Featured
-
-
Article |
 Identification and characterization of diverse coherences in the Fenna–Matthews–Olson complex
Identification and characterization of diverse coherences in the Fenna–Matthews–Olson complex
The implications of coherence signals for the transfer of energy within the Fenna–Matthews–Olson complex of photosynthetic green sulfur bacteria is a well debated topic. Now, polarization-controlled 2D spectroscopy — aided by vibronic exciton modelling — has enabled the characterization of all such coherences and determination of their physical origins; while electronic coherences dephase extremely rapidly, ground- and excited-state vibrational coherences dominate.
- Erling Thyrhaug
- , Roel Tempelaar
- & Donatas Zigmantas
-
Article |
 Understanding the quantum nature of low-energy C(3Pj) + He inelastic collisions
Understanding the quantum nature of low-energy C(3Pj) + He inelastic collisions
Collision-induced spin–orbit transitions involve multiple interaction potentials and are by nature non-adiabatic, complicating both their experimental and theoretical study. Crossed-beam experiments and non-Born–Oppenheimer quantum calculations for inelastic collisions of carbon atoms with helium atoms, down to energies corresponding to temperatures below 10 K, have now been performed. Quantum-dynamical resonances predicted by theory were experimentally detected.
- Astrid Bergeat
- , Simon Chefdeville
- & François Lique
-
Article |
 Cold quantum-controlled rotationally inelastic scattering of HD with H2 and D2 reveals collisional partner reorientation
Cold quantum-controlled rotationally inelastic scattering of HD with H2 and D2 reveals collisional partner reorientation
Scattering of molecules at low temperature that are prepared in single quantum states illuminates the mechanism of rotationally inelastic collisions and reveals the reorientation of partner molecules. By correlating each outgoing partial wave with the incoming waves, partial-wave analysis of the scattering angular distribution determines the dominant short- and long-range anisotropies of the interaction potential.
- William E. Perreault
- , Nandini Mukherjee
- & Richard N. Zare
-
News & Views |
 Sticky when wet
Sticky when wet
The aqueous hydronium cation diffuses about twice as fast as the aqueous hydroxide anion in liquid water, but the origin of this behaviour has been unclear. Now, state-of-the-art simulations provide an explanation for this long-standing conundrum.
- Ji Chen
- & Angelos Michaelides
-
Article |
 Hydroxide diffuses slower than hydronium in water because its solvated structure inhibits correlated proton transfer
Hydroxide diffuses slower than hydronium in water because its solvated structure inhibits correlated proton transfer
Even though the Grotthuss mechanism was proposed two centuries ago, it is still unclear why proton transfer via the hydroxide ion is slower than that via hydronium. Advanced ab initio molecular dynamics simulations now show that it is because proton transfer via hydroxide is less temporally correlated than transfer via hydronium.
- Mohan Chen
- , Lixin Zheng
- & Xifan Wu
-
Article |
 Control over phase separation and nucleation using a laser-tweezing potential
Control over phase separation and nucleation using a laser-tweezing potential
A low-power laser can cause phase separation or trigger the nucleation of a new phase in the proximity of a liquid–liquid critical point, or binodal, using a laser tweezing potential. This effect explains the physics behind non-photochemical laser-induced nucleation and suggests new ways of manipulating matter.
- Finlay Walton
- & Klaas Wynne
-
Article |
 Observation of correlated excitations in bimolecular collisions
Observation of correlated excitations in bimolecular collisions
Collisions between atoms and molecules are largely understood; however, our understanding of collisions between two molecules is lacking because they are significantly harder to study, Now, correlated rotational excitations have been observed in inelastic collisions between NO and O2 molecules. It is shown that the energy-gap law that governs atom–molecule collisions does not generally apply to bimolecular excitation processes.
- Zhi Gao
- , Tijs Karman
- & Sebastiaan Y. T. van de Meerakker
-
Article |
 Scattering resonances in bimolecular collisions between NO radicals and H2 challenge the theoretical gold standard
Scattering resonances in bimolecular collisions between NO radicals and H2 challenge the theoretical gold standard
Calculations at the theoretical gold standard generally yield accurate results for a variety of energy-transfer processes in molecular collisions. Using anti-seeding methods in a crossed-beam inelastic scattering experiment, a resonance structure is clearly resolved for NO–H2 collisions, pushing the required accuracy for theoretical potentials beyond the gold standard.
