Abstract
In the course of our screening program for new anti-methicillin-resistant Staphylococcus aureus antibiotics, four novel antibiotics, termed wychimicins A–D, were isolated from the culture broth of the rare actinomycete Actinocrispum wychmicini strain MI503-AF4. Wychimicins are spirotetronates possessing a macrocyclic 13-membered ring containing trans-decalin and β-d-xylo-hexopyranose moieties connected to C-17 by an O-glycosidic linkage according to MS, NMR and X-ray analyses. In X-ray crystal structure analysis, the Flack constant was 0.10 (11). The stereochemistry of the spirocarbon C-25 was R. Wychimicins had a minimum inhibitory concentration of 0.125–2 µg ml−1 against methicillin-resistant Staphylococcus aureus.
Similar content being viewed by others
Introduction
Natural products are considered to have pharmaceutical potential because of their pharmacophore-like structures and unique chemical spaces. According to Newman et al., natural products accounted for ~33% of all new drugs launched between 1980 and 2014, and ~32% of new drugs were developed with natural products as the lead compounds. Therefore, it is considered that 65% of drugs are derived from natural products [1]. In the field of infectious diseases in particular, natural products account for a large proportion of pharmaceuticals, and thus, it is expected that natural products can play an active role in development of treatments to overcome antimicrobial resistance (AMR).
Staphylococcus aureus is one of the most widespread bacterial pathogenic causes of nosocomial infections, causing both serious invasive infections and simple skin infections [2, 3]. It is a major cause of pneumonia and other respiratory tract infections, surgical-site infections, septic arthritis, cardiovascular infections, skin and soft tissue infections, and bacteremia [4]. S. aureus infections are particularly problematic because of the frequent development of AMR, with methicillin-resistant Staphylococcus aureus (MRSA) being the most clinically important antibiotic-resistant isolate [5]. MRSA has the highest mortality rate among all antibiotic-resistant isolates, being responsible for ~20,000 deaths in 2018 according to the CDC [6]. MRSA, a nosocomial drug-resistant S. aureus isolate, was first reported in 1961, the year after methicillin was launched [7]. Nosocomial MRSA has since spread rapidly and become a major cause of infection in immunocompromised inpatients, affecting medical facilities globally. During the recent SARS-CoV-2 pandemic, many patients requiring invasive mechanical ventilation were admitted to the intensive care unit (ICU) because of severe SARS-CoV-2–related respiratory failure. Increased entry into the ICU and the increased use of invasive mechanical ventilation have been reported to increase the risk of mechanical ventilation-related pneumonia (VAP) and bloodstream infection (BSI). In addition, treatment with tocilizumab and dexamethasone poses a potential risk of secondary or co-bacterial infections. This is associated with high mortality rates and medical costs and long ICU stays. S. aureus, particularly MRSA, is frequently involved in the etiology of VAP and BSI, thereby making treatment more difficult [8,9,10,11].
Vancomycin has been considered the antibiotic of last resort for MRSA infections. However, MRSA that has acquired resistance to vancomycin has been detected. In particular, vancomycin-intermediate-resistant S. aureus (VISA) strains are important because of their spread in many medical facilities around the world [12]. MRSA is also problematic because of the frequent acquisition of antibiotic resistance, and the strain has already acquired resistance to effective antibacterial agents launched in the 21st century. Therefore, the development of new antibacterial agents effective against multidrug-resistant MRSA strains such as VISA/vancomycin-resistant S. aureus (VRSA) is desired.
The majority of the scaffolding structures that comprise antibiotics were obtained from microbial metabolites. It is believed that 99.9% of naturally occurring microorganisms have not yet been discovered, and many naturally occurring microorganisms provide scaffolding structures for new antibiotics. Even microorganisms in soil, which are often studied as sources of antibiotic isolation, represent vast reserves of chemical diversity [13,14,15,16].
