Abstract
Insect pests have developed resistance to chemical insecticides, insecticidal toxins as bioinsecticides or genetic protection built into crops. Consequently, novel, orally active insecticidal toxins would be valuable biological alternatives for pest control. Here, we identified a novel insecticidal toxin, parasporal crystal toxin (PC), from Bacillus bombysepticus (Bb). PC shows oral pathogenic activity and lethality towards silkworms and Cry1Ac-resistant Helicoverpa armigera strains. In vitro assays, PC after activated by trypsin binds to BmAPN4 and BtR-175 by interacting with CR7 and CR12 fragments. Additionally, trypsin-activated PC demonstrates cytotoxicity against Sf9 cells expressing BmAPN4, revealing that BmAPN4 serves as a functional receptor that participates in Bb and PC pathogenicity. In vivo assay, knocking out BtR-175 increased the resistance of silkworms to PC. These data suggest that PC is the first protein with insecticidal activity identified in Bb that is capable of causing silkworm death via receptor interactions, representing an important advance in our understanding of the toxicity of Bb and the contributions of interactions between microbial pathogens and insects to disease pathology. Furthermore, the potency of PC as an insecticidal protein makes it a good candidate for inclusion in integrated agricultural pest management systems.
Similar content being viewed by others
Introduction
During the past decade, interest in isolating bacterial toxins with the hope of identifying novel properties that are particularly suited for the control of agronomically important pests has increased. These toxins have the potential to reduce the use of insecticide sprays, to promote pest suppression and to involve the incorporation of transgenes that encode these proteins in plants1. Many emerging and re-emerging bacterial pathogens have been screened to identify such toxins2,3. The pore-forming toxins (PFTs) are the largest family of bacterial toxins. According to the type of structure used to interact with the lipid bilayer, PFTs are classified into α-PFT and β-PFT. Colicins4 or Bacillus thuringiensis (Bt) Cry toxins5 are representative members of the α-PFT family. Clostridium perfringens enterotoxin6 and Vcc from Vibrio cholerae7 are members of the β-PFT family. Additionally, many bacterial toxins that do not fully qualify as PFT are known as AB toxins; in these toxins, the B subunit is responsible for binding to the target cell and for translocating the A subunit into the cytoplasm8.
The modes of action of bacterial toxins include damaging cell membranes2, inhibiting protein synthesis9, activating second messenger pathways10 and activating the host immune response11. Generally, PFTs bind to a specific receptor, diffuse towards target cell membranes, form multimers and undergo a conformational change leading to the formation of a pore in the target cell membrane that damages the extracellular matrix8. This damage not only results in the loss of membrane function and direct cell lysis but also facilitates bacterial dissemination throughout tissues.
Most bacterial toxins, such as biomphalysin, possess haemolytic function but not oral activity2. Therefore, these bacterial toxins are unlikely to be useful insecticides. However, OAIP-1 isolated from venom of the Australian tarantula is highly biologically stable, which likely contributes to its oral activity; therefore, this toxin might have potential as a “standalone” bioinsecticide12. Notably, Cry toxins isolated from the bacterium Bt have been shown to be orally lethal to some insects. Therefore, Cry toxins have had a widespread and revolutionary impact on insecticide use and crop production, resulting in reduced chemical insecticide use and improved yields13,14. Nevertheless, the remarkable ability of insects to adapt to insecticides suggests that the evolution of resistance threatens the continued success of Bt Cry toxin15,16. Thus, the development of novel insecticidal toxins that could function as valuable biological alternatives in pest control measures to improve resistance management is of significant interest.
Many species of bacteria that belong to the genus Bacillus produce toxins and establish a systemic infection in a variety of hosts17. Among them, the pathogen Bacillus bombysepticus (Bb), which was first identified from sick silkworm larvae cadavers by Hartman18, leads to silkworm bacterial black chest septicaemia. However, the pathogenesis of Bb is not well understood19. Through 16S rRNA gene sequence analysis, Bb has been shown to be closely related to Bacillus cereus and to Bt20. Similar to Bt, Bb also produces spores and parasporal crystal toxin (PC)20. Bacterial toxins are often important virulence factors for pathogens21. However, no information is available regarding whether PC has pathogenic properties.
Bb toxins are thought to poison the silkworm via a mechanism similar to that of the Bt Cry toxin, as shown by a genome-wide survey of the host during Bb infection20. Bb-mediated poisoning occurs in the midgut. After oral infection, the PC produced by Bb can be digested by gut proteases to generate a toxin fragment. The digested PC can pass through the peritrophic membrane (PM) to bind to the receptors of midgut epithelia cells and damage them. Bb can spread to the haemolymph from the damaged midgut. However, no information is available regarding the role of receptors for PC during the pathogenesis of Bb.
The interaction between the silkworm, a model lepidopteran organism and Bb, a Bacillus organism, represents an important host-pathogen dynamic that could provide insights into the relationship between lepidopteran insects and microorganisms. Here, we isolated an orally active toxin, PC from Bb, which plays an important role in insecticidal action. Exposure to PC induces silkworm and Helicoverpa armigera death. Our results indicate that interactions with the midgut receptors APN and BtR-175 are key contributors to PC pathogenicity and may be targets for the engineering of an antibacterial silkworm that would reduce economic losses in sericulture production. We propose a schematic model for the process by which Bb damages the silkworm midgut and haemolymph, causing death. Specifically, PC binds to midgut receptors, which disrupts homeostasis and material exchange throughout the insect body. Our work, along with studies on Bt Cry and other bacteria toxins, suggests that toxin production is a common strategy of entomopathogenic bacteria that interferes with insect guts through interactions with receptors. However, the oral potency of Bb PC and its insecticidal effects on Bt-resistant H. armigera makes it a good candidate for deployment with Bt Cry toxins against lepidopterans or other pests in integrated pest management systems that provide levels of crop protection that are more durable.
Results
PC is pathogenic to silkworms
Silkworm larvae were orally infected with Bb to induce larval death, leading to black chest septicaemia: a peutz appears on the thoracoabdominal region or the 1st to 3rd segment of the abdomen (Fig. 1Ab) and then expands to encompass the entire body (Fig. 1Ac). To determine whether or not the pathogenic mechanism of Bb was associated with PC, PC was purified from bacterial cultures using methods similar to those used to isolate Bt parasporal crystals (Fig. 1Ba). Because the protoxin can be hydrolyzed by midgut proteases to form an activated molecule that causes lethal toxicity to insect larvae22, the purified PC was digested with trypsin at 37 °C for 6 h and then separated by SDS-PAGE (Fig. 1Bb). All of the bands were excised from the gel and subjected to matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry analysis. The filled triangle band represented PC after trypsin digestion and MALDI-TOF analysis identified only one protein component in this band, namely, a recombinant PC protein that was expressed and purified (Fig. 1C). To confirm the pathogenicity of PC protein, mortality was determined by oral infection of the 5th-instar silkworms with 50 μg PC protein per larva. Beginning at 15 h after treatment, the PC-treated larvae began to die (Fig. S1B), but the wild-type (WT) larvae survived (Fig. S1A). We found that PC induced approximately 29% mortality compared to 0% in WT within 4 days (Fig. 1D), with an LD50 of 140.512 μg per larva for oral administration at the 5th-instar stage. Furthermore, the mortality rate of 3rd-instar larvae was 40.3% after 4 days at a dose of 50 μg PC protein per larva. The approximate LD50 value calculated for 3rd-instar silkworms was 77.35 μg per larva. These results indicated that PC is pathogenic to silkworms.
