Abstract
Rice bacterial blight (BB) is a devastating rice disease. The Xa21 gene confers a broad and persistent resistance against BB. We introduced Xa21 into Oryza sativa L ssp indica (rice 9311), through multi-generation backcrossing and generated a nearly isogenic, blight-resistant 9311/Xa21 rice. Using next-generation sequencing, we profiled the transcriptomes of both varieties before and within four days after infection of bacterium Xanthomonas oryzae pv. oryzae. The identified differentially expressed (DE) genes and signaling pathways revealed insights into the functions of Xa21. Surprisingly, before infection 1,889 genes on 135 of the 316 signaling pathways were DE between the 9311/Xa21 and 9311 plants. These Xa21-mediated basal pathways included mainly those related to the basic material and energy metabolisms and many related to phytohormones such as cytokinin, suggesting that Xa21 triggered redistribution of energy, phytohormones and resources among essential cellular activities before invasion. Counter-intuitively, after infection, the DE genes between the two plants were only one third of that before the infection; other than a few stress-related pathways, the affected pathways after infection constituted a small subset of the Xa21-mediated basal pathways. These results suggested that Xa21 primed critically important genes and signaling pathways, enhancing its resistance against bacterial infection.
Similar content being viewed by others
Introduction
Rice (Oryza sativa) is one of the most widely consumed staple crops in the world, feeding about half of the world population. Rice bacterial blight (BB), caused by infection of Xanthomonas oryzae pv. oryzae (Xoo), is a devastating disease of rice, resulting in 20% to 30% annual reduction of rice production worldwide1,2. Chemical pesticides and biocontrol agents, such as plant extracts3 and chitosan solutions4, have been used to control BB. Besides the environmental and food safety issues that these biochemical agents can cause, the field effects of these agents are far from satisfactory and their effectiveness diminishes over time of usage. To date, the most effective and economic means to control BB disease is to introduce disease resistant genes into rice plants1.
A total of 22 dominant and 9 recessive BB resistant genes have been identified2,5, some of which have been widely used in rice production. Among these genes, Xa21 is the most well studied. Through phosphorylation6,7 and cleavage of its intracellular kinase domain8, Xa21—a cell membrane receptor—perceives the presence of Xoo and relays the signal to the nucleus through multi-step signal cascades involving some key proteins such as XA21 Binding Protein 3 (XB3)9, mitogen-activated protein kinase 5 (MAPK5)10, MAPK1210 and transcription factors (TFs) including OsWRKY62 and OsWRKY7610,11,12 in the nucleus. Some Xoo resistant genes, such as xa5, are transcription factors13,14. Furthermore, many effectors from Xoo belong to the transcription activator-like (TAL) family, which facilitate injection into rice cells to activate susceptibility genes in the host to exert their functions15. The known Xoo effectors include avrxa516, avrXa717, avrXa1018 and avrXa2719, which trigger xa5-, Xa7-, Xa10- and Xa27-mediated resistance, respectively. Recently, avrXa23 was cloned and shown to be a TAL effector20. Overall, this transcription-activating characteristic of the Xoo effectors suggested their role of disrupting gene transcription regulation after Xoo infection, resulting in reprograming of the transcriptome of the host, as observed by microarray gene profiling of resistant and susceptible genotypes21,22,23.
In light of the broad disease resistance spectrum and endurance as well as distinct metabolite profiles24, it is necessary to conduct an independent study that focuses primarily on the mechanisms of Xa21-conferred BB resistance. In this study, we exploited two isogenic rice genotypes, one with and the other without the Xa21 gene, profiled their transcriptomes before and within the first 96 hours after Xoo infection and contrasted the variations of the transcriptomes of the two rice lines in reference to the transcriptome of the normal rice plants. The analyses of the large quantities of gene expression profiling data from deep sequencing and a de novo genome sequencing result revealed the genes, biological processes and signaling pathways that are responsible for the resistance to rice blight infection.
Results And Discussion
A system for study of Xa21 mediated BB resistance
To establish a platform for studying Xa21, we generated a nearly isogenic line (NIL) of rice indica variety 9311 that carries the desirable Xa21 gene. In order to minimize or eliminate the possible impact of diverse genetic background on gene expression, we used the 9311 rice, which is susceptible to Xoo, as the recurrent parent and the CBB23 rice, which carries Xa21, as the donor and successively applied more than 15 generations of backcrossing to introduce Xa21 into 9311, creating a new line of 9311/Xa21 rice. Fifteen generations of backcrossing are more than double the minimum of 6 backcrossing generations typically required to recover the phenotypes of the recurrent parental line and eliminate most of the donor chromosome fragments linked with the target Xa21 gene. As a result, the 9311/Xa21 rice, referred to as the R(esistant) plant, should have nearly the same genetic background as the 9311 rice, denoted as the S(usceptible) plant, except that the former carried Xa21 and its linked genomic fragment.
