Abstract
Plants exhibit a wide variety of disease symptoms in response to pathogen attack. In general, most plant symptoms are recognized as harmful effects on host plants and little is known about positive aspects of symptoms for infected plants. Herein, we report the beneficial role of purple top symptoms, which are characteristic of phytoplasma-infected plants. First, by using plant mutants defective in anthocyanin biosynthesis, we demonstrated that anthocyanin accumulation is directly responsible for the purple top symptoms and is associated with reduction of leaf cell death caused by phytoplasma infection. Furthermore, we revealed that phytoplasma infection led to significant activation of the anthocyanin biosynthetic pathway and dramatic accumulation of sucrose by about 1000-fold, which can activate the anthocyanin biosynthetic pathway. This is the first study to demonstrate the role and mechanism of the purple top symptoms in plant–phytoplasma interactions.
Similar content being viewed by others
Introduction
Phytoplasmas are plant-pathogenic bacteria that inhabit phloem sieve elements in host plants and belong to the class Mollicutes1,2. Phytoplasmas are transmitted between plants by phloem-feeding insects belonging to the order Hemiptera. Plants infected with phytoplasmas exhibit a variety of symptoms, including stunted growth, yellowed foliage, purple top (reddening), witches' blooms (proliferation of branches and leaves), virescence (development of green flowers and the loss of pigments) and phyllody (conversion of flowers into leaves)2,3. A number of mechanisms by which phytoplasmas induce disease symptoms have been proposed to date. Phytoplasma-secreted proteins, or so-called effectors, are predicted to play important roles in host-parasite interactions and pathogenicity and to cause disease symptoms4. In fact, some phytoplasma effectors have been identified as inducers of witches' bloom symptoms or leaf-like flower development5,6,7. On the other hand, stunting and yellowing induced by phytopathogenic mollicutes can be associated with the utilization of sugars in the phloem8. It has been shown that Spiroplasma citri, which also belongs to the class Molllicutes and is restricted to the cytoplasm of phloem cells in plants, preferentially utilizes fructose and leads to abnormally high glucose concentrations in the phloem, resulting in symptoms such as yellowing and stunting9. On the other hand, the mechanism causing purple coloration of leaves, or so-called “purple top”, which is characteristic of phytoplasma-infected potato, grapevine, corn, maize and clover1,10,11,12,13, is still largely unknown.
Anthocyanins are plant secondary metabolites synthesized by the flavonoid pathway and play important roles in flower pigmentation, allelopathy, or UV protection14,15. They are also important as antioxidant molecules to protect plant cells against damage by reactive oxygen species16,17,18. Indeed, the production of anthocyanins in autumn leaves reduces the risk of photo-oxidative damage and delays leaf senescence19,20. Anthocyanin biosynthesis genes have been identified in several plant species, including Arabidopsis thaliana and Petunia hybrida14,21,22. The biosynthetic pathway for anthocyanin production can be divided into upstream and downstream sections23. Chalcone synthase (CHS), one key enzyme in the upstream reactions, is responsible for the first step committed to flavonoid synthesis. Dihydroflavonol 4-reductase (DFR) serves within the downstream section to synthesize anthocyanin derivative molecules. In the Arabidopsis genome, most flavonoid pathway genes including CHS and DFR are present as single copies14. Therefore, in Arabidopsis both chs and dfr mutants are completely devoid of anthocyanins14. Anthocyanin accumulation is regulated by various environmental factors, such as light, temperature, nutrient and osmotic stress24,25,26. It has been reported that anthocyanin biosynthesis genes including CHS and DFR are induced in a sucrose-dependent manner27,28.
Flavonoids, including anthocyanins, are recognized as a significant contributor to plant defense against microbial pathogens15. Flavonoid induction in response to pathogen attack has been reported in grapevine and poplar29,30. Recently, global transcription profiles in grapevine infected with Bois noir phytoplasma have revealed that the genes involved in the flavonoid biosynthetic pathway are up-regulated in phytoplasma-infected leaves31,32. However, the roles of anthocyanins and their biosynthesis genes in the phytoplasma-infected plants are not clear.
In the present study, we demonstrate the induction of anthocyanin biosynthesis in response to an infection by ‘Candidatus Phytoplasma asteris’ OY-W strain (OY-W phytoplasma). By using anthocyanin-deficient Arabidopsis mutants, we examined the effects of anthocyanin accumulation on leaf cell death and phytoplasma propagation. Furthermore, we investigated the concentrations of several sugars in phytoplasma-infected plants and discuss the relationships between sucrose content and purple top symptoms during OY-W phytoplasma infection.