Bb parasporal crystal toxin (PC) identified from Bb bacterium is pathogenic and causes virulence.
(A) Bb oral infection causes silkworm cuticle peutz and death. The phenotypes of wild-type (a), Bb treatments at 30 h (b) and Bb treatments at 40 h (c). (B) SDS-PAGE analysis of purified PC from Bb (a) and trypsin-activated PC protein (b). PC was extracted from Bb cultures according to the purification method used for Bt toxin and was digested by trypsin at 37 °C for 6 h. The filled triangle band represents PC after trypsin digestion. (C) Purification of PC from a prokaryotic expression system. (D) Mortality of newly exuviated 5th-instar silkworm larvae following oral infection with PC. WT, unchallenged. (E) Mortality observed feeding PC into Bt-susceptible Helicoverpa armigera (LF) strains. (F) The mortality rate of three Cry1Ac-resistant Helicoverpa armigera strains characterized to show different resistance levels after oral infection with PC. Error bars depict ±SEM. Statistically significant differences from control samples are indicated; **P < 0.01.
Identification of PC pathogenicity towards Bt-resistant H. armigera
Bt crops represent one of the most widespread and controversial applications of pest management23. Many studies have addressed pest resistance to Bt crops and resistant pests that can overcome the lethality of Bt protein24. To investigate the pathogenic capacity of PC towards Bt-resistant insects for future pest control applications, we performed bioassays on larvae from one Bt-susceptible (LF) and three Cry1Ac-resistant H. armigera strains (LF5, LF10 and L240). Cry1Ac-resistant H. armigera strains were established by Prof. Kongming Wu et al. by screening field-collected populations with different doses of Cry1Ac toxins (LF5: 5 μg Cry1Ac/mL artificial diet, LF10: 10 μg Cry1Ac/mL artificial diet and LF240: 240 μg Cry1Ac/mL artificial diet) under laboratory conditions25. PC was fed to 1st-instar larvae at a dose of 50 μg toxin/mL artificial diet. PC produced a mortality rate above 60% in the LF species within 5 days (Fig. 1E). Interestingly, three Cry1Ac-resistant strains showed pathogenicities similar to the LF species (Fig. 1F). The mortality rate analysis after oral infection with resistant larvae showed that ~70% of the LF5, LF10 and LF240 infected with PC succumbed within 5 days. The insecticide activity of PC in Cry1Ac-resistant strains indicated its potential use as a bioinsecticide or transgenic crop gene to target Bt-resistant insects.
Molecular characterization of PC
The first 29 amino acids of PC corresponded to a putative signal peptide as predicted by the SignalP program. An analysis of putative post-translational modifications using the NetNglyc and YinOYang servers suggested the absence of N-linked glycosylation and a putativeO-(beta)-GlcNAc glycosylation event at G74. To further explore the relationship between PC and Bt Cry toxins (Cry1A–Cry28A), we used ClustalX and Mega 4 to visualize phylogenetic relationships. Sequence alignment showed that some sites of PC are conserved with Bt Cry toxins (Fig. S2), showing an identity of 8.6% with Bt Cry6Aa. However, as to Bt Cry6Aa, Bt Cry15Aa and Bt Cry23A, phylogenetic tree showed that PC resides on its own deep branch (Fig. S3), indicating that it should be considered a distinct toxin. The deduced amino acid sequence of PC displayed limited sequence similarity (≤11.4%) with a range of bacterial pore-forming toxins as determined by a BlastP search.
Binding assays to detect PC interaction with BmAPN4
Bt Cry toxins kill many insects by interacting with receptors such as APN or cadherin, leading to pore formation and ultimately cell lysis14,22,26. Bb toxins induce larvae poisoning responses in the midgut that are similar to those induced by Bt20. Surprisingly, PC accumulation in host intestinal epithelial cells and pores could be observed in the optical membrane of the larvae midgut after Bb bacterium infection20. A gene encoding an APN protein (BmAPNN4) was obtained by biopanning against Bb bacterium from a silkworm midgut T7 phage display cDNA library27. Additionally, some midgut-expressed APNs can be modulated by Bb infection20,28. Therefore, we hypothesized that silkworm APNs interact with PC to cause pathogenicity.
To test our hypothesis, far-western blot analysis was performed. The gene encoding BmAPN4 was cloned by RT-PCR and recombinant BmAPN4 protein with a glutathione S-transferase (GST) tag at the N-terminus was expressed and purified. The trypsin-activated PC proteins were separated and transferred to PVDF membranes. The membranes were separately incubated with BmAPN4 protein before the addition of anti-GST antibody (Fig. 2Aa). Membranes incubated with GST or bovine serum albumin (BSA) protein were used as negative controls (Fig. 2Ab,c) and another negative control, a membrane without PC, was incubated with BSA to exclude the possibility of a direct interaction between the anti-GST antibody and the PVDF membrane (Fig. 2Ad). Positive bands were observed only when trypsin-activated PC/BmAPN4 complexes were present (Fig. 2Aa). Far-western blot analysis with trypsin-activated PC and BmAPN4 showed that trypsin-activated PC was capable of interacting with silkworm BmAPN4. We used co-immunoprecipitation (Co-IP) assays to determine whether BmAPN4 interacted with trypsin-activated PC. As shown in Fig. 2B, the Co-IP assay indicated that PC bound to BmAPN4. Furthermore, ELISA binding saturation assays showed that PC bound to BmAPN4 (Fig. 2C), which confirmed the interaction between PC and BmAPN4.
Binding assays for trypsin-activated PC with BmAPN4.
(A) Far-western blot analysis of trypsin-activated PC and BmAPN4. The trypsin-activated PC was separated by 12% (w/v) SDS–PAGE and transferred to PVDF membranes for far-western blot analysis, in which the recombinant BmAPN4 (a), GST (b) and BSA (c) proteins were incubated with the membranes before adding the anti-GST antibody. The membrane was used for direct immunoblotting using anti-GST antibody (d); positive bands were observed only when trypsin-activated PC/BmAPN4 complexes were present. (B) A Co-IP assay for trypsin-activated PC and BmAPN4. Lane 1 shows trypsin-activated PC incubated with BmAPN4 that was immunoprecipitated with antibody. Lane 2 shows trypsin-activated PC incubated with antibody as control; the positive band indicates trypsin-activated PC. (C) ELISA binding saturation assays of trypsin-activated PC and BmAPN4. Error bars depict ±SEM. Statistically significant differences from the control samples are indicated; **P < 0.01.