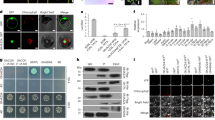
The nearly isogenic property between the R and S plants was further tested and validated using multiple means, including phenotyping, genotyping, whole genome sequencing and transcriptome profiling. The inclusion of Xa21 in the R plants was first indicated by the substantially smaller lesions on the R plants than on the S plants after Xoo inoculation (Fig. 1A) and the presence of a molecular marker co-segregated with Xa21 in the R plants (Fig. 1B). Furthermore, three lines of evidence showed the similar genetic backgrounds of the R and S plants except a small region encompassing the Xa21 gene. The R and S plants shared many similar agronomic traits (Supplemental Table S1), exhibited similar AFLP (Amplified Fragment Length Polymorphism) profiles (Fig. 1C) and had nearly identical genome sequences, based on re-sequencing of their genomes (see Methods), on all chromosomes except a ~2 Mbp region encompassing Xa21 on chromosome 11 of the R plants (Fig. 1D). In short, these results showed that the R and S rice lines were nearly isogenic.
(A) Comparison of in-field phenotypes of the R and S lines after inoculation of the P6 strain of Xoo. (B) PCR validation of Xa21 being introduced into the genome of the R (9311/Xa21) plants using the molecular marker U1/I2 that was co-segregated with Xa21. (C) The AFLP result of the R and S plants using 24 pairs of AFLP primers. (D) Genotypic variations across chromosome 11 of the R rice line. (E) The expression of Xa21 in the R plants at various time points before and within the first four days after Xoo inoculation. (F) Comparison of the amount of bacteria, measured by the number of bacterial colonies, in the R and S plants in the first 10 days post inoculation of Xoo.
The ~2 Mbp long Xa21-hosting region, which was inevitably introduced into the R plants along with Xa21 through backcrossing, had a high rate of genotypic variation (Fig. 1D) and harbored a total of 250 genes, among which 90 had non-synonymous mutations. It is noteworthy that only one of the 90 non-synonymously mutated genes, except Xa21, was expressed. We suspect the functional impact of the genes in the Xa21-hosting region except Xa21 is minimal. In support of this belief, we showed in a previous comparable study that a transgenic rice line and a rice line generated by backcrossing, both of which carry Xa21, were substantially equivalent at the transcriptome level25. Moreover, as validated by real time PCR, Xa21 in the R plants was constitutively expressed across all of the eight time points before and after Xoo infection that we profiled (Fig. 1E). In contrast, the regulatory role of expressed Xa21 on downstream genes was implicated by transcription factor OsWRKY62, which had a much lower abundance in the R plants than in the S plants before Xoo infection (Table S2). OsWRKY62 directly interacts with the Xa21 protein and acts as a negative regulator against Xoo in rice by suppressing defense-related genes11. Together, these results clearly showed that most of the genetic backgrounds of the R plant had been substituted with those of the S plants by backcrossing and the R and S plants formed an ideal system for studying the functions of Xa21.
Xa21 suppressed Xoo growth
Our first step to characterizing the functions of Xa21 was to profile the in planta growth of pathogen Xoo within both R and S plants in the first 10 days after pathogen inoculation (see Methods). No apparent difference of pathogen growth was observed between the R and S lines within the first 3 days while the pathogen was making its way into the host cells. However, starting on day 4 the growth of Xoo was substantially suppressed in the R plants with respect to the growth in the S plants (Fig. 1F). The suppression was statistically significant (t-test, p-value < 0.05) and persisted at least for 10 days after inoculation. This in planta observation revealed Xa21 as a suppressor of Xoo growth so as to curtail its virulence.
Xoo triggered broad perturbation in rice transcriptomes
In order to investigate how Xa21 responded to Xoo infection, we adopted high-throughput deep-sequencing to profile the transcriptomes of both R and S lines before Xoo infection and at seven time points within the first 4 days after pathogen inoculation (see Methods). Sequencing profiling produced more than 594 million raw reads from 19 plant samples (Table S3). About 82.24% (±1.29%) of the raw reads can be mapped to the rice reference genome and more than 92.10% (±1.26%) of the qualified reads (after removing low-mapping-quality reads) can be mapped to the exon regions of annotated genes (Table S3). This sequencing-based profiling provided a deep and broad map of transcriptome variations of the R and S rice plants in the process of Xoo infection.
Gene expression profiles of biological duplicates of the R and S plants before Xoo inoculation were produced to assess the quality of deep-sequencing based gene expression profiling. The result showed that the expression profiles were highly reproducible, with the Pearson correlation coefficients between the duplicates of the R plants and between the duplicates of the S plants being 0.8532 and 0.8052, respectively (Fig. 2A).
(A) Correlations between the duplicates of the R plants (left) and of the S plants (right). (B) The scheme for differential expression analysis. A circle represents a type of three libraries, i.e. Xoo susceptible 9311 (S), Xoo resistant 9311/Xa21 (R) and mock control 9311 (M). An edge represents a DE comparison, i.e., R vs S, S vs M and R vs M. Different types of comparison are shown in different colors. (C) The number of unique DE genes in each DE comparison. (D) The numbers of overlapped DE genes among the comparisons. (E) The number of DE genes at each time point. (F) The numbers of overlapped DE genes among the comparisons at each time point.