Results
Distribution of phytoplasma in purple colored leaves
The infection by OY-W phytoplasma induced purple discoloration in Arabidopsis and Petunia plants (Fig. 1). In the OY-W infected Arabidopsis, the lower, rather than the upper, surface of rosette leaves turned almost entirely purple within 1 to 2 weeks following inoculation (Fig. 1a, 1b). Similarly, the OY-W infected Petunia (cv. Vakara Blue) exhibited foliar purple discoloration beginning 3 to 4 weeks after inoculation (Fig. 1c).
Purple top symptom and phytoplasma distribution.
Typical samples of OY-W-infected leaves exhibiting purple coloration symptom in Arabidopsis wild-type (a, b) and Petunia Vakara Blue (c). The leaves were healthy (each three leaves in left sides) and infected with OY-W phytoplasma (each three leaves in right sides). (a) shows upper surface of rosette leaves of Arabidopsis and (b) shows lower surface. Bars = 1 cm. (d) Phytoplasma accumulation in Petunia leaves exhibiting purple coloration symptoms. Petunia leaves exhibiting symptoms were divided into three tissues; midveins, purple-colored leaf margins (purple-colored) and other non-colored leaf margins (non-colored). OY-W populations were estimated by real-time PCR for OY-W tufB gene. Each bar represents the average of three biological replicates (±SE). P. hybrida glyceraldehyde-3-phosphate dehydrogenase gene (PhGAPDH) was used for normalization. Different alphabets indicate significant differences between them (P < 0.05).
Phytoplasmas generally inhabit phloem elements in plants. However, in infected Petunia leaves, purple coloration was observed along leaf margins around midveins rather than along midveins (Fig. 1c). We investigated the relationships between the area of purple-pigmentation and phytoplasma localization. First, Petunia leaves that exhibited purple coloration were divided into three tissues (midveins, pigmentation area and other non-pigmentation area) and the phytoplasma populations therein were quantified using real-time PCR. As a result, phytoplasma accumulation in the midveins was significantly high compared with other tissues, whereas there was no significant difference between tissues with and without pigmentation (Fig. 1d). This suggests that OY-W phytoplasma accumulated mainly along the veins in Petunia leaves. Moreover, this result suggests that the purple coloration was not related to the localization of phytoplasma.
The activation of anthocyanin biosynthesis by OY-W phytoplasma infection
To test the hypothesis that phytoplasma-induced purple pigmentation is due to anthocyanin accumulation by host plants, chs mutant plants, which are anthocyanin deficient, were inoculated with OY-W phytoplasma. After 10 days post-inoculation, wild-type leaves showed full purple coloration (Fig. 1a, 1b), while chs leaves showed a little yellowing at leaf tips but did not show purple-colored symptoms (Fig. 2a). This suggests that the phytoplasma-induced purple top symptoms resulted from ectopic anthocyanin accumulation.
Purple top symptom is due to plant anthocyanin biosynthesis.
(a) Typical samples of healthy and OY-W infected rosette leaves of Arabidopsis chs mutant. Experimental conditions on phytoplasma-infection and observation were the same to Fig. 1a, b. Bar = 1 cm. (b) Expression analyses of anthocyanin synthesis genes, CHS and DFR, in Arabidopsis and Petunia. Transcriptional levels were obtained by qRT-PCR in healthy and OY-W-infected leaves. Each bar represents the average of three biological replicates(±SE). The average expression levels of healthy leaves were set as 1.0. Significant differences between healthy and infected leaves are indicated as * (P < 0.05) or ** (P < 0.01).
To examine whether OY-W phytoplasma infection activates the anthocyanin biosynthetic pathway, we quantified mRNA accumulation of the early and late anthocyanin biosynthetic genes, CHS and DFR, respectively, in the leaves of Arabidopsis and Petunia plants exhibiting purple-coloration symptoms. CHS expression was increased significantly by 8.8- and 3.1-fold in infected Arabidopsis and Petunia, respectively, compared to healthy controls (Fig. 2b, upper panels). DFR transcripts were also greatly increased in both infected plants, especially in the OY-W–infected Petunia, by 24-fold (Fig. 2b, lower panels). These results suggested that OY-W phytoplasma infection strongly induces the expression of both early and late anthocyanin biosynthetic genes in both Arabidopsis and Petunia.