Cytotoxic activity of PC in Sf9 cells expressing BmAPN4
Having determined that PC binds to silkworm BmAPN4, we investigated the biological significance of silkworm BmAPN4 in silkworms infected with Bb. To ascertain whether silkworm APNs are involved in PC pathogenicity, the cytotoxic activity of PC in Sf9 cells transfected with BmAPN4 gene was tested. BmAPN4 was expressed in the membrane fractions of Sf9 cells transfected with psl1180[hr3-BmAct4-BmAPN4-SV40] vectors (Fig. 3A, lane 2). Additionally, immunofluorescence was used to evaluate the location of BmAPN4 in cells (Fig. 3B). Separately, the incubation of live, viable cells expressing BmAPN4 with trypsin-activated PC at 50 μg/mL induced distinct morphological changes. A significant proportion of the cells exhibited dramatic cytological changes, including swelling and lysis (Fig. 3Cd). Such effects were not observed in the control Sf9 cells (Fig. 3Ca), Sf9 cells transfected with silkworm BmAPN4 that were not exposed to toxin (Fig. 3Cb), or control Sf9 cells incubated with trypsin-activated PC (Fig. 3Cc), suggesting that the expressed BmAPN4 directly interacted with trypsin-activated PC to induce morphological aberrations and cell lysis. The number of cells with such alterations after PC administration were counted in 50 different optical fields, indicating that the proportion of viable cells was reduced to ~15.8% (Fig. 3D). Susceptibility to trypsin-activated PC was further confirmed using a cytotoxicity assay based on the extracellular detection of LDH activity. Trypsin-activated PC caused a 35.3% increase in the release of total intracellular LDH in BmAPN4-transfected Sf9 cells into the culture medium (Fig. 3E). Untransfected Sf9 cells were not affected by PC. Together with the results of the binding assay (Fig. 2), this finding indicates that BmAPN4 primarily serves as a functional receptor involved in PC pathogenicity.
Cytotoxic activity of trypsin-activated PC-induced BmAPN4-expressing Sf9 cells death.
(A) Western blot analysis of a Sf9 cell line expressing BmAPN4. Membrane proteins were extracted from Sf9 cells after 48 h post-transfection with BmAPN4 expression vector. Lane 1 shows native Sf9 cells as a control. Lane 2 shows BmAPN4 was expressed by Sf9 cells; tubulin was used as an internal control. (B) Immunocytochemical analysis of BmAPN4 in Sf9 cells. Signals for Sf9 cells and BmAPN4 were detected under blue and red fluorescence. (C) The cytotoxic activity of trypsin-activated PC proteins on Sf9 cells transfected with the gene encoding the BmAPN4 receptor from Bombyx mori. Photomicrographs of healthy uninfected cells (a), BmAPN4-expressing Sf9 cells (b), healthy uninfected Sf9 cells treated with trypsin-activated PC (c) and BmAPN4-expressing Sf9 cells treated with trypsin-activated PC (d) are shown. Cells that expressed BmAPN4 that were incubated with trypsin-activated PC showed swelling and lysis. (D) The number of cells with such alterations after trypsin-activated PC-treated healthy and BmAPN4-expressing Sf9 cells is shown. (E) trypsin-activated PC-induced cell death was measured based on the extracellular release of LDH activity after incubation for 6 h. Error bars depict ±SEM. Statistically significant differences from the control samples are indicated; **P < 0.01.
Interaction of PC with Bt-R175
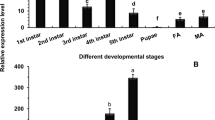
The interaction with APN concentrates Bt Cry toxin in the midgut brush border membrane vesicles (BBMV), where the toxin is able to bind with high affinity to cadherin29. Binding to cadherin is complex and involves three cadherin fragments that correspond to the extracellular regions CR7, CR11 and CR1230. Therefore, to determine whether cadherin is involved in Bb infection in silkworms, qRT-PCR was performed to analyse the expression pattern of cadherin (BtR-175) in the midgut after Bb infection. Similar to Bt infection, the induced peaks of the gene encoding BtR-175 appeared at 3 and 24 h, corresponding to the induced expression profiles of the gene encoding BmAPN4 after Bb infection (Fig. 4A) and suggesting that BtR-175 expression is associated with responses in Bb-infected silkworms. However, whether BtR-175 is involved in PC pathogenicity is unknown.
Interaction analysis of trypsin-activated PC with BtR-175 cadherin fragments.
(A) The induced expression profiles of BtR-175 after Bb and Bt challenge by qRT-PCR. (B) Far-western blot analysis of trypsin-activated PC and BtR-175 cadherin fragments. The trypsin-activated PC was separated using 12% (w/v) SDS–PAGE and transferred to PVDF membranes for far-western blot analysis, in which recombinant His-CR7, His-CR11 and His-CR12 proteins (lane 1) were incubated with membranes. The membrane was either incubated with BSA (lane 2) or directly immunoblotted (lane 3) as a negative control. Positive bands were observed only when trypsin-activated PC/CR7 and trypsin-activated PC/CR12 complexes were present. (C) His-tag pull-down assays for trypsin-activated PC and BtR-175 cadherin fragments. Lane 1 shows BtR-175 cadherin fragments incubated with PC that were digested with trypsin. The bands indicate the pull-down proteins of PC that bound to PC (CR7 or CR12) complexes. Lane 2 shows an agarose gel incubated with trypsin-activated PC as a control. (D) ELISA binding saturation assays of trypsin-activated PC and BtR-175 cadherin fragments. Error bars depict ±SEM. Statistically significant differences from the control samples are indicated; **P < 0.01; *P < 0.05.
To examine the role of BtR-175 in the process of toxin pathogenicity, we cloned three cadherin fragments that correspond to CR7 (residues M811–A927), CR11 (residues L1257–T1398) and CR12 (residues N1368–G1484) of silkworm BtR-175. The recombinant CR7, CR11 and CR12 proteins with His tags at N-termini were purified for use in binding assays. Far-western blot analysis of trypsin-activated PC and His-CR7, His-CR11, or His-CR12 resulted in positive bands that reacted with anti-His antibody only when trypsin-activated PC/His-CR7 or trypsin-activated PC/His-CR12 complexes were present, indicating that PC bound to CR7 and CR12 cadherin fragments, but not to CR11 (Fig. 4B). The purified His-CR7, His-CR11 and His-CR12 proteins were each incubated with trypsin-activated PC. After incubation, His-CR7-bound protein(s), His-CR11-bound protein(s) and His-CR12-bound protein(s) were purified by affinity chromatography. As a negative control, Ni-NTA beads incubated with trypsin-activated PC were used to exclude the possibility of PC directly binding to the NTA resin. As shown in Fig. 4C, PC was not detected by His-CR11 pull down but could be observed in the CR7 and CR12 pull-down assays. Furthermore, ELISA binding assays confirmed that trypsin-activated PC could bind to CR7 or CR12, but not to CR11 (Fig. 4D). These results showed that PC interacted with silkworm BtR-175 by binding to the cadherin fragments CR7 and CR12.
Effect of BtR-175 on PC pathogenicity in B. mori
To further characterize BtR-175 biological function during the toxin pathogenicity, we used CRISPR/Cas9 genome editing to knock out the BtR-175 gene for bioassay. A guiding sequence of BtR-175 gRNA was synthesized and inserted into pUC57-gRNA to form pUC57-BtR-175. The plasmids pUC57-hA4-Cas9 and pUC57-BtR-175 were introduced into silkworm embryos by co-microinjection to induce BtR-175 mutagenesis. Finally, homozygous mutants were obtained from G3 moths using T7EI genotyping and TA clone sequencing to reveal the exact mutant sequences (Fig. 5A). A homozygous mutant (ΔBtR-175) was inserted into 2 bp with disrupted BtR-175 leading to translation termination and loss-of-function (Fig. 5B). In total, 50 μg PC per larva was used to calculate the mortality of 3rd-instar ΔBtR-175 strain for oral administration toxicity assays. Remarkably, ΔBtR-175 silkworms were much more potent against PC. The mortality of WT silkworms orally infected with toxins at 4 days (40.2 ± 2.4%) was significantly elevated (**P < 0.01) compared to that of the ΔBtR-175 strain (5.9 ± 3.25%) (Fig. 5C). Moreover, no significant mortality was observed in WT silkworms (2.8 ± 2.4%) and in the ΔBtR-175 strain. This result indicated that knocking out BtR-175 gene increased the resistance of silkworms to PC. Together with the results of binding assay (Fig. 4), these data show that BtR-175 is involved in PC pathogenicity.