We analyzed gene expression levels to characterize transcriptome variations in the period of the first four days of infection (see Methods) between the R plants inoculated with Xoo and the 9311 rice (the S line) grown under the normal condition—the Mock—and between the S plants infected with Xoo and the Mock (Fig. 2B). Particularly, 5,802 and 6,534 differentially expressed (DE) genes existed for at least one time point in the first 4 days after pathogen inoculation in the R and S plants with respect to the Mock, respectively (Fig. 2C), indicating that Xoo infection triggered, in genome scales, transcriptomic perturbations in these two plants. All of these DE genes are listed in a file available at http://www.cse.wustl.edu/~zhang/software/xa21DEgenes.zip.
Remarkably, the two transcriptomic perturbations were incompatible, even if the difference between the two rice lines was only due to the ~2 Mbp Xa21-hosting region in the R plants. A total of 3,496 genes exhibited significant expression variations between the R and S plants for at least one of the seven time points after Xoo infection (Fig. 2C,D and Table 1) and most of these DE genes were aggregated into 187 of the 316 (59.18%) annotated pathways in rice (RiceCyc pathway database, version 3.3, Dharmawardhana, Ren et al. 2013) (Table S4); these 187 DE-gene containing pathways were referred to as the Xa21-induced pathways. These results of distinct transcriptomic responses suggested that Xoo activated different signaling pathways in the two rice plants, resulting in their distinct Xoo resistances (Fig. 1A,F).
Since the R rice exhibits stable resistance to Xoo throughout all stages of rice development, it is viable to hypothesize that genes continuously up-regulated or down-regulated across the R and S plants were most likely to be related to the resistance of Xa21. Along the seven time points profiled during the infection of Xoo, 12 genes were consistently differentially expressed between the R and S plants, where 4 genes were highly induced and the remaining 8 suppressed in the R plants (Fig. 3A). Four of these 12 genes (LOC_Os02g18140, LOC_Os11g36180, LOC_Os11g35710, LOC_Os11g36160) were further examined by real-time PCR for their expression variations across the R and S plants before Xoo inoculation (Fig. 3B). Note that LOC_Os02g18140 encodes a NBS type disease resistance protein and the other three genes are on chromosome 11 where Xa21 resides. LOC_Os11g36160 and LOC_Os11g36180 were particularly interesting since they reside in the neighborhood of Xa21, were highly induced in the R plants and were annotated to be receptor kinases.
Xa21 mediated complex basal signaling pathways to prepare for Xoo infection
Besides the broad, distinct perturbations to the transcriptomes of the R and S rice caused by Xoo infection, the most surprising result of the transcriptome profiling was a great deal of transcriptomic difference between the R and S rice before bacterial infection. Precisely, 1,889 genes, involving in 135 signaling pathways, exhibited significant expression variations between the R and S plants before Xoo inoculation (Fig. 2E). This is remarkable as it clearly indicated that Xa21 was already functional before the infection. These 135 pathways, referred to as the Xa21-mediated basal pathways, were related to various types of material and energy matabolisms (Table S4). Among them, 28, 26 and 4 Xa21-mediated basal pathways were related to basic material and energy metabolisms, cellular components and synthesis metabolisms, respectively. In contrast, based on Gene Ontology, many Xa21-induced processes after infection were directly related to stress responses and infection (Table 2), including all kinds of phytohormones and phytoalexins, whereas the first stress related biological process was only ranked the 24th among the Xa21-mediated basal processes (Table S5).
It is worthwhile to mention that at any of the seven time points after Xoo infection that we profiled, the degrees of differential expression between the R and S plants were substantially less than before the infection (Fig. 2E, the first columns of all time points). For example, at 8 hours post inoculation (hpi), 116 genes were differentially expressed, which were only 6.25% of the 1,889 DE genes before the infection; these 116 DE genes were involved in 10 signaling pathways (Table 3), in sharp contrast to the 135 Xa21-mediated basal pathways. On the other hand, approximately 66.6% of the Xa21-mediated basal pathways overlapped with the Xa21-induced pathways (Table S4). This high degree of overlap suggested that, even before Xoo infection, Xa21 had prepared the R rice well so that it was able to respond to the presence of Xoo as effectively and quickly as possible, as illustrated by its strong resistance to Xoo (Fig. 1).
Together, these results suggested that before Xoo invasion, Xa21 in the R rice effectively reallocated energy and resources among many house-keeping cellular activities to prepare the plant and after infection, Xa21 adjusted or activated stress related pathways according to a given Xoo strain and the time of pathogenesis.
Cytokinins contribute to Xa21 resistance to Xoo
The most notable enriched pathways between the R and S plants at various time points after Xoo inoculation were the ones related to phytohormones (Table 4 and S4). The pathways related to jasmonic acid (JA) and ethylene (ET), two classic phytohormones, had many DE genes in two of the eight time points profilied. Meantime, the pathways of some other members of hormone families, such as brassinosteroids (BR), gibberellic acid (GA) and cytokinins (CK), were also enriched (Table 4). Some phytohormones regulated themselves through different pathways at different time points. For example, at 4 hpi, the GA biosynthesis pathway had a substantial number of DE genes, however, at 24 hpi, the pathway for gibberellin inactivation was enriched. Furthermore, different DE genes were involved in the same pathways of the same hormones and showed distinct expression patterns in the R and S plants at different time points in the first four days of infection (Table S4).