Reduction of cell death in leaves accumulated with anthocyanin
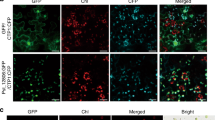
To investigate the roles of anthocyanin accumulation in phytoplasma-infected leaves, we compared symptoms between two OY-W–infected Petunia cultivars, Vakara Blue, which blooms with blue flowers and exhibits purple-colored leaves during phytoplasma infection (Fig. 1c) and Vakara White, which blooms with white flowers and has defects in the anthocyanin biosynthesis pathway. After 4 weeks post-inoculation, the OY-W–infected Vakara Blue exhibited foliar purple coloration along leaf margins; however, the leaves of Vakara White did not exhibit purple coloration and instead showed yellowing from the leaf edges (Fig. 3f). Furthermore, infected leaves of Vakara White appeared to undergo accelerated senescence compared with those of Vakara Blue (Fig. 3b, 3f). In fact, we often observed necrosis of the leaf margins in the infected leaves of Vakara White (Fig. 3f). To examine the relationship between purple coloration and necrosis of leaf margins during phytoplasma infection, we identified dead cells in Petunia plants using trypan blue staining. The leaf margins of OY-W–infected Vakara White were strongly stained dark blue (Fig. 3h), while those of Vakara Blue were not stained (Fig. 3d). This suggests that cell death was induced by OY-W phytoplasma infection along leaf margins in Vakara White, but was suppressed in Vakara Blue. Together with the finding that anthocyanin accumulates along leaf margins in the OY-W–infected Vakara Blue (Fig. 1c, 3b), these data suggest that anthocyanin may be responsible for the suppression of the leaf cell death.
Anthocyanin accumulation reduces cell death in Petunia and Arabidopsis.
(a–h) Differences in disease symptoms between two Petunia cultivars, Vakara Blue and Vakara White. (i) Trypan blue staining assays in healthy and OY-W infected Arabidopsis wild type and chs mutant. The values of signal intensity detected by trypan blue staining were calculated by image J. The relative signal intensities are means of 3 samples, with standard error of the mean. Significant difference between infected WT and infected chs is indicated as * (P < 0.05).
However, these experiments cannot exclude the possibility that this finding might be dependent on the genetic background of Petunia in addition to anthocyanin accumulation. To test the hypothesis that anthocyanin accumulation contributes to reduce cell death in phytoplasma-infected leaves, we used Arabidopsis wild type (WT) and an anthocyanin-deficient mutant (chs) for the trypan blue staining. Entire surfaces along leaf veins in both infected WT and infected chs were darkly stained (Fig. 3i). However, the statistical analyses of the staining intensity revealed a significantly high number of dead cells in infected chs compared with infected WT (Fig. 3j).
Additionally, to assess the effects of anthocyanin on the accumulation of phytoplasma, we quantified the titer of phytoplasma in WT and two anthocyanin-deficient mutants, chs and dfr, infected with OY-W after 3 weeks. However, there were no significant differences between the WT and the mutants (Fig. 4).
The effect of anthocyanin on phytoplasma accumulation.
Phytoplasma accumulation in Arabidopsis plants with or without anthocyanin biosynthesis. The relative levels of phytoplasma accumulations in Arabidopsis wild type, chs and dfr were detected by real-time PCR for OY-W tuf B gene. Arabidopsis β-tubulin gene was used for normalization.
Sugar accumulation during phytoplasma infection
Recent studies revealed that sucrose is associated with the accumulation of anthocyanins, in addition to high light intensity and mineral depletion27,28. Additionally, previous reports have shown that phytoplasma infection affects the sugar content33,34,35,36. However, the relationship between anthocyanin synthesis and the sugar accumulation induced by phytoplasma infection remains unknown. Therefore, to examine this relationship, we measured the fructose, glucose, sucrose and trehalose contents of healthy and OY-W infected leaves of Vakara Blue and Vakara White, which were subjected to the same experimental condition as those shown in Fig. 3-a, -b, -e and -f. In both OY-W–infected Vakara Blue and Vakara White leaves, all sugars accumulated to a greater extent than those in the respective healthy leaves (Table 1, Fig. 5). Fructose and glucose contents in infected leaves were higher than those in healthy leaves by 10- to 40-fold. In particular, the sucrose contents of OY-W–infected leaves of both Vakara Blue and Vakara White were much higher than those of healthy leaves, by ~1000-fold (Table 1, Fig. 5). These results showed that the significant elevation of sugars in response to phytoplasma infection occurred independent of anthocyanin accumulation.
The ratio of sugar contents in infected leaves to those in healthy leaves.
The graphs were visualized from the ratio (Infected/Healthy) data of table 1 with displayed on a log10 scale.