We consider newly discovered microorganisms to be important sources of bioactive natural products in the search for interesting molecules and new therapeutic agents. As part of our efforts to overcome AMR, we conducted exploratory research on antibiotics with a novel skeleton and efficacy against MRSA. As a screening source, actinomycetes isolated from soil were used for molecular phylogenetic analyses of bacteria using a phenotype and 16 S rDNA to eliminate similar microorganisms and collect more novel microorganisms. As a result, Actinocrispum, a novel genus containing a rare actinomycete, was reported [17]. In this report, we discovered new 13-membered macrocyclic spirotetronates with a trans-decalin glycoside moiety termed wychimicins A–D (Fig. 1) exhibiting strong antibacterial activity against MRSA strains including VRSA in the culture broth of A. wychmicini strain MI503-A4 collected from soil in Kuroishi-shi, Aomori prefecture, Japan [17]. This paper describes the isolation, structure determination, and biological properties of these compounds.
Results and discussion
Fermentation and isolation of wychimicins A, B, C and D
A slant culture of MI503-A4 was inoculated into a 500 ml baffled Erlenmeyer flask containing 110 ml of medium consisting of galactose, dextrin, Bacto Soytone, corn steep liquor, glycerol, (NH4)2SO4, and CaCO3. The culture (5 l) was incubated on a rotary shaker (200 rpm) at 30 °C for 7 days. Active compounds were isolated from the extracts of mycelium cakes with MeOH and ethyl acetate followed by fermentation broth with ethyl acetate. The organic layer was purified by silica gel column chromatography and eluted with ethyl acetate:methanol:formic acid (100:0:0, 40:1:0.1, 36:4:0.1, and 40:10:0.125) via reversed-phase octadecylsilyl silica gel column chromatography using a Sephadex LH20 column. The active fractions were further purified via reversed-phase octadecylsilyl silica gel HPLC to obtain four novel compounds, which were termed wychimicins A (82.8 mg), B (47.0 mg), C (62.8 mg), and D (35.5 mg).
Structure elucidation of wychimicins A–D
The physicochemical properties of these compounds are summarized in the supplementary information (S-1). Wychimicins were brown solid compounds. The UV spectra of wychimicins revealed absorption maxima at 281–283 nm in acidic methanol and bathochromic shifts to 317–327 nm in alkaline conditions, as presented in Table S1. The molecular formulae of wychimicins A (1), B (2), C (3), and D (4) were C47H60ClNO11, C47H61ClNO11, C46H58ClNO11, and C46H59NO11, respectively, as determined by HRESI-MS and NMR spectra. 1H and 13C NMR data are summarized in Tables S2–5.
Structure determination of 1
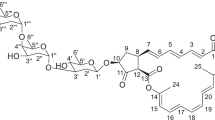
1 has 47 carbons consisting of 14 fully substituted carbons, 20 methine groups, 5 methylene groups, and 8 methyl groups as confirmed by the 1H, 13C, DEPT135, and HMQC spectra (Figs. S1–6), and six exchangeable protons were indicated by these data and the molecular formula. NMR analyses of 1 were summarized in Fig. 2. Spin coupling systems from 5-H (δH 3.57, t 9.8 Hz) to the olefinic proton 11-H (δH 5.63), from 6-H (δH 1.85) to 28-H (δH 0.65), and from 5-H to 10-H (δH 2.23) were established by 1H-1H COSY, as presented in Fig. S4. According to the 13C NMR chemical shifts, oxygen atoms were substituted at C-8 (δC 69.9) and C-9 (δC 75.2) in the substructure. The long-range couplings from 5-H to C-3 (δC 208.7), C-4 (δC 53.6), and C-27 (δC 16.7); from 11-H to C-13 (δC 57.2) and C-29 (δC 27.5); from 27-H (δH 1.20) to the carbonyl carbon C-3, C-4, C-5 (δC 37.9), and C-13; and the from ethyl group of 29-H (δH 1.80, 1.97), which was connected with 30-H (δH 0.95) to C-11 (δC 118.8), C-12 (δC 138.7), and C-13, were confirmed to reflect an 11, 12-dehydro-4, 6, 8, 9, 12, 13-substituted trans-decalin moiety.