Among the phytohormones detected by the transcriptome profiling, CK was the most pronounced. The DE genes residing on the CK pathways appeared at five of the seven time points after Xoo infection, four of which were the highest ranked among all pathways detected (Table S4), alluding to CK’s involvement in Xa21-mediated resistance to Xoo. These CK pathways included that for cytokinins-O-glucoside biosynthesis (PWY-2902), cytokinins-9-N-glucoside biosynthesis (PWY-2901) and cytokinins-7-N-glucoside biosynthesis (PWY-2881), which were responsible for CK conjugation and thus for inactivating cytokinins26. CK is known to be involved in plant disease resistance27 and in biotic28 and abiotic29,30,31 stress responses. The DE genes on the cytokinins-glucoside biosynthesis pathways exhibited a relatively coherent pattern of expression (Fig. 4A). Before Xoo infection, most of the DE genes, i.e., 15 of the 16 (93.75%), had lower abundances in the R plants than in the S plants. In contrast, at the 12, 24 and 72 hpi, 26 of the 27 (96.30%) DE genes on the cytokinins-glucoside biosynthesis pathways had higher abundances in the R plants than in the S plants. At 96 hpi, all (100%) of the 18 DE genes on the cytokinins-glucoside biosynthesis pathway had lower abundance in the R plants. For example, the low expression abundances of 4 genes in the CK-related pathways were validated by real time PCR (Fig. 4B). Such a high degree of coherent expression pattern over the course of Xoo infection suggested that the role of endogenous CK in Xa21-mediated resistance is complex and is difficult to be imitated by a constant amount of exogenous CK or by constantly inducing/repressing CK-related genes, which was often adopted in the previous studies on the functions of endogenous phytohormones.
(A) The relative fold-change of cytokinins genes that were significantly differentially expressed for at least one time point. Red and blue corresponds to down-regulated or up-regulated fold-change, respectively, for each gene in the first 96 hours post infection. (B) Real-time quantitative PCR validated the expression levels of four genes in the cytokinins-related pathways, which were identified as significantly DE at a given time point.
Pterocarpan phytoalexins might function to repress Xoo in the R plants
Maackiain, together with medicarpin, is the main pterocarpan phytoalexin in chickpea and occurs exclusively in a (-)-(6aR,11aR)-configuration32. Two adjacent genes on rice chromosome 1, LOC_Os01g01650 and LOC_Os01g01660, encode the enzymes for production of medicarpin and maackiain, respectively. These two genes had similar expression patterns in the R and S plants before Xoo infection. At the initial Xoo infection, their expressions were dramatically repressed at 4 hpi in both R and S plants. The suppression of these two genes persisted in the S plants throughout the 96 hours of infection. These results suggested that Xoo suppressed the synthesis of pterocarpan phytoalexins to promote its growth and disease progression in the S plants. However, the expressions of these two genes in the R plants elevated dramatically after 12-hpi and peaked at 24-hpi, which were further validated by real time PCR (Fig. 5A,B). The expression levels were suppressed again after 24-hpi and dropped to nearly 0 at 96-hpi. The increased expression of the two genes in the R plants might promote the synthesis of pterocarpan phytoalexin to control BB disease within the early stage of 24-hpi.
(A,B) Real-time quantitative PCR validated two genes in the pathway of pterocarpan phytoalexin. LOC_Os01g01650 and LOC_Os01g01660 were significantly induced in the R plants at 12 and 24 hpi. (C) Real-time quantitative PCR validated three genes in enterobactin biosynthesis pathway. LOC_Os07g46870, LOC_Os11g30560 and LOC_Os04g33240 were significantly repressed in the R plants without the infection.
Notably, these two genes were not highly expressed in a rice line that is susceptible to rice blast fungal infection after the fungal infection (data not shown), suggesting that pterocarpan phytoalexins responded differently to fungal and bacterial infections. In addition, mock infection with water did not induce these two genes in the S plants either, confirming that they responded to Xoo infection.
Involvement of iron in Xa21-mediated disease responses
Iron is a key nutrient for bacterial growth and the usable form of iron for microorganisms is usually siderophore33. As Xoo colonizes within rice xylem, where siderophores are derived, the host typically exploits the essentiality and toxicity of transition metals to defend against bacterial invaders34. Before Xoo infection, the pathway of the enterobactin biosynthesis, a catecholate siderophore, was significantly enriched with 12 DE genes (FDR = 0.08703, Table S4), among which, 11 genes had lower abundance in the R plants. The experimental validation of three of the DE genes, using real-time quantitative PCR was consistent with the result from sequence profiling (Fig. 5C). The reduced expressions of these genes on the enterobactin biosynthesis pathway might help lower the amount of siderophores, which in turn restricted Xoo colonization in the R plants after Xoo infection. Because of iron uptake deficiency, a mutation in the Xoo feoB gene causes severe virulence deficiency and constitutive production of a siderophore35. A defect in siderophores formation in Dickeya dadantii, a plant soft-rotting enterobacterium, leads to symptoms localized to inoculated leaves, indicating that the siderophores are required for bacteria to spread to the other parts of the plant36,37. The R plants had limited blight lesions on inoculated leaves (Fig. 1), indicating that Xoo was curtailed in the R plants. Since most known mechanisms of disease resistance through iron-withholding are realized by regulation of iron-binding proteins33,38,39, it is viable to hypothesize that siderophores were also restricted in the R plants through iron-withholding. The way to restrict active iron in the form of siderophore seemed to be rare; this may be a double-edged sword because the lack of active iron is harmful to Xoo as well as to the rice plant at the same time.
