Abstract
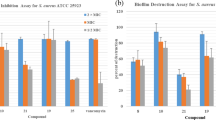
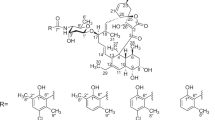
FOLLOWING on the isolation in these Laboratories of 6-aminopenicillanic acid1, we have used it to synthesize many novel penicillins, with particular emphasis on structures which resist inactivation by staphylococcal penicillinase. One class of penicillins possessing this valuable property has been obtained by acylating 6-aminopenicillanic acid with aromatic or heteroaromatic carboxylic acid chlorides substituted in both ortho positions. When the ring structure of the side-chain acid is six-membered, almost any pair of ortho substituents confer marked stability towards penicillinase, but when it is five-membered one or both of the substituents must be relatively bulky, for example, phenyl or substituted phenyl. Such findings illustrate the primary importance of steric effects in determining stability towards penicillinase. In this respect the chemical constitution of the side-chain is of much less significance, although it has a profound influence on antibacterial activity and on stability towards acids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Batchelor, F. R., Doyle, F. P., Nayler, J. H. C., and Rolinson, G. N., Nature, 183, 257 (1959). Doyle, F. P., Nayler, J. H. C., and Rolinson, G. N., Brit. Pat. Spec. 870, 396 (1961).
Doyle, F. P., Hardy, K., Nayler, J. H. C., Soulal, M. J., Stove, E. R., and Waddington, H. R. J., J. Chem. Soc. (in the press).
Doyle, F. P., and Nayler, J. H. C., U.S. Patent Spec. 2, 996, 501 (1961).
Doyle, F. P., Nayler, J. H. C., Smith, H., and Stove, E. R., Nature, 191, 1091 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DOYLE, F., LONG, A., NAYLER, J. et al. New Penicillins Stable towards Both Acid and Penicillinase. Nature 192, 1183–1184 (1961). https://doi.org/10.1038/1921183a0
Issue Date:
DOI: https://doi.org/10.1038/1921183a0
This article is cited by
-
Development of β-lactams with antipseudomonal activity
Journal of Infection and Chemotherapy (1996)
-
6-Aminopenicillanic acid and its derivatives (literature review)
Pharmaceutical Chemistry Journal (1971)
-
A Rapid Procedure for Screening Unusual Penicillin-resistant Clinical Strains of Staphylococci
Nature (1964)
-
Isoxazolyl Penicillins and Penicillinase
Nature (1962)
-
Chemistry, Toxicology, Pharmacology and Microbiology of a new Acid-Stable Penicillin, Resistant to Penicillinase (BRL.1621)
Nature (1962)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.