Effect of BtR-175 knock-out in silkworm on PC pathogenicity.
(A) Cas9/gRNA-induced mutations at the BtR-175 locus in silkworm. Schematic representation of BtR-175 and gRNA target sequences (sequences at the bottom). The wild type sequence (Ref) is shown. gRNA sites are highlighted in red and PAM sequences are shown in green. Sequences of mutations at the targeted BtR-175 locus in G3 silkworm (ΔBtR-175) and 2 bp sequence was inserted into the target site by TA-clone sequencing. (B) Amino acid sequences of mutations. *: terminator. (C) The mortality of 3rd-instar ΔBtR-175 larvae after oral infection with PC. Error bars depict ±SEM. Statistically significant differences from the control samples are indicated; **P < 0.01.
Discussion
Many Gram-positive and Gram-negative bacterial pathogens produce toxins that contribute to their virulence31. Bb, a typical natural pathogen of silkworms, is a model of insect host-pathogen interactions based on triggering a strong host response20. Here, we described the cloning and functional characterization of a novel toxin that contributes to the virulence of Bb, which we named PC. Bioinformatic analysis showed that PC does not exhibit any homology to other bacterial toxins and appears to be distinct from previously identified insecticidal PFTs such as Cry toxins (Fig. S3). Nevertheless, amino acid sequence analysis showed the presence of conserved residues in PC and Bt Cry toxins. Bioassays based on oral activity confirmed that this toxin isolated from Bb is an insecticidal toxin and that this toxin was lethal to B. mori (Figs 1 and 5).
How might PC induce silkworm death? The insect midgut represents the first line of resistance and triggers the immune response, which deploys multiple innate defence mechanisms to fight microbial intruders. However, many bacterial pathogens are equipped with clever infectious stratagems that circumvent these defence systems. The interplay between pathogens and their hosts involves the specific recognition of surface molecules that modulate cell recognition, membrane insertion, or internalization, which determine the fate of bacterial infections and disease outcomes. Toxins are important virulence factors for bacterial pathogenicity. Interactions with single receptors are a general strategy, although several toxins such as diphtheria, Cry, or aerolysin toxins target more than one surface molecule in their binding activities and action modes8. Here, we demonstrated that binding to BmAPN4 provokes the pathogenicity of PC (Figs 2 and 3), although the kinetic parameters of the interaction between PC with APN have not been fully analysed. APNs are known to play key roles in the mechanisms responsible for the toxicity of various classes of Bt Cry toxins32. Both the monomer and oligomer Bt Cry toxins interact with the APN receptor. The binding of oligomer to APN induces its insertion into cell membrane, leading to pore formation and cell lysis, which is a key determinant of toxicity26,33. APNs are highly abundant proteins that are anchored to membranes by a glycosyl phosphatidylinositol anchor34. In Trichoplusia ni, different APNs are associated with resistance to Bt Cry1Ac toxin35. In Spodoptera exigua, the absence of APN causes resistance to Bt Cry1Ca toxin36. In B. mori, BmAPN4 is specifically expressed in the midgu28,37, which explains why the injection of PC in the body cavity at high doses had no impact on silkworm survival (data not shown), while oral infection induced silkworm death. In this study, we report the discovery that BmAPN4, which is specifically expressed in the midgut, functions as a receptor and is involved in Bb recognition and toxin-related pathogenicity. Additionally, the binding of toxin to cell surface is the key step in causing toxicity. Thus, the loss or alteration of this receptor could play a pivotal role in the natural development of toxin resistance in silkworms.
The pathogenicity of Bt Cry toxin is the result of sequential interactions with at least two receptor molecules, cadherin and APN26. Interactions with APN concentrate Bt Cry toxin at the midgut brush border membrane, where the toxin can bind to cadherin receptor33. The binding of Bt Cry toxin to cadherin triggers toxin oligomerization to form a pre-pore structure, which interacts with APN and results in pore formation that leads to insect death14,26,38. Pores form in the silkworm intestinal epithelium after Bb oral infection, after which the infiltration becomes prominent and material exchange throughout silkworm body is disrupted similar to Bt Cry toxin poisoning20. Nevertheless, whether the formation of pores related to APN occurs following the binding of PC to BmAPN4 is unknown. However, in this study we found that PC interacts with two regions of silkworm BtR-175 receptor that are located in the CR7 and CR12 cadherin repeats (Fig. 4) and does not bind to CR11. Cadherin repeats have been recognized as important regions of cadherin that mediate Bt Cry toxicity. In Manduca sexta, Bt Cry1A toxins bind to cadherin Bt-R1 receptor via interactions with CR7, CR11 and CR12 cadherin repeats, which are essential for toxicity30. Resistance to Bt Cry toxin in some insects is linked with mutations that disrupt a toxin-binding cadherin protein39. Using a CRISPR/Cas9 system to knock out the binding region of BtR-175 gene for bioassay, we identified that BtR-175 mutant exhibited reduced PC pathogenicity in vivo (Fig. 5). The enhanced activity of PC in the presence of silkworm BtR-175 correlates with the mechanism of action of the toxin. These demonstrated that PC bound to silkworm BtR-175, which is located on the luminal membrane of midgut epithelial cells40, to induce host death.
Bacterial toxins secreted by pathogens diffuse towards the target cell by binding to specific receptors that are ultimately inserted in cell membranes and that form a pore8. Aerolysin, a pore-forming toxin, initially interacts with a high-abundance and low-affinity molecule and subsequently interacts with a low-abundance and high-affinity binding site41. A “ping pong” binding mechanism has been reported in which Bt Cry toxin is involved in the first binding event with the high-abundance and low-affinity APN receptor and then interacts with the high-affinity cadherin receptor33. APN is abundant in the M. sexta midgut, in contrast to cadherin, which is present at a much lower concentration42. Therefore, we speculate that PC binds to the high-abundance BmAPN4 receptor before interacting with Bt-R175. However, little is known regarding the structure of PC or its loop regions and receptor binding regions. In Bt Cry toxin, differences in sequence, length and loop conformation are thought to be key determinants of its insect midgut BBMV binding ability and toxin selectivity. Thus, mapping the specificity of binding regions in PC will help to improve the applications of PC to insect pest control with Bt toxin.
The results shown here indicate that PC, a novel toxin, is pathogenic to silkworms. BmAPN4 primarily acts as a functional receptor that participates in PC pathogenicity and BtR-175 can interact with the toxin to affect pathogenicity. Based on our data, a schematic overview of Bb infection in the silkworm20 and previous studies of the mechanism of action of Cry toxins in lepidopterans at the molecular level22,26, we propose a schematic model for the process by which Bb damages the silkworm midgut and the haemolymph (Fig. 6). Larvae ingest Bb, which produce PC. PC can be digested by gut proteases. The digested PC passes through the PM to bind to the high-abundance APN receptor, after which the toxin is in close proximity to the membrane of the midgut. This interaction is followed by high-affinity binding to the BtR-175 receptor. Interactions with BtR-175 trigger oligomerization of the toxin, which then binds to the receptors and leads to pore formation (Pathway 1). This sequence of events is similar to the sequential model for Cry toxins activity proposed by Bravo et al.22,26,43. Additionally, unknown toxins might play a part in larval death (Pathway 2). In these models, all of the receptors would work together to disrupt the balance of infiltration and material exchange throughout the insect, resulting in larval death caused by Bb infection.