- Sjoerd N. Vogels
- , Tijs Karman
- & Sebastiaan Y. T. van de Meerakker
-
Article |
 Ultrafast dynamics of low-energy electron attachment via a non-valence correlation-bound state
Ultrafast dynamics of low-energy electron attachment via a non-valence correlation-bound state
The capture of an electron by a molecule represents one of the most fundamental chemical transformations, but its mechanism at very low energies remains unclear. Now, it is shown that low-energy electron attachment to hexafluorobenzene is mediated by a non-valence correlation-bound state of the anion.
- Joshua P. Rogers
- , Cate S. Anstöter
- & Jan R. R. Verlet
-
News & Views |
 The view from a transition state
The view from a transition state
Ejecting electrons from negative ions using light can create structures that very closely resemble the transition states of bimolecular reactions. Now, using this technique, trapped quantum states, or 'resonances', have been observed in a seven-atom reaction, and theory has been shown to be up to the task of capturing such complex phenomena.
- Robert E. Continetti
-
Article |
 Direct mapping of the angle-dependent barrier to reaction for Cl + CHD3 using polarized scattering data
Direct mapping of the angle-dependent barrier to reaction for Cl + CHD3 using polarized scattering data
Two important properties in an activated chemical reaction are the barrier height and its geometrical dependence. Now, a method has been developed to directly map the angle-dependent barrier to reaction from polarized scattering data for the Cl + CHD3 reaction. The method should be applicable to many other direct reactions with a colinear barrier.
- Huilin Pan
- , Fengyan Wang
- & Kopin Liu
-
Article |
 Feshbach resonances in the exit channel of the F + CH3OH → HF + CH3O reaction observed using transition-state spectroscopy
Feshbach resonances in the exit channel of the F + CH3OH → HF + CH3O reaction observed using transition-state spectroscopy
The transition state governs how bonds form and cleave during a reaction — its direct characterization is a long-standing challenge. Now, the F + CH3OH → HF + CH3O reactive surface has been studied using photoelectron velocity-map imaging spectroscopy of cryo-cooled anions, revealing vibrational Feshbach resonances and bound states supported by the post-transition-state potential well. The experiments agree well with quantum dynamical calculations.
- Marissa L. Weichman
- , Jessalyn A. DeVine
- & Daniel M. Neumark
-
News & Views |
 React with nobility
React with nobility
Helium, the 'most noble' of the noble gases, had only been coaxed into forming molecular ions or van der Waals compounds. It has now been seen in a stable solid compound, Na2He, under high pressure.
- Maosheng Miao
-
Article |
 Observation of electron-transfer-mediated decay in aqueous solution
Observation of electron-transfer-mediated decay in aqueous solution
Electron-transfer-mediated decay (ETMD) is a recently discovered type of electronic relaxation that involves the refilling of a core hole by an electron from a neighbouring species. It has now been observed in LiCl solution, when previously it had only been seen in rare-gas clusters. Spectra generated during ETMD are observed to be sensitive to the immediate environment of the initially ionized ion.
- Isaak Unger
- , Robert Seidel
- & Nikolai V. Kryzhevoi
-
Article |
 Tuning underwater adhesion with cation–π interactions
Tuning underwater adhesion with cation–π interactions
Cation–π interactions are critical for the adhesion proteins of marine organisms, yet the energetics of cation–π interactions in underwater environments remains uncharted. Nanoscale force measurements and NMR spectroscopy reveal that interfacial confinement fundamentally alters the energetics of cation–π mediated assembly.
- Matthew A. Gebbie
- , Wei Wei
- & Jacob N. Israelachvili
-
Article |
 A stable compound of helium and sodium at high pressure
A stable compound of helium and sodium at high pressure
Helium is generally recognized as being chemically inert. A thermodynamically stable compound of helium and sodium, Na2He, has been predicted computationally and then synthesized at high pressure. It exists as an electride, where strongly localized electrons serve as anions located at the centre of Na8 cubes.
- Xiao Dong
- , Artem R. Oganov
- & Hui-Tian Wang
-
Article |
 Ionic solutions of two-dimensional materials
Ionic solutions of two-dimensional materials
Isolating nanoscale species in liquids permits their scalable manipulation, enabling numerous fundamental and applied processes. Thus, achieving true dissolution of 2D materials is particularly desirable. Now, ionic salts of a range of important layered materials have been shown to spontaneously dissolve, yielding solutions of charged, monodisperse, undamaged and easy-to-manipulate 2D nanosheets.