Discussion
In this study, we demonstrated the activation of the anthocyanin biosynthesis in response to OY-W phytoplasma infection, resulting in purple top symptoms. To our knowledge, this is the first study at the molecular level to show that purple-coloration symptoms in phytoplasma-infected plants are caused by anthocyanin biosynthesis, based on the following evidence. First, the Arabidopsis mutant chs leaves did not exhibit purple coloration following OY-W phytoplasma infection (Fig. 2a). Second, the OY-W phytoplasma infection of Arabidopsis and blue Petunia plants induced significant upregulation of both CHS and DFR, which are involved in anthocyanin biosynthesis (Fig. 2b). Together, these data revealed that OY-W phytoplasma infection activates the anthocyanin biosynthesis pathway in host plant leaves, resulting in a purple coloration. Furthermore, we found no association between the area of purple coloration and phytoplasma distribution (Fig. 1d). This result suggests that the induction of anthocyanin accumulation does not correlate with phytoplasma titer.
Our studies found that phytoplasma infection induced a high number of cell death in chs leaves in compared to WT leaves (Fig. 3j). This result suggests that flavonoids including anthocyanins could reduce cell death caused by infection. Cell death by infection occurred in the leaf vein and the leaf margin (Fig. 3). Leaf vein necrosis was observed in both infected Petunia and infected Arabidopsis (Fig. 3d, 3h, 3i). Phytoplasma diseases generally induce phloem necrosis3 and OY-W phytoplasma have also been reported to cause phloem necrosis37. Therefore, the leaf vein necrosis observed in this study is considered to be the phloem necrosis. On the other hands, the cell death from the leaf edge was observed in infected white Petunia (Fig. 3f, 3h). The mechanisms underlying such cell death remain unclear, because, to our knowledge, cell death at leaf margin has never been examined in phytoplasma-infected plants. However, considering the significant high-content sugars in infected leaves (Table 1, Fig. 5), this symptom might be a type of senescence that can be triggered by high sugar content because sugars are known as positive regulators of leaf senescence38. Both kinds of cell death were reduced in leaves accumulated with anthocyanins (Fig. 3). By using Arabidopsis chs mutant, which cannot generate flavonoids, it was demonstrated that flavonoids is involved in the reduction of leaf cell death. Flavonoids including anthocyanins are known to act as antioxidant molecules14,16 and have been shown to delay leaf senescence and contribute to the longevity of leaves20,39,40. Our findings are consistent with the role of flavonoids as antioxidant molecules in OY-W phytoplasma infected plants.
On the other hand, the accumulation of flavonoids including anthocyanins did not influence phytoplasma titer (Fig. 4). Flavonoids including anthocyanins have long been recognized as defense-related compounds15,41,42. In fact, the accumulation of anthocyanins was shown to reduce the susceptibility of tomatoes to Botrytis cinerea by altering dynamics of the ROS burst generated by infection43. B. cinerea is a necrotrophic pathogen that requires the induction of cell death for growth. In contrast, phytoplasma is a biotrophic pathogen and requires living cells. Therefore, flavonoids-mediated repression of cell death would not affect OY-W phytoplasma propagation. A recent study suggested that anthocyanin biosynthesis inhibits plant basal immunity in Arabidopsis44. In addition, it has been reported that pathogen attack and host immunity represses anthocyanin accumulation44,45,46. The interaction of host immunity and phytoplasma infection is still unclear and further studies are needed to determine how anthocyanin biosynthesis affects host plant–phytoplasma interactions.
The abnormal sucrose level in infected leaves (Table 1, Fig. 5) likely contributes to anthocyanin induction for the following reasons. Sugar (such as sucrose, glucose and fructose)-dependent upregulation of anthocyanin biosynthesis has often been reported and in particular, the induction of sucrose-specific anthocyanin synthesis has been also demonstrated27,28. This study revealed that the concentration of sucrose in infected purpling leaves was ~1000-fold higher than that in healthy control plants (Table 1, Fig. 5). The finding that the sucrose concentration in the infected leaves of white Petunia, Vakara White, was also significantly increased suggests that the sucrose increment is an upstream signal of anthocyanin accumulation or occurs via an anthocyanin-independent pathway. It has been shown that 15 mM exogenous sucrose was sufficient to enhance anthocyanin accumulation in Arabidopsis seedlings28, however, under the same conditions, the absorbed sucrose concentration inside plant tissue has not been investigated. In this study, the sucrose concentration in infected purpling leaves was increased to 3.5 mM (Table 1), while that in control leaves was 3.8 μM. This concentration inside the leaves may be sufficient to induce anthocyanin synthesis. Collectively, our findings suggest that sucrose is a factor in anthocyanin induction during phytoplasma infection.