A schematic representation of the model for B. bombysepticus or PC causing damage to the silkworm midgut and translocating into the hemolymph.
The larvae ingest B. bombysepticus, which produces parasporal crystal toxin (PC). PC can be digested by gut proteases. The digested PC pass through the peritrophic membrane (PM) to bind the high-abundance APN receptor, allowing the toxin to be located in close proximity to the membrane. This interaction is followed by high-affinity binding to the BtR-175 receptor by the cadherin fragments CR7 and CR12. Interactions with BtR-175 trigger the oligomerization of toxin that binds to the receptors and leads to pore formation (Pathway 1). However, unknown toxin(s) might also induce larvae death (Pathway 2). All of these toxins and receptors could work together, leading to aberrant gut infiltration and material exchange throughout the insect body, resulting in the death of larvae that is caused by B. bombysepticus infection.
Remarkably, PC exhibits oral toxicity against H. armigera (Fig. 1) similar to B. mori. H. armigera, which is one of the most severe insect pests in Asia, attacks wheat, corn, vegetables and cotton. The oral potency of PC and its insecticidal activity make it a good candidate for deployment against H. armigera and possibly other pest insect species. Because PC is a genetically encoded protein, engineering transgenes that encode this protein in plants should be possible. The most significant advancement in the use of bio-resources as bio-control agents in recent years is the use of transgenes encoding Bt Cry proteins, which have been incorporated into many crops to manage key insect pests successfully and to protect crops such as cotton and corn14. More than 420 million hectares are planted with Bt crops worldwide. Furthermore, in China, several Bt-producing crop varieties are waiting to be approved by the agricultural ministry for commercial use. However, to date, Bt Cry toxin resistance has been reported in some key pest species15,44,45 and the increasing use of Bt crops will accelerate the emergence of resistant insects and threaten long-term benefits. PC shows a pathogenic capacity towards three Cry1Ac-resistant H. armigera strains through oral infection (Fig. 1E), indicating that this toxin may provide farmers with a new weapon against the development of Bt Cry toxin resistance in insect pests. Thus, PC might be a good candidate for transgene engineering to replace or use in combination with Bt Cry toxins for integrated pest management systems with a strong biological control component.
The discovery of PC as a candidate for insecticides provides opportunities to develop PC into an effective insect pest control agent. Nevertheless, the most serious threat to the continued efficacy of PC is low insecticidal activity. PC has an LC50 value of 77 μg/per larvae. In contrast, Cry1A toxin has an LC50 value of 1 ng per square cm or a few hundred nanograms for B. mori instar larvae (less than 200 ng/cm2) indicating that PC is at least 400-fold less toxic8,46. The shortcoming of low toxicity of PC may be a limitation for its utility in pest control. Thus, one important future direction of research on PC is the provision of approaches to enhance its insecticidal efficacy. These possible strategies include the following. (i) The localization of the toxic portion of PC may be useful for enhancing PC activity. The goal of this strategy is to circumvent PC activation, resulting in improved toxicity against pests; thus, basic knowledge regarding the toxic portion of PC is helpful for the design of a toxin to control pests. (ii) Because the processing PC into its active form by insect proteases is crucial to its toxicity, enhanced processing by insect proteases may increase its efficacy against pests, such as the modification of PC to facilitate proteolytic activation. (iii) Binding to insect receptors is an important step in inducing toxicity; thus, increased binding to target insect receptors could improve insecticidal efficacy. Several approaches have been used to modify the binding affinity of Bt Cry toxins with the ultimate goal of producing designer toxins that target pests. According to these studies14,47, the alteration of PC binding affinity can be broken into the following categories: incorporation of binding fragments or domain swapping between PC and Bt Cry toxins and site-directed mutagenesis of PC. This approach has provided important insight into PC residues that interact with insect midgut receptors. (iv) Phage display screens for mutated PC offer a potentially powerful method for engineering PC and for the subsequent selection of mutant PC with enhanced activity. Overall, these strategies for toxin modification will help to increase PC insecticidal efficacy against pests. Incorporation of these approaches into current management strategies will maximize the use of PC as a tool for crop protection and pest management.
Insect pests that lead to crop losses are a significant limiting factor in food production. In modern agriculture, insect pest control has undergone a revolution. In the United States, China, India and Australia, the engineering of crop plants for enhanced resistance to insect pests has been a success for Bt crops that has resulted in reduced reliance on chemical insecticides. Second-generation Bt crops, termed pyramids, produce two or more Bt Cry toxins that are selected for resistance to one toxin that does not cause cross-resistance to the other toxins, which can delay or prevent the emergence of resistant insects48. In the past decade, farmers in Australia, India and the United States have switched from planting first-generation transgenic crops to using pyramids against a particular pest15. Therefore, the goal of using PC transgenes in crops with Bt Cry toxins could be achieved by designing the toxin to enhance the efficacy of insect- and Bt-resistant pests, thus providing levels of crop protection that are more durable.
Methods
Insect and bacterial strains
Silkworm larvae (DaZao P50 strain) were reared on fresh mulberry leaves at a stable temperature of 25 °C. The H. armigera strains (LF, LF5, LF10 and LF240) were kindly provided by Prof. Kongming Wu (State Key Laboratory for Biology of Plant Diseases and Insect Pests, Chinese Academy of Agricultural Science, China) and reared using methods reported by Liang et al.49. The LF strain was started with approximately 100 3rd to 6th instars collected from Bt cotton in Langfang, Hebei Province, China in 1998. The LF550, LF10 and LF240 strains were generated with resistance by selecting insects from LF strains grown with different concentrations of MVPII (Dow AgroSciences), a commercial formulation of Cry1Ac protoxin incorporated in the diet, over more than a decade. Bb was kindly provided by Prof. Yangwen Wang (Silkworm Disease Laboratory of Shandong Agriculture University, China).
Purification of PC from Bb
The method for purifying PC was based on the procedure used for Bt Cry toxin purification51,52,53. Bb was grown on 100 μg/mL ampicillin at 37 °C. Cells were centrifuged at 8000 rpm for 20 min and the pellet was washed three times with ice-cold 1 M NaCl and then washed five times with distilled water. The PC was solubilized in 50 mM sodium carbonate buffer (pH 9.5) containing 0.2% 2-mercaptoethanol and 25 mM EDTA on ice for12 h54. After the mixture was incubated, it was centrifuged at 8000 rpm for 30 min at 4 °C. The supernatant was subjected to a second round of centrifugation to remove insoluble components. The remaining supernatant was adjusted to pH 4.5 using 4 M CH3COONa-CH3COOH buffer and acetic acid and was maintained on ice for 4 h. After the sample was centrifuged at 12,000 rpm for 30 min at 4 °C to remove the supernatant, the pellet was washed two times with distilled water. The PC protein was solubilized in 50 mM sodium carbonate buffer (pH 10) at 4 °C.