Concluding remarks
The 9311 rice (the S line) and its nearly isogenic line with the Xa21 gene (the R line) that we used form a robust tool for studying the function of Xa21. In combination of deep sequencing-based transcriptome profiling and bioinformatics analysis, our results provided remarkable genome-wide profiles of gene expression and related signaling pathways and biological processes that significantly differed in the two rice genotypes. The significant difference between the transcriptomes of the two rice genotypes before Xoo infection revealed insights into the functions of Xa21 in priming various metabolic pathways so as to gain high and durable resistance to Xoo. After Xoo infection, Xa21 mediated DE genes and pathways were sharply reduced but more related to resistance to Xoo. Among them, the plant hormones, especially cytokinins, were broadly involved, suggesting complex mechanisms of hormones in Xa21-mediated resistance to Xoo.
Material and Methods
Rice varieties and growth condition
Rice 9311 variety, a popular indica rice restorer line40 and the backcrossing line 9311/Xa21 were used to study Xa21-mediated BB resistance. The 9311 rice was susceptible (the S genotype) and the 9311/Xa21 rice resistant (the R genotype) to Xoo infection. These two rice lines have identical genetic background except the latter carrying Xa21. Seeds of the two plants with no defects nor disease were surface-sterilized in 70% ethanol for 2 min, rinsed twice in deionized water, imbibed overnight at 30 °C and placed on moist filter paper overnight at 30 °C. Germinated seeds were then sown in UC potting mix. Pots with plants were kept in nutrient solution (0.25 × Hoagland’s solution) in a greenhouse in Hainan province (lat 20° 1’ N, long 110°19’ E). The greenhouse conditions in which the rice seedlings were cultivated were: ~30 °C/20 °C (day/night), ~80% RH, natural sunlight with a ~13 h/11 h light/dark photoperiod at ~40 W/m2 intensity.
Pathogen inoculation and evaluation
Xanthomonas oryzae pv. oryzae (Xoo) Philippine race 6 (P6) was used for pathogen inoculation. Xoo was subcultured at 28 °C on PSA (Potato-Sugar-Agar) medium (potato, 300 g/L; Ca(NO3)2•4H2O, 0.5 g/L; Na2HPO4•12H2O, 2.0 g/L; sugar, 15 g/L; agar, 15 g/L) for 3 days. Inoculums were prepared by suspending the bacterial cells in sterile water and adjusting the concentration to about 109 cells per milliliter. The last rice leaves were infected with P6 by using scissors dipped in bacterial suspensions to clip leaves 1–2 cm down from the tip of the leaf blade at the heading stage of 9311 and 9311/Xa2141. Mock-infected plants were treated in a similar fashion except that water substituted for P6. Fifteen days post inoculation, lesion length was measured from the cut surface at the tip to the distal-most position on the leaf that exhibited a grey, chlorotic or water-soaked lesion.
DNA and RNA isolation and genetic analysis
Fifteen randomly selected leaves were harvested at each time point (Table 1) and pooled to represent each treatment. After harvest, leaves were immediately frozen and stored in liquid nitrogen until use. About 100 mg samples were grinded to powder with liquid nitrogen for DNA and total RNA isolation using the Total DNA/RNA Isolation Kit (R6731, Omega, USA) following the manufacture’s protocols. The total RNA quality was measured using Agilent RNA 6000 Pico Kit (5067–1513, Agilent, USA). Only the total RNAs with RIN (RNA Integrity Number) no less than 7 were used for the subsequent experiments.
To investigate whether Xa21 was introduced into the genome of the R plant, we designed a molecular marker (Sequence listed in Table S6), named as U1/I2, which was co-segregated with Xa21. This marker was able to amplify a fragment of 575 bp in the R plant and a fragment of 445 bp in the S plant.
The method for analyzing the expression of Xa21 was described in our early report42; Amplified fragment length polymorphism (AFLP) was used for genetic background analysis for the R and S genotypes, using the methods of Vos et al.43. AFLP primers were given in our earlier report44.
Assay for quantification of bacterial growth
The bacterium population was determined using three P6-infected leaf samples collected at 0d (day), 1d, 2d, 3d, 4d, 6d, 8d and 10d after inoculation. The bacterial growth was analyzed according to Song et al.45 with three biological replicates.