In this study, the necrosis of leaf veins was observed in OY-W phytoplasma infected leaves of both Arabidopsis and Petunia (Fig. 3). Numerous previous studies have suggested that phloem necrosis due to phytoplasma infection may cause a blockage in phloem transport. For example, metabolomic and transcriptional analyses of several phytoplasma-infected plants provided evidence of the inhibition of phloem loading by phytoplasma infection31,32,33,34,44. Santi et al. showed decreased phloem loading through the inhibition of transport gene expression47. Together with these findings, the sucrose accumulation in OY-W infected leaves could be considered as a result of the reduction in phloem loading caused by phloem necrosis (Fig. 6).
Schematic model of possible mechanism underlying different leaves symptoms between wild type and anthocyanin deficient mutant in A. thaliana.
Phytoplasma infections cause phloem necrosis in both wild type and anthocyanin deficient mutant (ii). In those leaves, sucrose concentration is increased at high level by impaired phloem loading (iii). Sucrose accumulation activates the anthocyanin biosynthesis pathway in wild type, resulting in purple top symptoms (iv-left column). In anthocyanin deficient mutant, the leaf shows yellowing symptom and dies earlier than wild type (iv-right column). (Drawings by Misako Himeno).
Phytoplasma symptoms, such as stunted growth and leaf yellowing, are reported to be associated with phloem necrosis and phloem hyperplasia37,48,49,50. In periwinkle (Catharanthus roseus (L.) G. Don) source leaves infected with phytoplasmas, leaf bleaching (yellowing) may result from a high content of carbohydrates33. Moreover, leaf yellowing by a stolbur phytoplasma infection was associated with the repression of genes involved in sugar transport51. Similarly, the leaf yellowing observed in this study (Fig. 2a and 3f) was likely associated with the abnormal sugar contents due to decreased phloem loading.
Our study revealed that anthocyanin accumulation did not influence phytoplasma growth, as discussed above (Fig. 4). Rather, sucrose upregulation might facilitate the spread of phytoplasma infection, since many insects favor sucrose52 and insect vectors might be attracted to infected plants. Anthocyanin accumulation may also benefit phytoplasma, because the longevity of purple-colored leaves seems to enhance the preference of sucking insects that transmit disease. Further studies will be required to elucidate the benefits of sucrose and anthocyanin accumulation for phytoplasma survival.
Methods
Plant material and phytoplasma infection
Seeds of Arabidopsis thaliana, ecotype Landsberg erecta (Ler) and mutant lines CS84 and CS85 were provided by the Arabidopsis Biological Resource Center (ABRC) (Ohio State University, Columbus, OH). Petunia hybrida cultivars, Vakara Blue and Vakara White (Sakata Seeds Ltd, Yokohama, Japan), were used in this study. A. thaliana and P. hybrida plants were grown in growth chambers with 16-h-light/8-h-dark cycles at 25°C.
A wild strain of onion yellows phytoplasma (OY-W strain) has been maintained in a plant host, Chrysanthemum coronarium, using a leafhopper vector, Macrosteles striifrons as described previously37. Inoculations of Arabidopsis and Petunia with phytoplasma were performed as described previously5,53. Note that the “healthy” plants used in all experiments were fed on by healthy leafhoppers for 1 week.
DNA isolation and OY-W phytoplasma quantification
Total DNA was isolated from phytoplasma-infected tissues using the GENE PREP STAR PI-80X system (KURABO) according to the manufacturer's procedure. The accumulation of phytoplasma DNA was quantified using Thermal Cycler Dice real-time PCR system (TaKaRa) with SYBR Premix ExTaq (TaKaRa). The primer set for phytoplasma detection was designed from the elongation factor Tu gene of OY (tufB) as described previously54. Results were normalized using β-tubulin and PhGAPDH as host DNA genes in A. thaliana and P. hybrida, respectively53 (Table 2).
RNA isolation and real time quantitative RT-PCR
Total RNA was extracted from leaves using Sepasol–RNA I (Nacalai Tesque, Inc.) according to the manufacturer's protocol. Both DNase treatment and real-time quantitative RT-PCR were carried out as described previously53. The results were normalized using β-tubulin and PhGAPDH in A. thaliana and P. hybrida, respectively. The primer sets designed in this study are shown in Table 2. Each data point represents the mean of at least three biological replicates and three technical replicates.
