cirrhotic drug.
Future studies are aimed at clarifying the signalling pathways in HSC in this experimentally induced liver fibrosis.
References
Chojkier M, Houglum K, Solis-Herruzo J, et al. Stimulation of collagen gene expression by ascorbic acid in cultured human fibroblasts. J Biol Chem 1989;264:16957–16962.
Sporn MB, Roberts AB . Autocrine growth factors and cancer. Nature 1985;313:745–747.
Massaguè J . The transforming growth factors. Trends Biochem Sci 1985;10:237–240.
Ignotz RA, Massaguè J . Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem 1986;261:4337–4345.
De Bleser PJ, Niki T, Rogiers V, et al. Transforming growth factor-beta gene expression in normal and fibrotic rat liver. J Hepatol 1997;26:886–893.
Knittel T, Janneck T, Muller L, et al. Transforming growth factor beta 1-regulated gene expression of Ito cells. Hepatology 1996;24:352–360.
Armendariz-Borunda J, Seyer JM, Kang AH, et al. Regulation of TGFβ gene expression in rat liver intoxicated with carbon tetrachloride. FASEB J 1990;4:215–221.
Parola M, Pinzani M, Casini A, et al. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen α1 (I) gene expression in human liver fat-storing cells. Biochem Biophys Res Commun 1993;194:1044–1050.
Benedetti A, Comporti M, Esterbauer H . Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta 1980;620:281–296.
Leonarduzzi G, Scavazza A, Biasi F, et al. The lipid peroxidation end product 4-hydroxy-2, 3-nonenal up-regulates transforming growth factor β1 expression in the macrophage lineage: a link between oxidative injury and fibrosclerosis. FASEB J 1997;11:851–857.
Zamara E, Novo E, Marra F, et al. 4-hydroxynonenal as a selective pro-fibrogenic stimulus for activated human hepatic stellate cells. J Hepatol 2004;40:60–68.
Morrow JD, Harris TM, Roberts II LJ . Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal Biochem 1990;184:1–10.
Morrow JD, Awad JA, Boss HJ, et al. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci USA 1992;89:10721–10725.
Morrow JD, Roberts II LJ . The isoprostanes. Current knowledge and directions for future research. Biochem Pharmacol 1996;51:1–9.
Roberts II LJ, Morrow JD . Measurement of isoprostanes to assess oxidant stress status in vivo. In: Yoshikawa T, Toyokuni S, Yamamoto Y, Naito Y (eds). Free Radicals in Chemistry, Biology and Medicine. OICA International London: Nashville, TN, 2000, pp 329–340.
Roberts II LJ, Morrow JD . The generation and actions of isoprostanes. Biochim Biophys Acta 1997;1345:121–135.
Takahashi K, Nammour TM, Fukunaga M, et al. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 α, in the rat. Evidence for interaction with thromboxane A2 receptors. J Clin Invest 1992;90:136–141.
Morrow JD, Awad JA, Kato T, et al. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J Clin Invest 1992;90:2502–2507.
Comporti M, Signorini C, Leoncini S, et al. Plasma F(2)-isoprostanes are elevated in newborns and inversely correlated to gestational age. Free Radic Biol Med 2004;37:724–732.
Gressner AM . Mediators of hepatic fibrogenesis. Hepato-Gastroenterology 1996;43:92–103.
Pietrangelo A . Metals, oxidative stress, and hepatic fibrogenesis. Semin Liver Dis 1996;16:13–30.
Nourooz-Zadeh J, Gopaul NK, Barrow S, et al. Analysis of F2-isoprostanes as indicators of non-enzymatic lipid peroxidation in vivo by gas chromatography-mass spectrometry: development of a solid-phase extraction procedure. J Chromatogr B 1995;667:199–208.
Morrow JD, Roberts II LJ . Mass spectrometry of prostanoids: F2-isoprostanes produced by non-cyclooxygenase free radical-catalyzed mechanism. Oxygen radicals in biological systems, part C. In: Packer L (ed). Methods in Enzymology, Vol. 233. Academic Press: San Diego, 1994, pp 163–179.