- Patrick L. Cullen
- , Kathleen M. Cox
- & Christopher A. Howard
-
Article |
 Imaging quantum stereodynamics through Fraunhofer scattering of NO radicals with rare-gas atoms
Imaging quantum stereodynamics through Fraunhofer scattering of NO radicals with rare-gas atoms
Stereodynamics describes how the vector properties of molecules affect the probabilities of specific processes in molecular collisions. Measurements of irregular diffraction patterns for NO radicals colliding with rare-gas atoms reveal a previously unrecognized type of quantum stereodynamics and a ‘propensity rule’ for the magnetic quantum number (m) of the molecules.
- Jolijn Onvlee
- , Sean D. S. Gordon
- & Sebastiaan Y. T. van de Meerakker
-
Article |
 Lack of evidence for phase-only control of retinal photoisomerization in the strict one-photon limit
Lack of evidence for phase-only control of retinal photoisomerization in the strict one-photon limit
The degree to which light-induced processes are sensitive to the shape of an incident electromagnetic wave remains a hotly debated topic. Experiments performed at very low levels of light agree with seminal theoretical predictions that tuning the phase of the light field does not affect photochemical reactivity at the single-photon level.
- M. Liebel
- & P. Kukura
-
Article |
 Translational, rotational and vibrational relaxation dynamics of a solute molecule in a non-interacting solvent
Translational, rotational and vibrational relaxation dynamics of a solute molecule in a non-interacting solvent
Spectral broadening generally conceals the signatures of rotational and translational motion in solution-phase spectra. Now, using highly inert perfluorocarbon solvents, spectral broadening has been minimized allowing the translational, rotational and vibrational relaxation dynamics of highly excited CN solute molecules to be observed simultaneously.
- Michael P. Grubb
- , Philip M. Coulter
- & Michael N. R. Ashfold
-
Article |
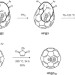 The dipolar endofullerene HF@C60
The dipolar endofullerene HF@C60
Hydrogen fluoride has been encapsulated in C60-fullerene using molecular surgery. The quantum rotor system has been studied by NMR and infrared spectroscopy as well as neutron scattering. The fullerene cage causes a small red-shift in the HF rotational and vibrational constants, and shields around 75% of its dipole.
- Andrea Krachmalnicoff
- , Richard Bounds
- & Richard J. Whitby
-
Article |
 In situ mapping of the energy flow through the entire photosynthetic apparatus
In situ mapping of the energy flow through the entire photosynthetic apparatus
Effective light capture in photosynthetic organisms depends on the efficiency of all energy-transfer steps in the photosynthetic unit. Two-dimensional electronic spectroscopy has now been used on intact cells in situ to reveal and characterize the functional connectivity between individual complexes in the photosynthetic apparatus of green sulfur bacteria.
- Jakub Dostál
- , Jakub Pšenčík
- & Donatas Zigmantas
-
News & Views |
 Between a rock and a soft place
Between a rock and a soft place
The critical step in water splitting is the formation of a peroxo bond; the mechanism, thought to involve oxyl radical formation, remains elusive. Now, experiments reveal a distinct bond vibration directly connected to an oxyl radical that is simultaneously coupled to both the semiconductor electronic states and the motion of the surrounding water.
- Heather Vanselous
- & Poul B. Petersen
-
Article |
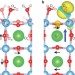 Detecting the oxyl radical of photocatalytic water oxidation at an n-SrTiO3/aqueous interface through its subsurface vibration
Detecting the oxyl radical of photocatalytic water oxidation at an n-SrTiO3/aqueous interface through its subsurface vibration
Water oxidation on titanate surfaces is thought to occur via an oxyl-radical intermediate (Ti–O•), which precedes O–O bond formation, and for which there is indirect evidence. Using ultrafast infrared spectroscopy and theoretical calculations of photocatalytic water oxidation at the n-SrTiO3/aqueous interface, the oxyl radical has now been detected through its subsurface vibration.