Trypan blue staining
Trypan blue staining was performed for the detection of cell death. Leaf samples were stained with lactophenol-trypan blue solution55 and cleared with chloral hydrate solution56. Signal intensity was quantified using Image J (National Institutes of Health) as described by Ishii et al.57 and values were normalized based on healthy A. thaliana leaves treated with the same trypan blue staining procedure.
Quantification of sugars by LC-TOF-MS
A total of 0.2 g of Petunia leaves was homogenized in liquid nitrogen and mixed with 200 μl pure water. After centrifugation, an equal volume of chloroform was added to the supernatant, mixed and centrifuged. The supernatant was stored at −80°C until required for analysis. The samples were diluted 1:20 by 75% acetonitrile and then filtered with a 0.2 μm syringe filter prior to injection into LC-TOF-MS.
LC-TOF-MS analysis was performed using an ACQUITY ultra performance liquid chromatographic system (Waters) and a LCT Premier XE system (Waters) equipped with 2.1 mm × 100 mm ACQUITY UPLC BEH Amide column maintained at 40°C and eluted with a two-step gradient at 0.13 ml/min. Mobile phase solutions were 0.25% ammonium water (A) and pure acetonitrile (B). The gradient was initiated with 75% of B and then reduced to 50% of B in a 10 min linear step. The injection volume was 5 μl and the samples were kept at 4°C throughout the analysis. Spectra were collected in both positive and negative ESI modes over a mass range m/z 50–1000.
Statistical analysis
Data are expressed as the means ± S.D. obtained for at least three independent experiments. Statistical analysis was performed by Welch's t-test because P < 0.05 was suggested by the F-test in all statistical analyses. P-values < 0.05 were considered to indicate statistical significance.
References
Lee, I. M., Davis, R. E. & Gundersen-Rindal, D. E. Phytoplasma: phytopathogenic mollicutes. Annu Rev Microbiol 54, 221–255 (2000).
Oshima, K., Maejima, K. & Namba, S. Genomic and evolutionary aspects of phytoplasmas. Front Microbiol 4, 230 (2013).
Hogenhout, S. et al. Phytoplasmas: bacteria that manipulate plants and insects. Mol Plant Pathol 9, 403–423 (2008).
Sugio, A. et al. Diverse targets of phytoplasma effectors: from plant development to defense against insects. Annu Rev Phytopathol 49, 175–195 (2011).
Hoshi, A. et al. A unique virulence factor for proliferation and dwarfism in plants identified from a phytopathogenic bacterium. Proc Natl Acad Sci USA 106, 6416–6421 (2009).
Sugio, A., Kingdom, H. N., MacLean, A. M., Grieve, V. M. & Hogenhout, S. A. Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proc Natl Acad Sci USA 108, E1254–1263 (2011).
MacLean, A. et al. Phytoplasma effector SAP54 induces indeterminate leaf-like flower development in Arabidopsis plants. Plant Physiol 157, (2011).
Hogenhout, S. & Loria, R. Virulence mechanisms of Gram-positive plant pathogenic bacteria. Curr Opin Plant Biol 11, 449–456 (2008).
Andre, A., Maucourt, M., Moing, A., Rolin, D. & Renaudin, J. Sugar import and phytopathogenicity of Spiroplasma citri: glucose and fructose play distinct roles. Mol Plant-Microbe Interact 18, 33–42 (2005).
Nault, L. Maize bushy stunt and corn stunt: A comparison of disease symptoms, pathogen host ranges and vectors. Phytopathol 70, 659–662 (1980).
Banttari, E., Orr, P. & Preston, D. Purple top as a cause of potato chip discoloration. Trans ASAE 33, 221–226 (1990).
Franova, J., Paltrinieri, S., Botti, S., Simkova, M. & Bertaccini, A. Association of phytoplasmas and viruses with malformed clovers. Folia Microbiol 49, 617–624 (2004).
Duduk, B. & Bertaccini, A. Corn with symptoms of reddening: New host of stolbur phytoplasma. Plant Disease 90, 1313–1319 (2006).
Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology and biotechnology. Plant Physiol. 126, 485–493 (2001).
Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol 7, 581–591 (2005).
Gould, K., McKelvie, J. & Markham, K. Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant Cell Environ 25, 1261–1269 (2002).
Neill, S., Gould, K., Kilmartin, P., Mitchell, K. & Markham, K. Antioxidant activities of red versus green leaves in Elatostema rugosum. Plant Cell Environ 25, 539–547 (2002).
Nagata, T. et al. Levels of active oxygen species are controlled by ascorbic acid and anthocyanin in Arabidopsis. J Agric Food Chem 51, 2992–2999 (2003).