The COSY spectrum revealed the sequence from 13-H of the decalin ring to the oxymethine proton 17-H (δH 3.83) through the olefinic proton 14-H (δH 5.51), 15-H (δH 5.05), and methylene (δH 2.27 and 2.33). Other spin coupling systems from the methyl proton 32-H (δH 1.08) to the olefinic proton 19-H (δH 4.84) via the methine proton 23-H (δH 2.81), olefinic proton 22-H (δH 5.68), 21-H (δH 5.45), and the methine proton 20-H (δH 3.65) and from 23-H to the methylene proton 24-H (δH 1.85, 1.91) were observed. Long-range couplings from the methyl proton 31-H (δH 1.73) to the oxymethine carbon C-17 (δC 84.5), olefinic carbon C-18 (δC 142.0), and C-19 (δC 122.6); from the methylene proton 24-H to the quaternary carbon C-25 (δC 84.5), methine carbon C-20 (δC 41.9), and C-26 (δC 199.9); and from 20-H to C-25 and C-26 established a C6 chain containing a 3-methylcyclohex-1-ene ring from C-13 of decalin. As another substructure, the sequence from the overlapping methine proton 1′-H (δH 4.84) to 6′-H (δH 1.24) through the methylene 2′-H (δH 1.89, 1.95), the methine 3′-H (δH 4.26), 4′-H (δH 4.04), and 5′-H (δH 3.74) and from 4′-H to the NH proton (δH 6.30) suggested a 2′, 4′, 6′-trideoxy-4′-animohexose moiety. The long-range correlations from 1′-H to C-5′ (δC 69.3) and C-17 and from 5′-H to C-1′ (δC 95.9) established the sugar moiety attached to the C6 chain from decalin. The remaining protons were the two singlet methyl protons 8″-H (δH 2.19) and 9″-H (δH 2.46) and the aromatic singlet proton 3″-H (δH 7.20). Long-range couplings were observed from 3″-H to C-1″ (δC 155.2), C-2″ (δC 125.9), C-4″ (δC 129.5), and C-5″ (δC 125.3); from the methyl proton 8″-H (δH 2.19) to C-1″, C-2″, and C-3″ (δC 133.6); from the methyl proton 9″-H to C-4″, C-5″, and C-6″ (δC 119.8); and from the sugar moiety NH proton to C-6″ and C-7″ (δC 169.3), thereby establishing 5-saturated 2-hydroxy-3,6-dimethylbenzoic acid connected with the sugar moiety at the 4′ position via an amide bond. The MS spectrum of 1 revealed the fragment m/z 312.1000 (C15H19NO4Cl), establishing that C-4″ should be Cl. The bathochromic shift under acidic condition in the UV spectra of 1, and the carbon signals of C-20 (δC 41.9), C-24 (δC 38.7), C-25 (δC 84.5), C-26 (δC 199.9), and three characteristic sp2 carbons C-1 (δC 166.8), C-2 (δC 105.6), and C-3 (δC 208.7) were related to those of tetrocarcin [18] and kijanimicin [19], respectively, suggested that 1 contains a spirotetronate moiety. Finally, 1 possesses a 13-membered macrocyclic ring with a spirotetronate containing trans-decalin and sugar moieties connected to C-17 by an O-glycosidic linkage.
Structure determination of 2
2 possesses 47 carbons consisting of 13 fully substituted carbons, 21 methine groups, 5 methylene groups, and 8 methyl groups as confirmed by the 1H, 13C, DEPT135, and HMQC spectra (Figs. S7–12). NMR analyses of 2 were summarized in Fig. 2. The molecular formula indicated that 2 lost a Cl atom compared with 1. The NMR spectra of 2 were similar to those of 1 excluding the phenyl moiety. The newly appeared protons 3″-H (δH 7.08) and 4″-H (δH 6.61) coupled with each other. Long-range coupling from the methyl proton 8″-H (δH 2.21) to C-1″ (δC 158.2), C-2″ (δC 124.6), and C-3″ (δC 133.2); from the methyl proton 9″-H (δH 2.48) to C-4″ (δC 122.0), C-5″ (δC 132.2), and C-6″ (δC 116.8); from 3″-H to C-1″ and C-5″; from 4″-H to C-2″ and C-6″; and from NH to C-6″ and C-7″ (δC 170.4) were established. The MS spectrum of 2 revealed the fragment m/z 278.1389 (C15H20NO4). From these data, 2 was determined to be a 4″-dechloro derivative of 1.