The solubilized PC protein was digested with trypsin at trypsin: toxin ratio of 1:25 (w/w) at 37 °C for 6 h. The proteins were purified by adding them to1/10 (v/v) 4 M CH3COONa-CH3COOH buffer and incubating them at 4 °C for 15 min before centrifugation at 12,000 rpm for 10 min. The pellet was then solubilized in distilled water. These steps were repeated five times. Finally, the pellet was solubilized in 50 mM sodium carbonate buffer. SDS–PAGE gels were run and then visualized using Coomassie Brilliant Blue (CBB) staining.
Bioinformatics of PC
Signal peptide prediction was performed using the SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/). The glycosylation sites were predicted by NetNGlyc 1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc/) and YinO Yang 1.2 (http://www.cbs.dtu.dk/services/YinOYang/). All sequences of Bt Cry toxin were downloaded from the databases (http://www.lifesci.sussex.ac.uk/home/Neil_Crickmore/Bt/index.html). A multipe sequence alignment and the neighbor-jioning (N-J) tree were constructed according to Lin et al.28.
Expression vector construction and recombinant protein expression
The truncated ORF of PC and the cadherin repeats CR7, CR11 and CR12 were cloned into pET-28a expression vectors with a 6×His tag on the N-terminal ends of the target sequences. BmAPN4 was cloned into a pGEX-4T-1 expression vector with a GST tag on the N-terminus. These recombinant plasmids were transformed into the Escherichia coli BL21 (DE3) strain to express PC, GST-tagged BmAPN4 (GST-BmAPN4), His-tagged CR7 (His-CR7), His-tagged CR11 (His-CR11) and His-tagged CR12 (His-CR12) proteins. The PC, His-CR7, His-CR11 and His-CR12 proteins were purified by affinity chromatography using Ni-NTA (GE Healthcare). GST-BmAPN4 was purified by GSTrapFF (GE Healthcare). All of these purified proteins were used for subsequent experiments.
Construction of knock-out mutations
The plasmids of pUC57-hA4-Cas9 and pUC57-gRNA were kindly provided by Dr. Sanyuan Ma (State Key Laboratory of Silkworm Genome Biology, Southwest University, China). Guiding sequence of BtR-175 gRNA were synthesized and inserted to BbsI treated pUC57-gRNA, forming pUC-BtR-175. The microinjection of B. mori embryos, crossing strategy, T7 endonuclease I assay and sequencing of the mutations were performed according to the method of Ma et al.55.
Determination of PC insecticidal activity on silkworm and H. armigera larvae
The mortality of silkworms after oral inoculation with PC was investigated. A fresh mulberry leaf was cut into square pieces 2 cm long and coated with a dose of 50 μg purified PC recombinant proteins per silkworm larva. Newly exuviated 3rd-instar and 5th-instar larvae that ate leaves with toxin were selected for continuous rearing. After 24 h, the larvae were transferred to a fresh diet (without toxin). Mortality was recorded after 4 days. We tested 30 larvae per test for each treatment and this bioassay was replicated three times. The LC50 was calculated using GraphPad Prism software version 5.0 for Windows (GraphPad Software, San Diego, CA). Statistical analysis was performed with GraphPad using Student’s t-tests. No significant differences between the sessions were observed. P > 0.05 and statistically significant differences are indicated; * P < 0.05, **P < 0.01.
For H. armigera bioassay, the method was based on the procedure by Cao et al.25. In brief, the 1th-instar susceptibility (LF) and three Cry1Ac-resistant larvae (LF5, LF10 and LF240) were placed individually on PC-contaminated artificial diet (doses of PC: 50 mg toxin/mL artificial diet) in plastic wells in 24-well plates. And 144 larvae were test per PC concentration. After five days, we recorded the mortality rate. The statistical analysis were performed according to above described.
Cell transfection, immunofluorescence and plasma membrane protein immunoblotting
Sf9 cells (Invitrogen) were cultivated in Grace medium (Gibco) supplemented with 10% foetal bovine serum, 0.35 g/L NaHCO3 and antibiotic-antimycotic (HyClone) at 27 °C. The ORF encoding the BmAPN4 gene was cloned into the pSL1180[hr3-BmAct4-DsRed-SV40] plasmid between the BamHI and NotI restriction sites, yielding the plasmid pSL1180[hr3-BmAct4-BmAPN4-SV40]. Cell transfection with the pSL1180[hr3-BmAct4-BmAPN4-SV40] plasmid was performed using Cellfectin II Reagent (Invitrogen) as described in a published protocol (Protocol Pub. No. MAN0007821). Immunofluorescence and immunoblotting of plasma membrane proteins were performed 48 h later. Cell membrane protein extraction from Sf9 cells was performed as described by Wang et al.56. Total membrane proteins were resolved by10% SDS-PAGE. After t the proteins were transferred to PVDF membranes, they were immunoblotted with anti-BmAPN4antibody (Invitrogen) or anti-Tubulin mAb (Sigma–Aldrich) following standard procedures. Immunofluorescence analysis was performed as described previously57 using FITC-conjugated goat anti-mouse IgG antibody.
Far-western blot, co-immunoprecipitation, pull-down assay and ELISA binding assay
Far-western blots were performed according to the method of Wu et al.58. A summary of the Co-IP protocol is described below. The proteins (100 μg) were incubated with IP antibody for 1 h at room temperature. The complexes were bound to Protein A magnetic beads (Thermo Fisher Scientific) for 1 h at room temperature and then BS3 (Thermo Fisher Scientific) was used for cross-linking. After washing, the beads were incubated with PC (100 μg) overnight at 4 °C. The complexes were eluted after washing. The methods used for His pull-down experiments were the same as those described for the Pierce Protein Interaction Pull-Down Kit (Thermo Fisher Scientific). A summary of the ELISA protocol used was described previously by Pacheco33.
Cytotoxicity assay of PC in Sf9 cells
Sf9 cells were harvested and transferred to a 48-well tissue culture dish (Costar) at 0.1 × 106 cells per well. After the cells settled, they were transfected with pSL1180[hr3-BmAct4-BmAPN4-SV40]. After 48 h, the cell layer was overlaid with PC at 50 μg/mL in PBS after three gentle washes with PBS. The cells were incubated with the proteins for 6 h, after which they were observed under an Olympus TH4-200 microscope. The total number of cells per field was counted. Final values represent an average of 50 fields that were randomly selected for each treatment; each treatment was replicated three times. This assay measures the amount of stable cytosolic lactate dehydrogenase (LDH) released from the lysed cells using a coupled enzymatic assay that results in the conversion of a tetrazolium salt into a red formazan product according to the methods of Wang et al.59 and Tsuda et al.60.
RNA extraction and quantitative real-time PCR after Bb challenge
RNA extraction and qRT-PCR analysis of the midgut expression pattern of the gene encoding BtR-175 after Bb infection were performed as described by Lin et al.28.
Additional Information
How to cite this article: Lin, P. et al. PC, a Novel Oral Insecticidal Toxin from Bacillus bombysepticus Involved in Host Lethality via APN and BtR-175. Sci. Rep. 5, 11101; doi: 10.1038/srep11101 (2015).
References
Cao, C.-W. et al. Response of the gypsy moth, Lymantria dispar to transgenic poplar, Populus simonii x P. nigra, expressing fusion protein gene of the spider insecticidal peptide and Bt-toxin C-peptide. J. Insect. Sci. 10, 200 (2010).