DNA and RNA library preparation, emulsion PCR and sequencing
About 1 μg genomic DNA from the R and S plants were used for DNA library preparation, using the SOLiD™ 5500 Fragment Library Core Kit (Part no. 4464412) according to its user guide. A total of 20 μg total RNA was used for two rounds of mRNA purification using Dynabeads (610.06, Invitrogen, USA). About 100 ng mRNA was fragmented using NEB Next Magnesium RNA Fragmentation Module (E6150, NEB), purified with an RNA clean up kit (R6247, Omega, USA), end repaired with T4 Polynucleotide Kinase (T4 PNK) (M0201, NEB) and cleaned up again with a kit (R6247, Omega). The end-repaired RNAs were used to prepare the strand specific transcriptome, using the Small RNA Sample Preparation kit (E6160, NEB) according to the manufacturer protocol with some minor modification, including the SR Primer F3 being replaced with barcode primers. The resulting DNA and RNA libraries were used for emulsion PCR to produce the beads for sequencing on the SOLiD 5500 machine, using 75 nt mode and 75 nt + 35 nt mode for the sequencing of DNA and RNA libraries, respectively. Biological duplicates of RNA libraries of the R and S plants before Xoo infections were profiled for quality assessment.
Analysis of genotypic variation
The DNA sequencing reads in the color-space format were mapped to the Oryza sativa Nipponbare reference genome and gene annotation from MSU Rice Genome Annotation Project (Release 746) using LifeScope (Life Technologies) software version 2.5.1.
Genotypic variations in the R rice line were analyzed using the genomic regions where the genome sequences of the S line and the 9311 reference genome were the same in order to rule out possible impact of natural mutations in the S plants. The analysis had a resolution of 5 Kbp, in which sequence variations within a 5 Kbp window were tallied. The calling of a genotypic variation at a genomic locus of the R line was subjected to a set of stringent criteria: the locus had distinct nucleotides in the R and S lines; and the sequencing must have at least 5X coverage of the same read at the locus to rule out or minimize possible sequencing error.
Gene expression and differential expression analysis
For RNA analysis, the reads mapped to each annotated gene were tallied using the whole transcriptome analysis workflow of LifeScope. Differential expression analysis was performed using the edgeR47 package. The reads count per gene was normalized using the TMM method in edgeR. An exact test, analogous to the Fisher’s exact test, was performed based on the normalized counts with the common dispersion factor being set to 0.1. Genes were considered to be differentially expressed if they had more than 10 read counts across all samples and their False Discovery Rates (FDR)48 of the exact test were no greater than 10%.
Functional and pathway enrichment analysis
The Gene Ontology49 Slim annotations of rice genes were downloaded from the MSU Rice Genome Annotation Project46. The association information of rice genes and pathways was retrieved from the RiceCyc pathway database (version 3.3, Dharmawardhana, Ren et al. 2013). Given a list of genes, the Fisher’s exact test was performed to measure the statistical significance of enrichment of the genes on a GO term or pathway; the resulting p value was subject to Benjamini-Hochberg multiple testing correction50 to derive the final FDR.
Gene expression validation
Real-time PCR was performed following the standard THUNDERBIRD SYBR qPCR Mix kit (QPS-201, Toyobo) protocol. The 20-μl reactions (0.3-μl first-stand cDNA product, 10-μl THUNDERBIRD qPCR Mix, 0.3 μM forward and reverse primers, 1 × ROX reference dye) were incubated in 0.2 ml tubes of Applied Biosystems StepOne™ Real-Time PCR machine as follows: 95 °C for 20 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The procedure ended by a melt-curve ramping from 60 to 95 °C, raised by 0.5 °C each step. The primers used for real time PCR are listed in Table S6. Data were normalized using the reference gene LOC_Os06g11170.1 (coding for a putative nucleic acid binding protein) with the same primers published by Narsai et al.51. Before performing expression analysis, the primers’ efficiency was estimated through a five-point standard curve. Only the target genes with amplification efficiencies between 90%–105% were chosen for expression validation. All PCR reactions were done in three biological replicas and three technical replicas. Ct values were exported and analyzed using Microsoft Excel 2010.
Additional Information
How to cite this article: Peng, H. et al. Rice Xa21 primed genes and pathways that are critical for combating bacterial blight infection. Sci. Rep. 5, 12165; doi: 10.1038/srep12165 (2015).
References
Mew, T. Current status and future prospects of research on bacterial blight of rice. Annual Review of Phytopathology 25, 359–382 (1987).
NiÑo, D. O., Ronald, P. C. & Bogdanove, A. J. Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol Plant Pathol 7, 303–324 (2006).
Nisha, S. et al. Effect of plant compounds on induced activities of defense-related enzymes and pathogenesis related protein in bacterial blight disease susceptible rice plant. Physiological and Molecular Plant Pathology 80, 1–9 (2012).
Li, B. et al. Antibacterial activity of two chitosan solutions and their effect on rice bacterial leaf blight and leaf streak. Pest Management Science, 10.1002/ps.3399 (2012).
Wang, C., Wen, G., Lin, X., Liu, X. & Zhang, D. Identification and fine mapping of the new bacterial blight resistance gene, Xa31 (t), in rice. European journal of plant pathology 123, 235–240 (2009).
Chen, X. et al. An ATPase promotes autophosphorylation of the pattern recognition receptor XA21 and inhibits XA21-mediated immunity. Proc Natl Acad Sci USA 107, 8029–8034 (2010).