Nourooz-Zadeh J . Gas chromatography-mass spectrometry assay for measurement of plasma isoprostanes. Oxidants and antioxidants, part B. In: Packer L (ed). Methods in Enzymology, Vol. 300. Academic Press: San Diego, 1999, pp 13–17.
Signorini C, Comporti M, Giorgi G . Ion trap tandem mass spectrometric determination of F2-isoprostanes. J Mass Spectrom 2003;38:1067–1074.
Kivirikko KI, Laitinen O, Prockop DJ . Modifications of a specific assay for hydroxyproline in urine. Anal Biochem 1967;19:249–255.
De Bleser P, Geerts A, van Eyken P, et al. Tenascin synthesis in cultured rat liver fat-storing cells. In: Wisse E, Knook D, McCusker R (eds). Cells of the Hepatic Sinusoid. Kupffer Cell Foundation: Rijswijk, The Netherlands, 1991, pp 218–221.
Boscoboinik D, Szewczyk A, Hensey C, et al. Inhibition of cell proliferation by α-tocopherol. Role of protein kinase C. J Biol Chem 1991;266:6188–6194.
Taylor CM, Blanchard B, Zava DT . A simple method to determine whole cell uptake of radiolabelled oestrogen and progesterone and their subcellular localization in breast cancer cell lines in monolayer culture. J Steroid Biochem 1984;20:1083–1088.
Peterkofsky B, Diegelmann R . Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry 1971;10:988–994.
Diegelmann RF, Peterkofsky B . Inhibition of collagen secretion from bone and cultured fibroblasts by microtubular disruptive drugs. Proc Natl Acad Sci USA 1972;69:892–896.
Morrow JD, Hill KE, Burk RF, et al. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci USA 1990;87:9383–9387.
Fukunaga M, Makita N, Roberts II LJ, et al. Evidence for the existence of F2-isoprostane receptors on rat vascular smooth muscle cells. Am J Physiol 1993;264:1619–1624.
Holt S, Marley R, Fernando B, et al. Acute cholestasis-induced renal failure: effects of antioxidants and ligands for the thromboxane A2 receptor. Kidney Int 1999;55:271–277.
Yura T, Fukunaga M, Khan R, et al. Free-radical-generated F2-isoprostane stimulates cell proliferation and endothelin-1 expression on endothelial cells. Kidney Int 1999;56:471–478.
Montero A, Munger KA, Khan RZ, et al. F2-isoprostanes mediate high glucose-induced TGF-β synthesis and glomerular proteinuria in experimental type I diabetes. Kidney Int 2000;58:1963–1972.
Comporti M, Saccocci C, Dianzani MU . Effect of CCl4 in vitro and in vivo on lipid peroxidation of rat liver homogenates and subcellular fractions. Enzymologia 1965;29:185–204.
Recknagel RO, Ghoshal AK . Lipoperoxidation as a vector in carbon tetrachloride hepatotoxicity. Lab Invest 1966;15:132–148.
Slater TF . Free Radical Mechanisms in Tissue Injury. Pion Limited: London, UK, 1972.
Recknagel RO, Glende EA . Carbon tetrachloride hepatotoxicity: an example of lethal cleavage. Crit Rev Toxicol 1973;2:263–297.
Comporti M . Lipid peroxidation and cellular damage in toxic liver injury. Lab Invest 1985;53:599–623.
Comporti M, Benedetti A, Chieli E . Changes in microsomal lipids of rat liver after chronic carbon tetrachloride intoxication. Experientià 1971;27:1155–1156.
Johnston DE, Kroening C . Stimulation of prostaglandin synthesis in cultured liver cells by CCl4 . Hepatology 1996;24:677–684.
Fukunaga M, Yura T, Grygorczyk R, et al. Evidence for the distinct nature of F2-isoprostane receptors from those of thromboxane A2. Am J Physiol 1997;272:477–483.
Yura T, Fukunaga M, Grygorczyk R, et al. Molecular and functional evidence for the distinct nature of F2-isoprostane receptors from those of thromboxane A2. Adv Prostaglandin Thromboxane Leukot Res 1995;23:237–239.
