Abstract
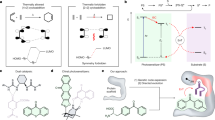
Despite their intriguing photophysical and photochemical activities, naturally occurring photoenzymes have not yet been repurposed for new-to-nature activities. Here we engineered fatty acid photodecarboxylases to catalyse unnatural photoredox radical C–C bond formation by leveraging the strongly oxidizing excited-state flavoquinone cofactor. Through genome mining, rational engineering and directed evolution, we developed a panel of radical photocyclases to facilitate decarboxylative radical cyclization with excellent chemo-, enantio- and diastereoselectivities. Our high-throughput experimental workflow allowed for the directed evolution of fatty acid photodecarboxylases. An orthogonal set of radical photocyclases was engineered to access all four possible stereoisomers of the stereochemical dyad, affording fully diastereo- and enantiodivergent biotransformations in asymmetric radical biocatalysis. Molecular dynamics simulations show that our evolved radical photocyclases allow near-attack conformations to be easily accessed, enabling chemoselective radical cyclization. The development of stereoselective radical photocyclases provides unnatural C–C-bond-forming activities in natural photoenzyme families, which can be used to tame the stereochemistry of free-radical-mediated reactions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available in the main text and the Supplementary Information.
References
Begley, T. P. Photoenzymes: a novel class of biological catalysts. Acc. Chem. Res. 27, 394–401 (1994).
Taylor, A., Heyes, D. J. & Scrutton, N. S. Catalysis by nature’s photoenzymes. Curr. Opin. Struct. Biol. 77, 102491 (2022).
Schoefs, B. & Franck, F. Protochlorophyllide reduction: mechanisms and evolution. Photochem. Photobiol. 78, 543–557 (2003).
Zhang, S. et al. Structural basis for enzymatic photocatalysis in chlorophyll biosynthesis. Nature 574, 722–725 (2019).
Heyes, D. J. et al. Photocatalysis as the ‘master switch’ of photomorphogenesis in early plant development. Nat. Plants 7, 268–276 (2021).
Archipowa, N., Kutta, R. J., Heyes, D. J. & Scrutton, N. S. Stepwise hydride transfer in a biological system: insights into the reaction mechanism of the light‐dependent protochlorophyllide oxidoreductase. Angew. Chem. Int. Ed. 57, 2682–2686 (2018).
Heyes, D. J., Sakuma, M. & Scrutton, N. S. Solvent‐slaved protein motions accompany proton but not hydride tunneling in light‐activated protochlorophyllide oxidoreductase. Angew. Chem. Int. Ed. 48, 3850–3853 (2009).
Dong, C. et al. Crystal structures of cyanobacterial light-dependent protochlorophyllide oxidoreductase. Proc. Natl Acad. Sci. USA 117, 8455–8461 (2020).
Zhang, M., Wang, L., Shu, S., Sancar, A. & Zhong, D. Bifurcating electron-transfer pathways in DNA photolyases determine the repair quantum yield. Science 354, 209–213 (2016).
Brettel, K. & Byrdin, M. Reaction mechanisms of DNA photolyase. Curr. Opin. Struct. Biol. 20, 693–701 (2010).
Li, J. et al. Dynamics and mechanism of repair of ultraviolet-induced (6–4) photoproduct by photolyase. Nature 466, 887–890 (2010).
Sorigué, D. et al. An algal photoenzyme converts fatty acids to hydrocarbons. Science 357, 903–907 (2017).
Sorigué, D. et al. Mechanism and dynamics of fatty acid photodecarboxylase. Science 372, eabd5687 (2021).
Moulin, S. L. Y. et al. Fatty acid photodecarboxylase is an ancient photoenzyme that forms hydrocarbons in the thylakoids of algae. Plant Physiol. 186, 1455–1472 (2021).
Aselmeyer, C. et al. Fatty acid photodecarboxylase is an interfacial enzyme that binds to lipid–water interfaces to access its insoluble substrate. Biochemistry 60, 3200–3212 (2021).
Huijbers, M. M. E., Zhang, W., Tonin, F. & Hollmann, F. Light‐driven enzymatic decarboxylation of fatty acids. Angew. Chem. Int. Ed. 57, 13648–13651 (2018).