Structure determination of 3
3 possesses 46 carbons consisting of 13 fully substituted carbons, 21 methine groups, 5 methylene groups, and 7 methyl groups confirmed as by the 1H, 13C, DEPT135, and HMQC spectra (Figs. S13–18). NMR analyses of 3 were summarized in Fig. 2. The molecular formula indicated that 3 lost a CH3 moiety compared with 1. The NMR spectra of 3 were similar to those of 1 excluding the phenyl moiety. The newly appeared protons 2″-H (δH 6.77) and 3″-H (δH 7.29) coupled with each other. Long-range couplings from the methyl proton 8″-H (δH 2.48) to C-4″ (δC 126.1), C-5″ (δC 132.8), and C-6″ (δC 121.0); from 2″-H to C-4″ and C-6″; from 3″-H to C-1″ (δC 156.5) and C-5″ (δC 132.8); and from NH (δH 6.32) to C-6″ and C-7″ (δC 168.7) were detected. The MS spectrum of 3 revealed the fragment m/z 298.0843 (C14H17NO4Cl). From these data, 3 was determined to be a 2″-demethyl derivative of 1.
Structure determination of 4
4 possesses 46 carbons consisting of 13 fully substituted carbons, 22 methine groups, 5 methylene groups, and 7 methyl groups as confirmed by the 1H, 13C, DEPT135, and HMQC spectra (Figs. S19–24). NMR analyses of 4 were summarized in Fig. 2. The molecular formula indicated that 4 lost a Cl atom compared with 1. The NMR spectra of 4 were similar to those of 1 except excluding the phenyl moiety. The protons 2″-H (δH 6.83), 3″-H (δH 7.20), and 4″-H (δH 6.70) were observed in the COSY spectrum. Long-range couplings from the methyl 8″-H proton (δH 2.51) to C-4″ (δC 122.7), C-5″ (δC 135.2), and C-6″ (δC 117.7); from 2″-H to C-4″ and C-6″; from 4″-H to C-2″ (δC 115.8) and C-6″; and from NH (δH 6.48) to C-6″ and C-7″ (δC 169.9) were observed. The MS spectrum of 4 revealed the fragment m/z 264.1235 (C14H18NO4). From these data, 4 was determined to be a 2″-demethyl-4″-dechloro derivative of 1.
Absolute structure of wychimicins
Because the complete structure of wychimicins including their stereochemistry could not be determined by NMR, X-ray crystallographic analysis was attempted. Crystallization of wychimicins A–D was performed in various solvents. Only 4 was able to obtain a single crystal in ethyl acetate/methanol, which was used to perform a single-crystal X-ray diffraction study. The Flack parameter of 0.10 (11) permitted definition of the configuration as 4S, 5R, 6S, 8S, 9R, 10S, 13R, 17R, 20S, 23S, 25R, 1’R, 3’S, 4’S, 5’R (Fig. 3a, b).
According to physicochemical analyses, including CD spectra (Figs. S29–33), and NMR studies, wychimicins have the same aglycone and sugar moiety. Only the phenyl moieties differ in structure. These results allowed the absolute configuration of wychimicins A–D to be determined in Fig. 1.
Wychimicins belong to a family of spirotetronates. This family of compounds has various biological activities, as exemplified by the antitumor agents tetrocarcin A1 [20] and kijanimicin [21]; the antiviral drugs JK-1, JK-2 [22], MM46115 [23], and quartromicins [24]; the antibacterial compounds chlorothricin [25], abyssomicin [26], and lobophorins [27]; and the cholecystokinin B inhibitor tetronothiodin [28]. The spiro moiety of wychimicins had the R configuration, similarly as abyssomicin.
