Abstract
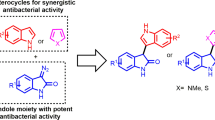
We report the synthesis and antimicrobial studies of a new series of naphthyl-substituted pyrazole-derived hydrazones. Many of these novel compounds are potent growth inhibitors of several strains of drug-resistant bacteria. These potent compounds have inclined growth inhibitory properties for planktonic Staphylococcus aureus and Acinetobacter baumannii, and its drug-resistant variants with minimum inhibitory concentration (MIC) as low as 0.78 and 1.56 µg ml−1, respectively. These compounds also show potent activity against S. aureus and A. baumannii biofilm formation and eradication properties. Time Kill Assay shows that these compounds are bactericidal for S. aureus and bacteriostatic for A. baumannii. The probable mode of action is the disruption of the bacterial cell membrane. Furthermore, potent compounds are nontoxic to human cell lines at several fold higher concentrations than the MICs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
CDC. 2019 AR threats report. Unites States: CDC; 2019. https://www.cdc.gov/drugresistance/biggest-threats.html.
Saini H, Vadekeetil A, Chhibber S, Harjai K. Azithromycin-ciprofloxacin-impregnated urinary catheters avert bacterial colonization, biofilm formation, and inflammation in a murine model of foreign-body-associated urinary tract infections caused by Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2017;61:e01906–16.
Roy R, Tiwari M, Donelli G, Tiwari V. Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018;9:522–54.
Worthington RJ, Richards JJ, Melander C. Small molecule control of bacterial biofilms. Org Biomolecular Chem. 2012;10:7457–74.
Santajit S, Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int. 2016;2016:2475067.
Joseph R, Naugolny A, Feldman M, Herzog IM, Fridman M, Cohen Y. Cationic pillararenes potently inhibit biofilm formation without affecting bacterial growth and viability. J Am Chem Soc. 2016;138:754–7.
Laverty G, McCloskey AP, Gilmore BF, Jones DS, Zhou J, Xu B. Ultrashort cationic naphthalene-derived self-assembled peptides as antimicrobial nanomaterials. Biomacromolecules. 2014;15:3429–39.
Chen YY, Gopala L, Bheemanaboina RRY, Liu HB, Cheng Y, Geng RX, et al. Novel naphthalimide aminothiazoles as potential multitargeting antimicrobial agents. Acs Med Chem Lett. 2017;8:1331–5.
Chang FY, Peacock JE, Musher DM, Triplett P, MacDonald BB, Mylotte JM, et al. Staphylococcus aureus bacteremia - recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine. 2003;82:333–9.
Muhlbacher JM. Naftifine - a topical allylamine antifungal agent. Clin Dermatol. 1991;9:479–85.
Gupta AK, Ryder JE, Cooper EA. Naftifine: a review. J Cutan Med Surg. 2008;12:51–8.
Ryder NS, Frank I, Dupont MC. Ergosterol biosynthesis inhibition by the thiocarbamate antifungal agents tolnaftate and tolciclate. Antimicrob Agents Chemother. 1986;29:858–60.
Asif M. Rifampin and their analogs: a development of antitubercular drugs. World J Org Chem. 2013;1:14–9.
Steinbach G, Lynch PM, Phillips RKS, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52.
Luttinger D, Hlasta DJ. Antidepressant agents. Annu Rep Med Chem. 1987;22:20–30.
Hampp C, Hartzema AG, Kauf TL. Cost-utility analysis of rimonabant in the treatment of obesity. Value Health. 2008;11:389–99.
Kameyama T, Nabeshima T. Effects of 1,3-diphenyl-5-(2-dimethylaminopropionamide)-pyrazole[difenamizole] on a conditioned avoidance-response. Neuropharmacology. 1978;17:249–56.
Kumar V, Aggarwal R, Tyagi P, Singh SP. Synthesis and antibacterial activity of some new 1-heteroaryl-5-amino-4-phenyl-3-trifluoromethylpyrazoles. Eur J Med Chem. 2005;40:922–7.
Aggarwal R, Kumar V, Tyagi P, Singh SP. Synthesis and antibacterial activity of some new 1-heteroaryl-5-amino-3H/methyl-4-phenylpyrazoles. Bioorgan Med Chem. 2006;14:1785–91.
Aslan HG, Ozcan S, Karacan N. The antibacterial activity of some sulfonamides and sulfonyl hydrazones, and 2D-QSAR study of a series of sulfonyl hydrazones. Spectrochim Acta A. 2012;98:329–36.