Zhang, W. et al. Hydrocarbon synthesis via photoenzymatic decarboxylation of carboxylic acids. J. Am. Chem. Soc. 141, 3116–3120 (2019).
Xu, J. et al. Light‐driven kinetic resolution of α‐functionalized carboxylic acids enabled by an engineered fatty acid photodecarboxylase. Angew. Chem. Int. Ed. 58, 8474–8478 (2019).
Li, D. et al. Engineering fatty acid photodecarboxylase to enable highly selective decarboxylation of trans fatty acids. Angew. Chem. Int. Ed. 60, 20695–20699 (2021).
Xu, J. et al. Light-driven decarboxylative deuteration enabled by a divergently engineered photodecarboxylase. Nat. Commun. 12, 3983 (2021).
Santner, P. et al. Optimization and engineering of fatty acid photodecarboxylase for substrate specificity. ChemCatChem 13, 4038–4046 (2021).
Xu, W. et al. Rational design of fatty acid photodecarboxylase enables the efficient decarboxylation of medium- and short-chain fatty acids for the production of gasoline bio-alkanes. Mol. Catal. 524, 112261 (2022).
Amer, M. et al. Low carbon strategies for sustainable bio-alkane gas production and renewable energy. Energy Environ. Sci. 13, 1818–1831 (2020).
Zhou, Q., Chin, M., Fu, Y., Liu, P. & Yang, Y. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450. Science 374, 1612–1616 (2021).
Fu, Y. et al. Engineered P450 atom-transfer radical cyclases are bifunctional biocatalysts: reaction mechanism and origin of enantioselectivity. J. Am. Chem. Soc. 144, 13344–13355 (2022).
Fu, W. et al. Enzyme-controlled stereoselective radical cyclization to arenes enabled by metalloredox biocatalysis. Nat. Catal. 6, 628–636 (2023).
Rui, J. et al. Directed evolution of nonheme iron enzymes to access abiological radical-relay C(sp3)−H azidation. Science 376, 869–874 (2022).
Cheng, L. et al. Stereoselective amino acid synthesis by synergistic photoredox-pyridoxal radical biocatalysis. Science 381, 444–451 (2023).
Emmanuel, M. A. et al. Photobiocatalytic strategies for organic synthesis. Chem. Rev. 123, 5459–5520 (2023).
Emmanuel, M. A., Greenberg, N. R., Oblinsky, D. G. & Hyster, T. K. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 540, 414–417 (2016).
Biegasiewicz, K. F. et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization. Science 364, 1166–1169 (2019).
Black, M. J. et al. Asymmetric redox-neutral radical cyclization catalysed by flavin-dependent ‘ene’-reductases. Nat. Chem. 12, 71–75 (2020).
Clayman, P. D. & Hyster, T. K. Photoenzymatic generation of unstabilized alkyl radicals: an asymmetric reductive cyclization. J. Am. Chem. Soc. 142, 15673–15677 (2020).
Page, C. G. et al. Quaternary charge-transfer complex enables photoenzymatic intermolecular hydroalkylation of olefins. J. Am. Chem. Soc. 143, 97–102 (2021).
Gao, X., Turek-Herman, J. R., Choi, Y. J., Cohen, R. D. & Hyster, T. K. Photoenzymatic synthesis of α-tertiary amines by engineered flavin-dependent “ene”-reductases. J. Am. Chem. Soc. 143, 19643–19647 (2021).
Nicholls, B. T. et al. Engineering a non‐natural photoenzyme for improved photon efficiency. Angew. Chem. Int. Ed. 61, e202113842 (2022).
Fu, H. et al. An asymmetric sp3–sp3 cross-electrophile coupling using ‘ene’-reductases. Nature 610, 302–307 (2022).
Fu, H., Qiao, T., Carceller, J. M., MacMillan, S. N. & Hyster, T. K. Asymmetric C-alkylation of nitroalkanes via enzymatic photoredox catalysis. J. Am. Chem. Soc. 145, 787–793 (2023).
Page, C. G. et al. Regioselective radical alkylation of arenes using evolved photoenzymes. J. Am. Chem. Soc. 145, 11866–11874 (2023).