Biological activities
Table 1 presents the antimicrobial activities of wychimicins against gram-positive and gram-negative bacteria. The minimum inhibitory concentrations of wychimicins were determined by the agar dilution method. All compounds exhibited excellent antimicrobial activities against gram-positive bacteria such as S. aureus (including MRSA) and Enterococcus faecalis/faecium (including VRE). However, only 1 and 3 exhibited strong antibacterial activity against S. aureus and E. faecalis/faecium, and lower activity were noted for 2 and 4. This result indicated that the presence of Cl at the 4″ position in the benzoic acid moiety enhances antibacterial activity.
Materials and methods
General
The optical rotations of the purified compounds were measured using a P-1030 polarimeter (JASCO, Tokyo, Japan). The UV spectra were recorded using a U-2800 UV-Vis spectrophotometer (Hitachi High-Technologies, Tokyo, Japan). The IR spectra were recorded using an FT/IR-4100 Fourier transform infrared spectrometer (JASCO). CD spectra were obtained using a J-1500 Circular Dichroism spectrometer (JASCO). The 1H and 13C NMR spectra were measured by an ECZ600R spectrometer (JEOL RESONANCE, Tokyo, Japan) at 25 °C using tetramethylsilane as an internal reference. The mass spectra were recorded using an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA) or an ACQUITY QDa mass detector (Waters, Milford, MA, USA).
Fermentation
A slant culture of A. wychmicini MI503-AF4 was inoculated into a 500 ml baffled Erlenmeyer flask containing 110 ml of a seed medium consisting of 2% (w/v) galactose, 2% (w/v) dextrin, 1% (w/v) Bacto Soytone (Becton Dickinson, Franklin Lakes, NJ, USA), 0.5% (w/v) corn steep liquor (Kogo Starch, Chiba, Japan), 1% (w/v) glycerol, 1.8% Daigo’s Artificial Seawater SP (Nihon Pharmaceutical, Tokyo, Japan), 0.2% (w/v) (NH4)2SO4, and 0.2% (w/v) CaCO3 in deionized water (pH 7.4 before sterilization). The seed culture was incubated on a rotary shaker (200 rpm) at 30 °C for 7 days. The seed culture (2.5 mL) of the strain was then transferred into a 500 ml baffled Erlenmeyer flask containing 110 ml of a producing medium consisting of 2.0% glycerin, 2.0% dextrin, 1.0% Bacto soytone, 0.3% yeast extract, 0.2 % (NH4)2SO4, and 0.2% CaCO3 in deionized water (pH 7.4 before sterilization). Fermentation was conducted on a rotary shaker (180 rpm) at 27 °C for 7 days.
Isolation of wychimicins
The fermentation broth (5 l) was separated into a mycelial cake and supernatant by centrifugation. The mycelial cake was extracted with MeOH (3 l). The MeOH solution was then subjected to the removal of MeOH in vacuo and combined with supernatant. The combined solution, including the active substance, was extracted with EtOAc and concentrated in vacuo to give 1.4 g of a brown oil. This oil was then subjected to silica gel chromatography on silica gel 60 column (Φ 48 mm × 110 mm, Merck Millipore, MA, US) developed with EtOAc:MeOH:HCOOH successively at ratios of 100:0:0, 40:1:0.1, 36:4:0.1, and 40:10:0.125. The active fraction was eluted from 40:1:0.1 to 36:4:0.1, collected, and concentrated in vacuo to give a light brown oil. The active oil was subjected to gel filtration chromatography on a Sephadex LH-20 (Φ 38 mm × 400 mm, GE Healthcare, WI, USA) column developed with MeOH. The active fraction was collected and concentrated in vacuo to give a pale brown solid. The active material was subjected to reversed-phase chromatography on a low-pressure ODS column (ODS-7515-12A, Φ 38 mm × 220 mm; Senshu Scientific Co., Ltd., Japan) developed with acetonitrile:water:trifluoroacetic acid at 60:40:0.01. The active fractions including 4, 3, and a mixture of 1 and 2 were collected and concentrated in vacuo to give 35.5 mg of pure 4, 81.5 mg of semi-pure 3, and a mixture of 1 and 2 (161.4 mg), respectively. The semi-pure 3 was subjected to further chromatography on reversed-phase HPLC (Capcell Pak UG120, Φ 30 mm × 250 mm; Shiseido Co., Ltd., Japan) developed with acetonitrile:water at a ratio of 60:40 and a flow rate of 18 ml min−1, collected, and concentrated in vacuo to yield 62.8 mg of pure 3. The mixture of 1 and 2 was subjected to further chromatography on reversed-phase HPLC (Capcell Pak UG120, Φ 30 mm × 250 mm) developed with acetonitrile:water:trifluoroacetic acid at a ratio of 60:40:0.01 and a flow rate of 18 ml min−1, collected, and concentrated in vacuo to yield 47.0 mg of pure 2 (rt: 40–41 min) and 82.8 mg of pure 1.