- David M. Herlihy
- , Matthias M. Waegele
- & Tanja Cuk
-
News & Views |
 Catch the carbon dioxide
Catch the carbon dioxide
Understanding the minute details of CO2 transport is key to finding new technologies that reduce the hazardous levels of CO2 in our atmosphere. Now, the observation that the transport of CO2 in molten calcium carbonate occurs faster than standard molecular diffusion brings us one step closer.
- Barbara Kirchner
- & Barbara Intemann
-
News & Views |
 All in a spin
All in a spin
A fundamental challenge in systems chemistry is to engineer the emergence of complex behaviour. The collective structures of metal cyanide chains have now been interpreted in the same manner as the myriad of magnetic phases displayed by frustrated spin systems, highlighting a symbiotic approach between systems chemistry and magnetism.
- Lucy Clark
- & Philip Lightfoot
-
Article |
 Carbon dioxide transport in molten calcium carbonate occurs through an oxo-Grotthuss mechanism via a pyrocarbonate anion
Carbon dioxide transport in molten calcium carbonate occurs through an oxo-Grotthuss mechanism via a pyrocarbonate anion
The solvation behaviour of CO2 in carbonate melts is important from both a geochemical point of view and with respect to its electroreduction. Now, simulations have shown that solvation of CO2 in molten CaCO3 leads to the formation of the pyrocarbonate anion, C2O52–, which significantly enhances the transport of CO2 via a Grotthuss-like mechanism.
- Dario Corradini
- , François-Xavier Coudert
- & Rodolphe Vuilleumier
-
Article |
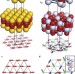 Encoding complexity within supramolecular analogues of frustrated magnets
Encoding complexity within supramolecular analogues of frustrated magnets
Competing metallophilic and electrostatic interactions between gold and/or silver cyanide chains govern their assembly into different structures. An analogy has now been drawn between these systems and two-dimensional magnets. Supramolecular interactions between the chains have been tuned to mimic different magnetic interactions, leading to the realization of complex states predicted for magnets.
- Andrew B. Cairns
- , Matthew J. Cliffe
- & Andrew L. Goodwin
-
Article |
 Molecular hydrogen interacts more strongly when rotationally excited at low temperatures leading to faster reactions
Molecular hydrogen interacts more strongly when rotationally excited at low temperatures leading to faster reactions
The rotational state of a molecule is not generally considered to play a role in how fast it reacts; however, when the temperature is low quantum effects become more important. Now, it is shown that at low temperatures rotationally excited H2 molecules react with He faster than non-rotating ground-state molecules — a process mediated by stronger long-range attraction.
- Yuval Shagam
- , Ayelet Klein
- & Edvardas Narevicius
-
Article |
 Sub-50-fs photoinduced spin crossover in [Fe(bpy)3]2+
Sub-50-fs photoinduced spin crossover in [Fe(bpy)3]2+
Fe(II) complexes display transitions between spin states that can be triggered externally. Now the light-induced ΔS = 2 transition upon excitation of the metal-to-ligand charge-transfer states of Fe(II)-polypyridine complexes has been investigated at high time-resolution in the visible and the ultraviolet range. It has been shown to occur in less than 50 fs — that is, on a sub-vibrational timescale.
- Gerald Auböck
- & Majed Chergui
-
Article |
 Roaming-mediated ultrafast isomerization of geminal tri-bromides in the gas and liquid phases
Roaming-mediated ultrafast isomerization of geminal tri-bromides in the gas and liquid phases
Roaming — a new and unusual reaction mechanism in gas-phase chemical transformations — is now shown to occur in solution. Following ultraviolet excitation of geminal tribromides, what initially seems to be the simple fission of a bond is in fact isomerization occurring through the roaming of molecular fragments.
- Andrey S. Mereshchenko
- , Evgeniia V. Butaeva
- & Alexander N. Tarnovsky
-
Article |
 Coulomb explosion during the early stages of the reaction of alkali metals with water
Coulomb explosion during the early stages of the reaction of alkali metals with water
The explosion of alkali metals in water is a typical high-school chemistry experiment, but its mechanism is not fully understood. Using high-speed cameras and molecular simulations it is now shown that a key early step in this reaction is the migration of electrons from the alkali metal into water, leading to a charging of the metal's surface and subsequent Coulomb explosion.