Nasr T, Bondock S, Youns M. Anticancer activity of new coumarin substituted hydrazide-hydrazone derivatives. Eur J Med Chem. 2014;76:539–48.
Senkardes S, Kaushik-Basu N, Durmaz I, Manvar D, Basu A, Atalay R, et al. Synthesis of novel diflunisal hydrazide hydrazones as anti-hepatitis C virus agents and hepatocellular carcinoma inhibitors. Eur J Med Chem. 2016;108:301–8.
Brider J, Rowe T, Gibler DJ, Gottsponer A, Delancey E, Branscum MD, et al. Synthesis and antimicrobial studies of azomethine and N-arylamine derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-methicillin-resistant Staphylococcus aureus agents. Medicinal Chem Res. 2016;25:2691–7.
Allison D, Delancey E, Ramey H, Williams C, Alsharif ZA, Al-khattabi H, et al. Synthesis and antimicrobial studies of novel derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-Acinetobacter baumannii agents. Bioorg Medicinal Chem Lett. 2017;27:387–92.
Zakeyah AA, Whitt J, Duke C, Gilmore DF, Meeker DG, Smeltzer MS. et al. Synthesis and antimicrobial studies of hydrazone derivatives of 4-[3-(2,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid and 4-[3-(3,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid. Bioorg Med Chem Lett. 2018;28:2914–9.
Whitt J, Duke C, Ali MA, Chambers SA, Khan MMK, Gilmore D. et al. Synthesis and antimicrobial studies of 4-[3-(3-fluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid and 4-[3-(4-fluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid as potent growth inhibitors of drug-resistant bacteria. ACS Omega. 2019;4:14284–93.
Whitt J, Duke C, Sumlin A, Chambers SA, Alnufaie R, Gilmore D. et al. Synthesis of hydrazone derivatives of 4-[4-formyl-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid as potent growth inhibitors of antibiotic-resistant Staphylococcus aureus and Acinetobacter baumannii. Molecules. 2019;24:2051
Chen CY, Nace GW, Irwin PL. A 6 × 6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J Microbiol Methods. 2003;55:475–9.
Foerster S, Unemo M, Hathaway LJ, Low N, Althaus CL. Time-kill curve analysis and pharmacodynamic modelling for in vitro evaluation of antimicrobials against Neisseria gonorrhoeae. BMC Microbiol. 2016;16:216.
Werby SH, Cegelski L. Design and implementation of a six-session CURE module using biofilms to explore the chemistry–biology interface. J Chem Educ. 2019;96:2050–4.
Halicki PCB, Radin V, von Groll A, Nora MV, Pinheiro AC, da Silva PEA, Ramos DF. Antibiofilm potential of arenecarbaldehyde 2-pyridinylhydrazone derivatives against Acinetobacter baumannii. Microbial Drug Resistance. 2019. https://doi.org/10.1089/mdr.2019.0185.
Abdelhady W, Bayer AS, Seidl K, Nast CC, Kiedrowski MR, Horswill AR, et al. Reduced vancomycin susceptibility in an in vitro catheter-related biofilm model correlates with poor therapeutic outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57:1447.
Stiefel P, Schmidt-Emrich S, Maniura-Weber K, Ren Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 2015;15:36.
Acknowledgements
This publication was made possible by the Research Technology Core of the Arkansas INBRE program, supported by a grant from the National Institute of General Medical Sciences, (NIGMS), P20 GM103429 from the National Institutes of Health to record the Mass Spectrometry data. This publication was made possible by the Arkansas INBRE program, supported by a grant from the National Institute of General Medical Sciences, (NIGMS), P20 GM103429 from the National Institutes of Health, grant number P20 GM109005 (AGB). ABI mini-grant 200027 also helped to accomplish this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41429_2020_341_MOESM1_ESM.docx
Design, and Synthesis of 4-[4-Formyl-3-(2-naphthyl)pyrazol-1-yl]benzoic acid Derivatives as Potent Growth Inhibitors of Drug-Resistant Staphylococcus aureus
Rights and permissions
About this article
Cite this article
Alnufaie, R., Alsup, N., KC, H.R. et al. Design and synthesis of 4-[4-formyl-3-(2-naphthyl)pyrazol-1-yl]benzoic acid derivatives as potent growth inhibitors of drug-resistant Staphylococcus aureus. J Antibiot 73, 818–827 (2020). https://doi.org/10.1038/s41429-020-0341-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-020-0341-2