References
Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod. 2016;79:629–61.
Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core surveillance (ABCs) MRSA I.S. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Jama-J Am Med Assoc. 2007;298:1763–71.
Rasigade JP, Dumitrescu O, Lina G. New epidemiology of Staphylococcus aureus infections. Clin Microbiol Infec. 2014;20:587–8.
Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin Microbiol Rev. 2015;28:603–61.
Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, Carugati, Holland TL, Fowler VGM. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17:203–18.
Kourtis AP, Hatfield K, Baggs J, Mu Y, See I, Epson, Nadle J, Kainer MA, Dumyati G, Petit S, Ray SM, Ham D, Capers C, Ewing H, Coffin N, McDonald LC, Jernigan J, Cardo DE. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections - United States. Mmwr-Morb Mortal W. 2019;68:214–9.
Jevons MP, Rolinson GN, Knox R. Celbenin-Resistant Staphylococci. Brit Med J. 1961;1:124–5.
Meawed TE, Ahmed SM, Mowafy SMS, Samir GM, Anis RH. Bacterial and fungal ventilator associated pneumonia in critically ill COVID-19 patients during the second wave. J Infect Public Heal. 2021;14:1375–80.
Giacobbe DR, Battaglini D, Ball L, Brunetti I, Bruzzone B, Codda G, Crea F, De Maria A, Dentone C, Di Biagio A, Icardi G, Magnasco L, Marchese A, Mikulska M, Orsi A, Patroniti N, Robba C, Signori A, Taramasso L, Vena A, Pelosi P, Bassetti M. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50:e13319.
Sturm LK, Saake K, Roberts PB, Masoudi FA, Fakih MG. Impact of COVID-19 pandemic on hospital onset bloodstream infections (HOBSI) at a large health system. Am J Infect Control. 2022;50:245–9.
Rothe K, Lahmer T, Rasch S, Schneider J, Spinner CD, Wallnöfer F, Wurst M, Schmid RM, Waschulzik B, Fuest K, Kriescher S, Schneider G, Busch DH, Feihl S, Heim MF. Dexamethasone therapy and rates of secondary pulmonary and bloodstream infections in critically ill COVID-19 patients. Multidiscip Respir Med. 2021;16:793.
Hiramatsu K, Kayayama Y, Matsuo M, Aiba Y, Saito M, Hishinuma, Iwamoto AT. Vancomycin-intermediate resistance in Staphylococcus aureus. J Glob Antimicrob Re. 2014;2:213–24.
Crits-Christoph A, Diamond S, Butterfield CN, Thomas BC, Banfield JF. Novel soil bacteria possess diverse genes for secondary metabolite biosynthesis. Nature. 2018;558:440–4.
Charlop-Powers Z, Owen JG, Reddy BVB, Ternei MA, Brady SF. Chemical-biogeographic survey of secondary metabolism in soil. Proc Natl Acad Sci USA. 2014;111:3757–62.
Gans J, Wolinsky M, Dunbar J. Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science. 2005;309:1387–90.
Takahashi Y, Omura S. Isolation of new actinomycete strains for the screening of new bioactive compounds. J Gen Appl Microbiol. 2003;49:141–54.