Park, C. J. et al. Rice XB15, a protein phosphatase 2C, negatively regulates cell death and XA21-mediated innate immunity. PLoS biology 6, e231 (2008).
Park, C. J. & Ronald, P. C. Cleavage and nuclear localization of the rice XA21 immune receptor. Nature communications 3, 920 (2012).
Wang, Y. S. et al. Rice XA21 binding protein 3 is a ubiquitin ligase required for full Xa21-mediated disease resistance. The Plant cell 18, 3635–3646 (2006).
Seo, Y. S. et al. Towards establishment of a rice stress response interactome. PLoS genetics 7, e1002020 (2011).
Peng, Y. et al. OsWRKY62 is a negative regulator of basal and Xa21-mediated defense against Xanthomonas oryzae pv. oryzae in rice. Molecular plant 1, 446–458 (2008).
Peng, Y., Bartley, L. E., Canlas, P. & Ronald, P. C. OsWRKY IIa Transcription Factors Modulate Rice Innate Immunity. Rice (N Y) 3, 36–42 (2010).
Jiang, G. H. et al. Testifying the rice bacterial blight resistance gene xa5 by genetic complementation and further analyzing xa5 (Xa5) in comparison with its homolog TFIIAgamma1. Mol Genet Genomics 275, 354–366 (2006).
Iyer, A. S. & McCouch, S. R. The rice bacterial blight resistance gene xa5 encodes a novel form of disease resistance. Mol Plant Microbe Interact 17, 1348–1354 (2004).
Yang, B., Sugio, A. & White, F. F. Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proceedings of the National Academy of Sciences 103, 10503–10508 (2006).
Bai, J., Choi, S. H., Ponciano, G., Leung, H. & Leach, J. E. Xanthomonas oryzae pv. oryzae avirulence genes contribute differently and specifically to pathogen aggressiveness. Mol Plant Microbe Interact 13, 1322–1329 (2000).
Vera Cruz, C. M. et al. Predicting durability of a disease resistance gene based on an assessment of the fitness loss and epidemiological consequences of avirulence gene mutation. Proc Natl Acad Sci USA 97, 13500–13505 (2000).
Zhu, W., Yang, B., Chittoor, J. M., Johnson, L. B. & White, F. F. AvrXa10 contains an acidic transcriptional activation domain in the functionally conserved C terminus. Mol Plant Microbe Interact 11, 824–832 (1998).
Gu, K. et al. R gene expression induced by a type-III effector triggers disease resistance in rice. Nature 435, 1122–1125 (2005).
Wang, C. L. et al. The broad bacterial blight resistance of rice line CBB23 is triggered by a novel transcription activator‐like (TAL) effector of Xanthomonas oryzae pv. oryzae. Mol Plant Pathol 15, 333–341 (2014).
Grewal, R. K., Gupta, S. & Das, S. Xanthomonas oryzae pv oryzae triggers immediate transcriptomic modulations in rice. BMC Genomics 13, 49 (2012).
Li, Q. et al. Expression profiling of rice genes in early defense responses to blast and bacterial blight pathogens using cDNA microarray. Physiological and Molecular Plant Pathology 68, 51–60 (2006).
Kottapalli, K. R. et al. Transcriptional profiling of indica rice cultivar IET8585 (Ajaya) infected with bacterial leaf blight pathogen Xanthomonas oryzae pv oryzae. Plant Physiology and Biochemistry 45, 834–850 (2007).
Wu, J. et al. Metabolite profiles of rice cultivars containing bacterial blight-resistant genes are distinctive from susceptible rice. Acta biochimica et biophysica Sinica 44, 650–659 (2012).
Gao, L. et al. Do transgenesis and marker-assisted backcross breeding produce substantially equivalent plants?—A comparative study of transgenic and backcross rice carrying bacterial blight resistant gene Xa21. BMC Genomics 14, 738 (2013).
Hou, B., Lim, E. K., Higgins, G. S. & Bowles, D. J. N-glucosylation of cytokinins by glycosyltransferases of Arabidopsis thaliana. J Biol Chem 279, 47822–47832 (2004).
O’Brien, J. A. & Benkova, E. Cytokinin cross-talking during biotic and abiotic stress responses. Frontiers in plant science 4, 451 (2013).
Novák, J. et al. High cytokinin levels induce a hypersensitive-like response in tobacco. Ann Bot (Lond) 112, 41–55 (2013).
Hu, L., Wang, Z. & Huang, B. Effects of Cytokinin and Potassium on Stomatal and Photosynthetic Recovery of Kentucky Bluegrass from Drought Stress. Crop Sci 53, 221–231 (2013).
Emam, Y., Karimzadeh Sureshjani, H., Moori, S. & Maghsoudi, K. Yield Response of Bread and Durum Wheat to Different Levels of Auxin and Cytokinin Application under Terminal Drought Stress Conditions. Isfahan University of Technology-Journal of Crop Production and Processing 3, 93–104 (2013).
Reguera, M. et al. Stress-Induced Cytokinin Synthesis Increases Drought Tolerance through the Coordinated Regulation of Carbon and Nitrogen Assimilation in Rice. Plant Physiol 163, 1609–1622 (2013).