Harrison, W., Huang, X. & Zhao, H. Photobiocatalysis for abiological transformations. Acc. Chem. Res. 55, 1087–1096 (2022).
Huang, X. et al. Photoenzymatic enantioselective intermolecular radical hydroalkylation. Nature 584, 69–74 (2020).
Huang, X. et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition. Nat. Catal. 5, 586–593 (2022).
Heyes, D. J. et al. Photochemical mechanism of light-driven fatty acid photodecarboxylase. ACS Catal. 10, 6691–6696 (2020).
Jasperse, C. P., Curran, D. P. & Fevig, T. L. Radical reactions in natural product synthesis. Chem. Rev. 91, 1237–1286 (1991).
Gilmore, K. & Alabugin, I. V. Cyclizations of alkynes: revisiting Baldwin’s rules for ring closure. Chem. Rev. 111, 6513–6556 (2011).
Gilmore, K., Mohamed, R. K. & Alabugin, I. The Baldwin rules: revised and extended. WIREs Comput. Mol. Sci. 6, 487–514 (2016).
Neveselý, T., Wienhold, M., Molloy, J. J. & Gilmour, R. Advances in the E → Z isomerization of alkenes using small molecule photocatalysts. Chem. Rev. 122, 2650–2694 (2022).
Litman, Z. C., Wang, Y., Zhao, H. & Hartwig, J. F. Cooperative asymmetric reactions combining photocatalysis and enzymatic catalysis. Nature 560, 355–359 (2018).
Miao, Y., Feher, V. A. & McCammon, J. A. Gaussian accelerated molecular dynamics: unconstrained enhanced sampling and free energy calculation. J. Chem. Theory Comput. 11, 3584–3595 (2015).
Soler, J., Gergel, S., Klaus, C., Hammer, S. C. & Garcia-Borràs, M. Enzymatic control over reactive intermediates enables direct oxidation of alkenes to carbonyls by a P450 iron-oxo species. J. Am. Chem. Soc. 144, 15954–15968 (2022).
Acknowledgements
This research is supported by the National Institutes of Health (NIH; R35GM128779 to P.L., R35GM147387 to Y.Y.), American Chemical Society Petroleum Research Fund (ACS PRF; 65807-DNI1) and the National Science Foundation (NSF; OCE-1756947 to D.L.V.). We acknowledge the NSF BioPACIFIC Materials Innovation Platform (MIP; DMR-1933487) and NSF Materials Research Science and Engineering Centers (MRSEC) at University of California, Santa Barbara (UCSB; DMR-2308708) for access to instrumentation. Computational studies were carried out at the University of Pittsburgh Center for Research Computing and the Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) programme, supported by NSF award numbers OAC-2117681 and OAC-2138259. We thank Y. Wang for critical reading of this manuscript.
Author information
Authors and Affiliations
Contributions
Y.Y. conceived and directed the project. S.J. performed all the enzyme engineering and substrate scope studies. S.J. and A.V.-E. performed enzyme mining and molecular cloning with Y.Y. and D.L.V., and J.W. provided guidance. D.L., S.J. and X.L. synthesized all the substrates and racemic products for analysis. B.K.M. carried out the computational studies with P.L. providing guidance. Y.Y., P.L., S.J. and B.K.M. wrote the manuscript with the input of all other authors.
Corresponding authors
Ethics declarations
Competing interests
Y.Y. and S.J. are inventors on a patent application submitted by the University of California Santa Barbara (UC case no. 2024-843) that covers compositions, methods and applications of evolved RAPs derived from natural FAPs. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Dunming Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Origin of stereoconvergent photobiocatalytic radical cyclisation with (E)- and (Z)-1a.
a, Stereoconvergent photobiotransformation of (E)-1a and (Z)-1a. b, Photoisomerisation of (Z)-1a with FAD (free cofactor). c, Initial rate and photoisomerisation with CvRAP1.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, Discussion and Tables 1–26.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ju, S., Li, D., Mai, B.K. et al. Stereodivergent photobiocatalytic radical cyclization through the repurposing and directed evolution of fatty acid photodecarboxylases. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01494-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01494-0