Hatano M, Kinoshita N, Igarashi M, Nomoto A. Actinocrispum wychmicini gen. nov., sp nov., a novel member of the family Pseudonocardiaceae, isolated from soil. Int J Syst Evol Micr. 2016;66:4779–84.
Tamaoki T, Kasai M, Shirahata K, Ohkubo S, Morimoto M, Mineura K, Ishii S, Tomita F. Tetrocarcin, novel antitumor antibiotics II. Isolation, characterization and antitumor activity. J Antibiot. 1980;33:946–50.
Mallams AK, Puar MS, Rossman RR. Kijanimicin 2. Structure and absolute stereochemistry of Kijanimicin. J Am Chem Soc. 1981;103:3940–3.
Tomita F, Tamaoki T, Shirahata K, Kasai M, Morimoto M, Ohkubo, Mineura K, Ishii SS. Novel antitumor antibiotics, tetrocarcins. J Antibiot. 1980;33:668–70.
Waitz JA, Horan AC, Kalyanpur M, Lee BK, Loebenberg D, Marquez, Miller G, Patel MGJA. Kijanimicin (Sch 25663), a novel antibiotic produced by Actinomadura kijaniata SCC 1256. Fermentation, isolation, characterization and biological properties. J Antibiot. 1981;34:1101–6.
Tanaka Y, Sato I, Iwai C, Kosaka T, Ikeda T, Nakamura T. Identification of human liver diacetyl reductases by nano-liquid chromatography/Fourier transform ion cyclotron resonance mass spectrometry. JP2001238692 A. 2001;293:157–68.
Ashton RJ, Kenig MD, Luk K, Planterose DN, Scott-Wood G. MM 46115, a new antiviral antibiotic from Actinomadura pelletieri. Characteristics of the producing cultures, fermentation, isolation, physico-chemical and biological properties. J Antibiot. 1990;43:1387–93.
Tsunakawa M, Tenmyo O, Tomita K, Naruse N, Kotake C, Miyaki T, Konishi M, Oki TT. Quartromicin, a complex of novel antiviral antibiotics. I. Production, isolation, physico-chemical properties and antiviral activity. J Antibiot. 1992;45:180–8.
Kawashima A, Nakamura Y, Ohta Y, Akama T, Yamagishi M, Hanada K. New cholesterol biosynthesis inhibitors MC-031 (O-demethylchlorothricin), -032 (O-demethylhydroxychlorothricin), -033 and -034. J Antibiot. 1992;45:207–12.
Riedlinger J, Reicke A, Zahner H, Krismer B, Bull AT, Maldonado, WARD AC, Goodfellow M, Bister B, Bischoff D, Sussmuth RD, Fiedler HPLA. Abyssomicins, inhibitors of the para-aminobenzoic acid pathway produced by the marine Verrucosispora strain AB-18-032. J Antibiot. 2004;57:271–9.
Jiang ZD, Jensen PR, Fenical W. Lobophorins A and B, new antiinflammatory macrolides produced by a tropical marine bacterium. Bioorg Med Chem Lett. 1999;9:2003–6.
Ohtsuka T, Kotaki H, Nakayama N, Itezono Y, Shimma N, Kudoh T, Kuwahara T, Arisawa M, Yokose K, Seto HT. Tetronothiodin, a novel cholecystokinin type-B receptor antagonist produced by Streptomyces sp. NR0489. II. Isolation, characterization and biological activities. J Antibiot. 1993;46:11–7.
Acknowledgements
We would like to pay tribute to the late Naoko Kinoshita, who deeply admired the actinomycetes. Her meticulous observation led to the discovery of whychimicins. We thank Y. Kubota, Y. Takahashi, and R. Nagasaka for providing technical assistance. We thank Joe Barber Jr, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kimura, T., Umekita, M., Hatano, M. et al. Wychimicins, a new class of spirotetronate polyketides from Actinocrispum wychmicini MI503-A4. J Antibiot 75, 535–541 (2022). https://doi.org/10.1038/s41429-022-00560-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-022-00560-4