Feild, T., Lee, D. & Holbrook, N. Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiol 127, 566–574 (2001).
Schaberg, P., Murakami, P., Turner, M., Heitz, H. & Hawley, G. Association of red coloration with senescence of sugar maple leaves in autumn. Trees 22, 573–578 (2008).
Holton, T. & Cornish, E. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 7, 1071–1083 (1995).
Grotewold, E. The genetics and biochemistry of floral pigments. Annu Rev Plant Biol 57, 761–780 (2006).
Deroles, S. Anthocyanin biosynthesis in plant cell cultures: A potential source of natural colourants. In: Kevin, G., Kevin, D. & Chris, W. (eds) Anthocyanins: Biosynthesis, functions and applications. 107–117 (2009).
Mendez, M., Jones, D. & Manetas, Y. Enhanced UV-B radiation under field conditions increases anthocyanin and reduces the risk of photoinhibition but does not affect growth in the carnivorous plant Pinguicula vulgaris. New Phytol 144, 275–282 (1999).
Krol, M. et al. Low-temperature stress and photoperiod affect an increased tolerance to photoinhibition in Pinus banksiana seedlings. Can J Bot 73, 1119–1127 (1995).
Rajendran, L., Ravishankar, G., Venkataraman, L. & Prathiba, K. Anthocyanin production in callus cultures of Daucus carota as influenced by nutrient stress and osmoticum. Biotechnol Lett 14, 707–712 (1992).
Teng, S., Keurentjes, J., Bentsink, L., Koornneef, M. & Smeekens, S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol 139, 1840–1852 (2005).
Solfanelli, C., Poggi, A., Loreti, E., Alpi, A. & Perata, P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140, 637–646 (2006).
Kortekamp, A. Expression analysis of defence-related genes in grapevine leaves after inoculation with a host and a non-host pathogen. Plant Physiol Biochem 44, 58–67 (2006).
Miranda, M. et al. The transcriptional response of hybrid poplar (Populus trichocarpa × P.deltoides) to infection by Melampsora medusae leaf rust involves induction of flavonoid pathway genes leading to the accumulation of proanthocyanidins. Mol Plant-Microbe Interact 20, 816–831 (2007).
Albertazzi, G. et al. Gene expression in grapevine cultivars in response to Bois Noir phytoplasma infection. Plant Science 176, 792–804 (2009).
Hren, M. et al. ‘Bois noir’ phytoplasma induces significant reprogramming of the leaf transcriptome in the field grown grapevine. BMC Genomics 10, (2009).
Lepka, P., Stitt, M., Moll, E. & Seemuller, E. Effect of phytoplasmal infection on concentration and translocation of carbohydrates and amino acids in periwinkle and tobacco. Physiol Mol Plant Pathol 55, 59–68 (1999).
Maust, B. et al. Changes in carbohydrate metabolism in coconut palms infected with the lethal yellowing phytoplasma. Phytopathol 93, 976–981 (2003).
Choi, Y. H. et al. Metabolic discrimination of Catharanthus roseus leaves infected by phytoplasma using 1H-NMR spectroscopy and multivariate data analysis. Plant Physiol 135, 2398–2410 (2004).
Giorno, F., Guerriero, G., Biagetti, M., Ciccotti, A. & Baric, S. Gene expression and biochemical changes of carbohydrate metabolism in in vitro micro-propagated apple plantlets infected by ‘Candidatus Phytoplasma mali’. Plant Physiol Biochem 70, 311–317 (2013).
Oshima, K. et al. Isolation and characterization of derivative lines of the onion yellows phytoplasma that do not cause stunting or phloem hyperplasia. Phytopathol 91, 1024–1029 (2001).
Wingler, A., Purdy, S., MacLean, J. A. & Pourtau, N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J Exp Bot 57, 391–399 (2006).
Park, J., Canam, T., Kang, K., Unda, F. & Mansfield, S. Sucrose phosphate synthase expression influences poplar phenology. Tree Physiol 29, 937–946 (2009).
Tallis, M. J. et al. The transcriptome of Populus in elevated CO2 reveals increased anthocyanin biosynthesis during delayed autumnal senescence. New Phytol 186, 415–428 (2010).
Dixon, R., Harrison, M. & Lamb, C. Early events in the activation of plant defense responses. Annu Rev Phytopathol 32, 479–501 (1994).
Field, B., Jordan, F. & Osbourn, A. First encounters - deployment of defence-related natural products by plants. New Phytol 172, 193–207 (2006).