- Philip E. Mason
- , Frank Uhlig
- & Pavel Jungwirth
-
Article |
 Discovering chemistry with an ab initio nanoreactor
Discovering chemistry with an ab initio nanoreactor
Computational chemistry is traditionally used to interpret experimental findings. Now its use in reaction discovery is described with the development of the ab initio nanoreactor — a highly accelerated, first-principles molecular dynamics simulation of chemical reactions that discovers new molecules and mechanisms without preordained reaction coordinates or elementary steps.
- Lee-Ping Wang
- , Alexey Titov
- & Todd J. Martínez
-
News & Views |
 May the electric force be with you
May the electric force be with you
Intense laser fields can apply strong forces to molecules, distorting molecular potentials. Now, these effects have been used to precisely control the branching ratios of a polyatomic photodissociation reaction.
- Albert Stolow
-
Article |
 Irreversible xenon insertion into a small-pore zeolite at moderate pressures and temperatures
Irreversible xenon insertion into a small-pore zeolite at moderate pressures and temperatures
Several solutions to the ‘missing xenon’ problem have been proposed that involve the selective sorption of Xe in minerals found in the Earth. It is now shown that a zeolite, Ag-natrolite, absorbs and retains 28 wt% Xe at 1.7 GPa and 250 °C, conditions found in subsurface Earth, through expulsion of metallic Ag(0).
- Donghoon Seoung
- , Yongmoon Lee
- & Yongjae Lee
-
Article |
 Reactions of xenon with iron and nickel are predicted in the Earth's inner core
Reactions of xenon with iron and nickel are predicted in the Earth's inner core
Studies of the Earth's atmosphere have shown that more than 90% of xenon is depleted — the so-called missing Xe paradox. Now a theoretical study shows that Xe and Fe/Ni can form inter-metallic compounds of XeFe3 and XeNi3 under conditions found in the Earth's inner core, and could provide a solution to the puzzle.
- Li Zhu
- , Hanyu Liu
- & Yanming Ma
-
News & Views |
 Isotope effects feel the cold
Isotope effects feel the cold
Kinetic isotope effects are widely used to elucidate reaction mechanisms and are generally interpreted in terms of simple kinetic models. Measurements of this effect for the Penning ionization reaction between helium and dihydrogen highlight the need for a quantum description of chemical reaction rates when sub-kelvin temperatures are approached.
- Mark Brouard
-
News & Views |
 Vibronic coherence unveiled
Vibronic coherence unveiled
Pigment assemblies with high-efficiency electronic energy transfer have recently been observed to show unusual and persistent coherence, but its origin is not fully understood. Now, a combination of 2D electronic spectroscopy and theoretical modelling has allowed the excitonic coherence signal of a strongly coupled homodimer to be isolated.
- Vivek Tiwari
- , William K. Peters
- & David M. Jonas
-
Article |
 State-resolved diffraction oscillations imaged for inelastic collisions of NO radicals with He, Ne and Ar
State-resolved diffraction oscillations imaged for inelastic collisions of NO radicals with He, Ne and Ar
When molecules collide with atoms or other molecules their quantum mechanical character can lead to the diffraction of matter waves. Making use of advances in molecular beam technology, such diffraction oscillations have now been observed with unprecedented sharpness and angular resolution in the benchmark NO + He, Ne, or Ar systems.
- Alexander von Zastrow
- , Jolijn Onvlee
- & Sebastiaan Y. T. van de Meerakker
-
Article |
 Observation of the isotope effect in sub-kelvin reactions
Observation of the isotope effect in sub-kelvin reactions
In cold chemistry, quantum phenomena in reactants' translational motion lead to the temporary trapping of reactants in a collisional complex. It is now shown that this metastable complex is responsible for a dramatic quantum kinetic isotope effect as observed in Penning ionization reactions at low temperatures.
- Etay Lavert-Ofir
- , Yuval Shagam
- & Edvardas Narevicius
-
Article |
 The rate of the F + H2 reaction at very low temperatures
The rate of the F + H2 reaction at very low temperatures
The reaction F + H2 → HF + H is the only source of interstellar HF, but studying it at relevant cold temperatures has proved problematic. Now, the rates of this reaction have been measured at various temperatures down to 11 K and their remarkable agreement with state-of-the-art quantum mechanical calculations has been shown. (Background © Image Asset Management/Alamy)
- Meryem Tizniti
- , Sébastien D. Le Picard
- & Ian R. Sims
-
Article |
 Two-dimensional spectroscopy of a molecular dimer unveils the effects of vibronic coupling on exciton coherences
Two-dimensional spectroscopy of a molecular dimer unveils the effects of vibronic coupling on exciton coherences
The observation of long-lived coherent oscillations in the nonlinear spectra of photosynthetic proteins has raised significant discussion on the role of quantum effects in biology. Using a model system, the signatures of inter-exciton coherence have been isolated, which has allowed the influence of vibronic coupling to be studied in unprecedented detail.