Manasherob, R., Ben-Dov, E., Zaritsky, A. & Barak, Z. Protozoan-enhanced toxicity of Bacillus thuringiensis var. israelensis delta-endotoxin against Aedes aegypti larvae. J. Invertebr. Pathol. 63, 244–248, doi:10.1006/jipa.1994.1047 (1994).
Opota, O. et al. Monalysin, a novel ß-pore-forming toxin from the Drosophila pathogen Pseudomonas entomophila, contributes to host intestinal damage and lethality. PLoS Pathog. 7, e1002259 (2011).
Hilsenbeck, J. L. et al. Crystal structure of the cytotoxic bacterial protein colicin B at 2.5 Å resolution. Mol. Microbiol. 51, 711–720 (2004).
Galitsky, N. et al. Structure of the insecticidal bacterial-endotoxin Cry3Bb1 of Bacillus thuringiensis. Acta. Crystallogr. D. Biol. Crystallogr. 57, 1101–1109 (2001).
Kitadokoro, K. et al. Crystal structure of Clostridium perfringens enterotoxin displays features of β-pore-forming toxins. J. Biol. Chem. 286, 19549–19555 (2011).
De, S. & Olson, R. Crystal structure of the Vibrio cholerae cytolysin heptamer reveals common features among disparate pore-forming toxins. Proc. Natl. Acad. Sci. U.S.A. 108, 7385–7390 (2011).
Endo, H. et al. Affinity maturation of Cry1Aa toxin to the Bombyx mori cadherin-like receptor by directed evolution based on phage display and biopanning selections of domain II loop 2 mutant toxins. Microbiology Open 3, 568–577, doi:10.1002/mbo3.188 (2014).
Iglewski, B. H. & Kabat, D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc. Natl. Acad. Sci. U.S.A. 72, 2284–2288 (1975).
Orth, J. H. et al. Pasteurella multocida toxin activation of heterotrimeric G proteins by deamidation. Proc. Natl. Acad. Sci. U.S.A. 106, 7179–7184 (2009).
Kennedy, A. D. et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J. Infect. Dis. 202, 1050–1058 (2010).
Hardy, M. C., Daly, N. L., Mobli, M., Morales, R. A. & King, G. F. Isolation of an orally active insecticidal toxin from the venom of an Australian tarantula. PloS One. 8, e73136 (2013).
Qaim, M. Benefits of genetically modified crops for the poor: household income, nutrition and health. N. Biotechnol. 27, 552–557 (2010).
Tajne, S., Boddupally, D., Sadumpati, V., Vudem, D. R. & Khareedu, V. R. Synthetic fusion-protein containing domains of Bt Cry1Ac and Allium sativum lectin (ASAL) conferred enhanced insecticidal activity against major lepidopteran pests. J. Biotechnol. 171, 71–75, doi:10.1016/j.jbiotec.2013.11.029 (2014).
Tabashnik, B. E., Brévault, T. & Carrière, Y. Insect resistance to Bt crops: lessons from the first billion acres. Nat. Biotechnol. 31, 510–521 (2013).
Tabashnik, B. E. et al. Efficacy of genetically modified Bt toxins against insects with different genetic mechanisms of resistance. Nat. Biotechnol. 29, 1128–1131 (2011).
Szarowicz, S. E. et al. Bacillus anthracis edema toxin impairs neutrophil actin-based motility. Infect. Immun. 77, 2455–2464 (2009).
Hartman, E. A Flacherie Disease of Silkworms Caused by Bacillus Bombysepticus. Lignan Science Journal. 10, 279–289 (1931).
Priyadharshini, P., Mahalingam, C. & Shashidhar, K. Identification and characterization of bacterial pathogens in silkworm, Bombyx mori L. Current Biotica. 2, 181–192 (2008).
Somwatcharajit, R., Tiantad, I. & Panbangred, W. Coexpression of the silent cry2Ab27 together with cry1 genes in Bacillus thuringiensis subsp. aizawai SP41 leads to formation of amorphous crystal toxin and enhanced toxicity against Helicoverpa armigera. J. Invertebr. Pathol. 116, 48–55, doi:10.1016/j.jip.2013.12.008 (2014).
Gilbert, R. Pore-forming toxins. Cell. Mol. Life. Sci. 59, 832–844 (2002).
Pardo-Lopez, L., Soberon, M. & Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS. Microbiol. Rev. 37, 3–22, doi:10.1111/j.1574-6976.2012.00341.x (2013).
Sanahuja, G., Banakar, R., Twyman, R. M., Capell, T. & Christou, P. Bacillus thuringiensis: a century of research, development and commercial applications. Plant. Biotechnol. J. 9, 283–300, doi:10.1111/j.1467-7652.2011.00595.x (2011).
Jakka, S. R., Knight, V. R. & Jurat-Fuentes, J. L. Fitness costs associated with field-evolved resistance to Bt maize in Spodoptera frugiperda (Lepidoptera: Noctuidae). J Econ Entomol. 107, 342–351 (2014).
Cao, G. et al. Quantitative Analysis of Fitness Costs Associated with the Development of Resistance to the Bt Toxin Cry1Ac in Helicoverpa armigera. Sci. Rep. 4, 5629; doi:10.1038/srep05629 (2014).
Vachon, V., Laprade, R. & Schwartz, J.-L. Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: A critical review. J Invertebr Pathol. 111, 1–12 (2012).
Wang, F., Hu, C., Hua, X., Song, L. & Xia, Q. Translationally Controlled Tumor Protein, a Dual Functional Protein Involved in the Immune Response of the Silkworm, Bombyx mori. PLoS One. 8, e69284 (2013).
Lin, P. et al. Structural, evolutionary and functional analysis of APN genes in the Lepidoptera Bombyx mori. Gene 535, 303–311, doi:10.1016/j.gene.2013.11.002 (2014).
Hua, G., Park, Y. & Adang, M. J. Cadherin AdCad1 in Alphitobius diaperinus larvae is a receptor of Cry3Bb toxin from Bacillus thuringiensis. Insect Biochem. Mol. Biol. 45, 11–7 (2013).
Pacheco, S., Gómez, I., Gill, S. S., Bravo, A. & Soberón, M. Enhancement of insecticidal activity of Bacillus thuringiensis Cry1A toxins by fragments of a toxin-binding cadherin correlates with oligomer formation. Peptides. 30, 583–588 (2009).
Los, F. C., Randis, T. M., Aroian, R. V. & Ratner, A. J. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 77, 173–207 (2013).
Fujii, Y. et al. Affinity maturation of Cry1Aa toxin to the Bombyx mori cadherin-like receptor by directed evolution. Mol. Biotechnol. 54, 888–899, doi:10.1007/s12033-012-9638-0 (2013).
Pacheco, S. et al. Domain II loop 3 of Bacillus thuringiensis Cry1Ab toxin is involved in a “ping pong” binding mechanism with Manduca sexta aminopeptidase-N and cadherin receptors. J. Biol. Chem. 284, 32750–32757 (2009).
Upadhyay, S. K. & Singh, P. K. Role of alkaline phosphatase in insecticidal action of Cry1Ac against Helicoverpa armigera larvae. Biotechnol. Lett. 33, 2027–2036 (2011).
Tiewsiri, K. & Wang, P. Differential alteration of two aminopeptidases N associated with resistance to Bacillus thuringiensis toxin Cry1Ac in cabbage looper. Proc. Natl. Acad. Sci. U.S.A. 108, 14037–14042, doi:10.1073/pnas.1102555108 (2011).