Zhang, Y. et al. Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold. Curr Biol 23, 1094–1100 (2013).
Serrano, M. et al. Repression of sucrose/ultraviolet B light-induced flavonoid accumulation in microbe-associated molecular pattern-triggered immunity in Arabidopsis. Plant Physiol 158, 408–422 (2012).
McLusky, S. et al. Cell wall alterations and localized accumulation of feruloyl-3′-methoxytyramine in onion epidermis at sites of attempted penetration by Botrytis allii are associated with actin polarisation, peroxidase activity and suppression of flavonoid biosynthesis. Plant J 17, 523–534 (1999).
Schenke, D., Böttcher, C. & Scheel, D. Crosstalk between abiotic ultraviolet-B stress and biotic (flg22) stress signalling in Arabidopsis prevents flavonol accumulation in favor of pathogen defence compound production. Plant Cell Environ 34, 1849–1864 (2011).
Santi, S., Grisan, S., Pierasco, A., De Marco, F. & Musetti, R. Laser microdissection of grapevine leaf phloem infected by stolbur reveals site-specific gene responses associated to sucrose transport and metabolism. Plant Cell Environ 36, 343–355 (2013).
Braun, E. & Sinclair, W. Histopathology of phloem necrosis in Ulmus americana. Phytopathol 66, 598–607 (1976).
Schneider, H. Indicator hosts for pear decline: Symptomatology, histopathology and distribution of mycoplasmalike organisms in leaf veins. Phytopathol 67, 592–601 (1977).
Uehara, T. et al. Histopathological studies on two symptom types of phytoplasma associated with lettuce yellows. Annu Phytopathol Soc Jpn 65, 465–469. (1999).
Jagoueix-Eveillard, S. et al. Catharanthus roseus genes regulated differentially by mollicute infections. Mol Plant-Microbe Interact 14, 225–233 (2001).
Chapman, R. F. Contact chemoreception in feeding by phytophagous insects. Annu Rev Entomol 48, 455–484 (2003).
Himeno, M. et al. Unique morphological changes in plant pathogenic phytoplasma-infected petunia flowers are related to transcriptional regulation of floral homeotic genes in an organ-specific manner. Plant J 67, 971–979 (2011).
Oshima, K. et al. Dramatic transcriptional changes in an intracellular parasite enable host switching between plant and insect. PLoS One 6 (2011).
Rate, D., Cuenca, J., Bowman, G., Guttman, D. & Greenberg, J. The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses and cell growth. Plant Cell 11, 1695–1708 (1999).
Komatsu, K. et al. Viral-induced systemic necrosis in plants involves both programmed cell death and the inhibition of viral multiplication, which are regulated by independent pathways. Mol Plant-Microbe Interact 23, 283–293 (2010).
Ishii, Y. et al. In the non-insect-transmissible line of onion yellows phytoplasma (OY-NIM), the plasmid-encoded transmembrane protein ORF3 lacks the major promoter region. Microbiol 155, 2058–2067 (2009).
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (category “s” of Scientific Research Grant 25221201), by the Funding Program for Next Generation World-Leading Researchers (project: GS005) initiated by the Council for Science and Technology Policy (CSTP) and by the Program for Promotion of Basic Research Activities for Innovative Bioscience (PROBRAIN).
Author information
Authors and Affiliations
Contributions
M.H., K.M., Y.Y., K.O. and S.N. designed research; M.H., Y.K. and T.Y. performed research; M.H. and K.O., analyzed data; and M.H. wrote the paper. All authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Himeno, M., Kitazawa, Y., Yoshida, T. et al. Purple top symptoms are associated with reduction of leaf cell death in phytoplasma-infected plants. Sci Rep 4, 4111 (2014). https://doi.org/10.1038/srep04111
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04111
This article is cited by
-
BoMYB2 plays a key role in anthocyanin accumulation in tobacco plants overexpressing BoMYB2, BoTT8 and BoTTG1 transcription factors from purple cauliflower
Plant Cell, Tissue and Organ Culture (PCTOC) (2023)
-
Molecular biological study on the survival strategy of phytoplasma
Journal of General Plant Pathology (2021)
-
Spatiotemporal dynamics and quantitative analysis of phytoplasmas in insect vectors
Scientific Reports (2020)
-
Assessment of the optimal spectral bands for designing a sensor for vineyard disease detection: the case of ‘Flavescence dorée’
Precision Agriculture (2019)
-
Brassica yellows virus’ movement protein upregulates anthocyanin accumulation, leading to the development of purple leaf symptoms on Arabidopsis thaliana
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.