- Alexei Halpin
- , Philip J. M. Johnson
- & R. J. Dwayne Miller
-
News & Views |
 Knowing your neighbours
Knowing your neighbours
Quantitatively studying how the rate of a chemical reaction is affected by a reactant's atomic-scale environment is extremely challenging. This has now been achieved at the single-molecule level using scanning tunnelling microscopy to monitor tautomerization in an atomically well-defined environment.
- Peter Liljeroth
-
Article |
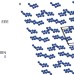 Calculations predict a stable molecular crystal of N8
Calculations predict a stable molecular crystal of N8
Polynitrogen compounds are of interest on a fundamental level and as potential high-energy-density materials. A crystalline solid that consists of two isomeric forms of N8 molecules held together by weak van der Waals interactions has now been predicted to exist, and to be stable even at low pressures.
- Barak Hirshberg
- , R. Benny Gerber
- & Anna I. Krylov
-
Article |
 Controlling intramolecular hydrogen transfer in a porphycene molecule with single atoms or molecules located nearby
Controlling intramolecular hydrogen transfer in a porphycene molecule with single atoms or molecules located nearby
The rate of an intramolecular hydrogen transfer reaction in a single porphycene molecule resting on a copper surface can be controlled by placing a copper adatom close to it. Cooperativity effects are also observed in rows of porphycene molecules, where the reaction rate of each individual molecule depends on the precise tautomer state of its neighbours.
- Takashi Kumagai
- , Felix Hanke
- & Leonhard Grill
-
Article |
 Singlet exciton fission in solution
Singlet exciton fission in solution
Singlet exciton fission produces two triplet excited states from one excited singlet through interchromophoric coupling, which is thought to require local order. Now, a triplet yield of 200% and diffusion-limited triplet formation are reported in solutions of TIPS pentacene. Kinetic studies revealed an excimer intermediate and enabled suggestions of design principles for the promotion of singlet fission.
- Brian J. Walker
- , Andrew J. Musser
- & Richard H. Friend
-
Article |
 Water vibrations have strongly mixed intra- and intermolecular character
Water vibrations have strongly mixed intra- and intermolecular character
Liquid water has the unique ability to mediate ultrafast energy transfer and relaxation in aqueous chemical reactions. Ultrafast broadband two-dimensional infrared spectroscopy that probes vibrations spanning the mid-infrared region with sub-70-femtosecond time resolution now provides evidence for highly intertwined intra- and intermolecular vibrations in water that act to efficiently dissipate vibrational energy.
- Krupa Ramasesha
- , Luigi De Marco
- & Andrei Tokmakoff
-
Article |
 Ultrafast above-threshold dynamics of the radical anion of a prototypical quinone electron-acceptor
Ultrafast above-threshold dynamics of the radical anion of a prototypical quinone electron-acceptor
Quinones are key electron acceptors in nature, however, the role of their excited states is not fully understood. Femtosecond spectroscopy and quantum calculations on the prototypical parabenzoquinone radical anion provide insight into quinones’ intrinsic electron-accepting ability, revealing how unbound electronically excited states relax to form the ground-state radical anion.
- Daniel A. Horke
- , Quansong Li
- & Jan R. R. Verlet
-
News & Views |
 In search of molecular movies
In search of molecular movies
Ultrafast chemical physics follows in the explosive wake of technological innovation, using light and radiation sources to study phenomena at timescales where the boundaries between physics and chemistry dissolve. UCP 2011, the second meeting in a series, explored the current state of the art in ultrafast time-resolved spectroscopy.
- Julia A. Weinstein
- & Neil T. Hunt

 Broadband 2D IR spectroscopy reveals dominant asymmetric H5O2+ proton hydration structures in acid solutions
Broadband 2D IR spectroscopy reveals dominant asymmetric H5O2+ proton hydration structures in acid solutions