Guo, L., Dixon, R. A. & Paiva, N. L. Conversion of vestitone to medicarpin in alfalfa (Medicago sativa L.) is catalyzed by two independent enzymes. Identification, purification and characterization of vestitone reductase and 7, 2’-dihydroxy-4’-methoxyisoflavanol dehydratase. Journal of Biological Chemistry 269, 22372–22378 (1994).
Fones, H. & Preston, G. M. The impact of transition metals on bacterial plant disease. FEMS Microbiology Reviews 37, 495–519 (2012).
Hood, M. I. & Skaar, E. P. Nutritional immunity: transition metals at the pathogen–host interface. Nature Reviews Microbiology 10, 525–537 (2012).
Pandey, A. & Sonti, R. V. Role of the FeoB protein and siderophore in promoting virulence of Xanthomonas oryzae pv. oryzae on rice. Journal of bacteriology 192, 3187–3203 (2010).
Dellagi, A. et al. Siderophore‐mediated upregulation of Arabidopsis ferritin expression in response to Erwinia chrysanthemi infection. The Plant Journal 43, 262–272 (2005).
Franza, T., Mahé, B. & Expert, D. Erwinia chrysanthemi requires a second iron transport route dependent of the siderophore achromobactin for extracellular growth and plant infection. Molecular microbiology 55, 261–275 (2005).
Expert, D., Franza, T. & Dellagi, A. in Molecular Aspects of Iron Metabolism in Pathogenic and Symbiotic Plant-Microbe Associations 7–39 (Springer, 2012).
Franza, T. & Expert, D. Role of iron homeostasis in the virulence of phytopathogenic bacteria: an ‘à la carte’menu. Mol Plant Pathol 14, 429–438 (2013).
Yu, J. et al. A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science (New York, N.Y 296, 79–92 (2002).
Century, K. S. et al. Developmental control of Xa21-me diated disease resistance in rice. Plant J 20, 231–236 (1999).
Gao, L. et al. Generation of marker‐free, bacterial blight‐resistant transgenic sterile line and hybrid rice with Xa21. Plant Breeding 130, 438–443 (2011).
Vos, P. et al. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23, 4407–4414 (1995).
Peng, H., Zhang, J. & Xie, Q. A quinclorac sensitive lethality rice mutant: its discovery, genetics and potential application. Plant Breeding 127, 490–493 (2008).
Song, W. Y. et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science (New York, N.Y 270, 1804–1806 (1995).
Ouyang, S. et al. The TIGR rice genome annotation resource: improvements and new features. Nucleic acids research 35, D883–D887 (2007).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Hirochika, H., Sugimoto, K., Otsuki, Y., Tsugawa, H. & Kanda, M. Retrotransposons of rice involved in mutations induced by tissue culture. Proc. Natl. Acad. Sci. 93, 7783–7788 (1996).
Ashburner, M. et al. Gene Ontology: tool for the unification of biology. Nature genetics 25, 25–29 (2000).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300 (1995).
Narsai, R., Ivanova, A., Ng, S. & Whelan, J. Defining reference genes in Oryza sativa using organ, development, biotic and abiotic transcriptome datasets. BMC Plant Biol 10, 56 (2010).
Acknowledgements
The work was supported in part by Natural Science Foundation of China (31300999), Youth and Technology Morning Program in Wuhan (2014072704011257), the Talent Development Program of Wuhan, the municipal government of Wuhan, Hubei, China (2014070504020241) and an internal research grant of Jianghan University, Wuhan, China, as well as by United States National Institutes of Health (R01GM100364) and United States National Science Foundation (DBI-0743797). We thank Sharlee Climer for a critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
H.P. and W. Zhang perceived the research and designed the experiments; Z.X., J.Z., L.G., L.C., L.L., T.L. and Z.F. grew rice plants and recorded resistance phenotypes; J.Z. constructed sequencing libraries and preformed sequencing profiling; Z.C. and Z.F carried out computational analysis and created figures and tables; L.G. performed PCR validation; W.Zhai provided some of the rice seeds used in the study; H.P., Z.C and W. Zhang analyzed the data and results and wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Peng, H., Chen, Z., Fang, Z. et al. Rice Xa21 primed genes and pathways that are critical for combating bacterial blight infection. Sci Rep 5, 12165 (2015). https://doi.org/10.1038/srep12165
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12165
This article is cited by
-
Dual Role of Potassium Silicate and Salicylic Acid: Plant Growth Promotor and Plant Immunity Booster Against Bakanae Disease of Rice
Silicon (2024)
-
Pyramiding of blast and bacterial blight resistance genes in premium quality rice variety, BRRI dhan63 through marker-assisted breeding approach
Euphytica (2024)
-
Rice immune sensor XA21 differentially enhances plant growth and survival under distinct levels of drought
Scientific Reports (2020)
-
Application of high-throughput amplicon sequencing-based SSR genotyping in genetic background screening
BMC Genomics (2019)
-
1k-RiCA (1K-Rice Custom Amplicon) a novel genotyping amplicon-based SNP assay for genetics and breeding applications in rice
Rice (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.