Praticò D, Smyth EM, Violi F, et al. Local amplification of platelet function by 8-epi prostaglandin F2alpha is not mediated by thromboxane receptor isoforms. J Biol Chem 1996;271:14916–14924.
Lahaie I, Hard P, Hou X, et al. A novel mechanism for vasoconstrictor action of 8-isoprostaglandin F2α on retinal vessels. Am J Physiol 1998;274:1406–1416.
Reynaud X, Dorey CK . Extraretinal neovascolarization induced by hypoxic episodes in the neonatal rat. Invest Ophthalmol Vis Sci 1994;35:3169–3177.
Phelps DL, Rosenbaum AL . Effects of marginal hypoxemia on recovery from oxygen induced retinopathy in the kitten model. Pediatrics 1984;73:1–6.
Flynn JT, Bancalari E, Snyder ES, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med 1992;326:1050–1054.
Davi G, Ciabattoni G, Consoli A, et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation 1999;19:224–229.
Gopaul NK, Anggard EE, Mallet AI, et al. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett 1995;368:225–229.
Pinzani M, Marra F . Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis 2001;21:397–416.
Ramadori G, Saile B . Portal tract fibrogenesis in the liver. Lab Invest 2004;84:153–159.
Desmouliere A, Darby IA, Gabbiani G . Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest 2003;83:1689–1707.
Lorena D, Darby IA, Reinhardt DP, et al. Fibrillin-1 expression in normal and fibrotic rat liver and in cultured hepatic fibroblastic cells: modulation by mechanical stress and role in cell adhesion. Lab Invest 2004;84:203–212.
Kinnman N, Francoz C, Barbu V, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest 2003;83:163–173.
Uchio K, Tuchweber B, Manabe N, et al. Cellular retinal-binding protein-1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Lab Invest 2002;82:619–628.
Desmouliere A, Darby I, Costa AM, et al. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab Invest 1997;76:765–778.
Comporti M, Hartman A, Di Luzio NR . Effect in vivo and in vitro ethanol administration on liver lipid peroxidation. Lab Invest 1967;16:616–624.
Meagher EA, Barry OP, Burke A, et al. Alcohol-induced generation of lipid peroxidation products in humans. J Clin Invest 1999;104:805–813.
Lieber CS . Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv Pharmacol 1997;38:601–628.
Moore K . Isoprostanes and the liver. Chem Phys Lipids 2004;128:125–133.
Huseby NE, Strømme JH . Practical points regarding routine determination of gamma-glutamyl transferase (gamma-GT) in serum with a kinetic method at 37°C. Scand J Clin Lab Invest 1974;34:57–63.
Acknowledgements
This research was supported by a grant from the Italian Ministery of University and Scientific Research (Program of National Relevance 2003 Prot. 2003060145). Additional funds were derived from an MIUR-FIRB grant and from the Siena University Research Plan (2003). We thank the Siena Hospital Administration for the mass-spectrometer purchase. The cooperation of Dr D Vecchio for part of the experiments is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Duality of interest
None declared.
Rights and permissions
About this article
Cite this article
Comporti, M., Arezzini, B., Signorini, C. et al. F2-isoprostanes stimulate collagen synthesis in activated hepatic stellate cells: a link with liver fibrosis?. Lab Invest 85, 1381–1391 (2005). https://doi.org/10.1038/labinvest.3700332
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.3700332
Keywords
This article is cited by
-
A novel murine model for non-alcoholic steatohepatitis developed by combination of a high-fat diet and oxidized low-density lipoprotein
Laboratory Investigation (2012)
-
Attenuated progression of diet-induced steatohepatitis in glutathione-deficient mice
Laboratory Investigation (2010)
-
Redox mechanisms in hepatic chronic wound healing and fibrogenesis
Fibrogenesis & Tissue Repair (2008)
-
Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence
Breast Cancer Research (2008)
-
F2-isoprostane receptors on hepatic stellate cells
Laboratory Investigation (2008)