Herrero, S., Gechev, T., Bakker, P. L., Moar, W. J. & de Maagd, R. A. Bacillus thuringiensis Cry1Ca-resistant Spodoptera exigua lacks expression of one of four Aminopeptidase N genes. BMC genomics. 6, 96, doi:10.1186/1471-2164-6-96 (2005).
Oliveira, G. R. et al. Improving Cry8Ka toxin activity towards the cotton boll weevil (Anthonomus grandis). BMC biotechnol. 11, 85, doi:10.1186/1472-6750-11-85 (2011).
Bravo, A. et al. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim. Biophys. Acta. 1667, 38–46 (2004).
Yang, Y., Chen, H., Wu, Y., Yang, Y. & Wu, S. Mutated cadherin alleles from a field population of Helicoverpa armigera confer resistance to Bacillus thuringiensis toxin Cry1Ac. Appl. Environ. Microbiol. 73, 6939–6944, doi:10.1128/AEM.01703-07 (2007).
Nagamatsu, Y. et al. Identification of Bombyx mori midgut receptor for Bacillus thuringiensis insecticidal CryIA (a) toxin. Biosci. Biotechnol. Biochem. 62, 718–726 (1998).
Abrami, L. et al. Sensitivity of polarized epithelial cells to the pore-forming toxin aerolysin. Infect. immun. 71, 739–746 (2003).
Chen, J., Brown, M. R., Hua, G. & Adang, M. J. Comparison of the localization of Bacillus thuringiensis Cry1A δ-endotoxins and their binding proteins in larval midgut of tobacco hornworm, Manduca sexta. Cell. Tissue. Res. 321, 123–129 (2005).
Bravo, A. & Soberon, M. How to cope with insect resistance to Bt toxins? Trends. biotechnol. 26, 573–579, doi:10.1016/j.tibtech.2008.06.005 (2008).
Gassmann, A. J. et al. Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Proc. Natl. Acad. Sci. U.S.A. 111, 5141–5146 (2014).
Jin, L. et al. Dominant resistance to Bt cotton and minor cross - resistance to Bt toxin Cry2Ab in cotton bollworm from China. Evol. Appl. 6, 1222–1235 (2013).
Shitomi, Y. et al. A novel 96-kDa aminopeptidase localized on epithelial cell membranes of Bombyx mori midgut, which binds to Cry1Ac toxin of Bacillus thuringiensis. J. biochem. 139, 223–233 (2006).
Walters, F. S., deFontes, C. M., Hart, H., Warren, G. W. & Chen, J. S. Lepidopteran-active variable-region sequence imparts coleopteran activity in eCry3.1Ab, an engineered Bacillus thuringiensis hybrid insecticidal protein. Appl. environ. microbiol. 76, 3082–3088, doi:10.1128/AEM.00155-10 (2010).
Brevault, T. et al. Potential shortfall of pyramided transgenic cotton for insect resistance management. Proc. Natl. Acad. Sci. U.S.A. 110, 5806–5811, doi:10.1073/pnas.1216719110 (2013).
Liang, G.-M. et al. Changes of inheritance mode and fitness in Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae) along with its resistance evolution to Cry1Ac toxin. J. invertebr. pathol. 97, 142–149 (2008).
Liu, C. et al. Cis-mediated down-regulation of a trypsin gene associated with Bt resistance in cotton bollworm. Sci. Rep. 4, 7219, doi:10.1038/srep07219 (2014).
Höfte, H. et al. Structural and functional analysis of a cloned delta endotoxin of Bacillus thuringiensis berliner 1715. Eur. J. biochem. 161, 273–280 (1986).
Lee, M. K., Milne, R., Ge, A. & Dean, D. Location of a Bombyx mori receptor binding region on a Bacillus thuringiensis delta-endotoxin. J. Biol. Chem. 267, 3115–3121 (1992).
Muñoz-Garay, C. et al. Permeability changes of Manduca sexta midgut brush border membranes induced by oligomeric structures of different Cry toxins. J. membr. biol. 212, 61–68 (2006).
Gómez, I., Oltean, D. I., Gill, S. S., Bravo, A. & Soberón, M. Mapping the Epitope in Cadherin-like Receptors Involved inBacillus thuringiensis Cry1A Toxin Interaction Using Phage Display. J. Biol. Chem. 276, 28906–28912 (2001).
Ma, S. et al. CRISPR/Cas9 mediated multiplex genome editing and heritable mutagenesis of BmKu70 in Bombyx mori. Sci. Rep. 4, 4489, doi:10.1038/srep04489 (2014).
Wang, F., Herzig, C., Ozer, D., Baldwin, C. L. & Telfer, J. C. Tyrosine phosphorylation of scavenger receptor cysteine - rich WC1 is required for the WC1 - mediated potentiation of TCR - induced T - cell proliferation. Eur. j. immunol. 39, 254–266 (2009).
Kelsey Downie, G. A. a. E. B. C. Characterization of protein-protein interaction domains within the baculovirus Autographa californica multiple nucleopolyhedrovirus late expression factor J. Gen. Virol. 94, 2530–2536 (2013).
Wu, Y., Li, Q. & Chen, X.-Z. Detecting protein–protein interactions by far western blotting. Nat. Protoc. 2, 3278–3284 (2007).
Wang, S.-W. & McCarthy, W. J. Cytolytic activity of Bacillus thuringiensis CryIC and CryIAc toxins to Spodoptera sp. midgut epithelial cells in vitro. In. Vitro. Cell. Dev. Biol. Anim. 33, 315–323 (1997).
Tsuda, Y. et al. Cytotoxic activity of Bacillus thuringiensis Cry proteins on mammalian cells transfected with cadherin-like Cry receptor gene of Bombyx mori (silkworm). Biochem. J. 369, 697–703 (2003).
Acknowledgements
We are grateful to Pro. Kongming Wu and Dr. Chaoxin Hu (State Key Laboratory for Biology of Plant Diseases and Insect Pests, Chinese Academy of Agricultural Science, China) for providing the Helicoverpa armigera larvae and giving a helpful comments on the procedure of Helicoverpa armigera bioassay. This work was supported by National Basic Research Program of China (973 program, 2012CB114600), National Natural Science Foundation of China (no. 31001034), Chongqing Fundamental and Advanced Research Projects (CSTC2014JCYJA80004) and Fundamental Research Funds for the Central Universities (XDJK2013C004).
Author information
Authors and Affiliations
Contributions
P.L., T.C.C. and Q.Y.X. conceived and designed the study. P.L., J.S.K., Y.Q.W., B.H.F. and R.W.L. performed the study. P.L., T.C.C., P.Z. and Q.Y.X analyzed data. P.L., T.C.C. and Q.Y.X. wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lin, P., Cheng, T., Jin, S. et al. PC, a Novel Oral Insecticidal Toxin from Bacillus bombysepticus Involved in Host Lethality via APN and BtR-175. Sci Rep 5, 11101 (2015). https://doi.org/10.1038/srep11101
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep11101
This article is cited by
-
Bacteria as genetically programmable producers of bioactive natural products
Nature Reviews Chemistry (2020)
-
Increasing the yield of middle silk gland expression system through transgenic knock-down of endogenous sericin-1
Molecular Genetics and Genomics (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.