Abstract
Upper tract urothelial carcinoma (UTUC) is rare but aggressive with poor prognosis. We aimed to find effective predictive markers for recurrence and prognosis in UTUC patients. In this retrospective study, we included 88 UTUC patients treated with radical neprhoureterectomy (RNU) and analyzed their clinicopathological parameters. For study of incidence of metachronous bladder tumor, models were adjusted with inclusion of prophylactic intravesical instillation chemotherapy. The mean follow-up was 28.59 months (2 to 82 mo). Lack of gross hematuria (RR 0.060, 95%CI 0.008–0.468), tumor located at ureter (RR 0.037, 95%CI 0.004–0.378), advanced stage and higher p53 expression were independent factors for worse survival. Recurrence of bladder cancer occurred 20% of patients at median follow-up of 37.65 months (5 to 82 mo). Higher tumor grade (RR 5.998, 95%CI 1.359–26.479) and presence of ipsilateral non-functioning kidney at diagnosis (RR 5.982, 95%CI 1.338–26.750) were predictors for recurrence. The present study identified several parameters with predictive value in the prognosis and intravesicle recurrence in UTUCand shed light on the better monitoring and management of the disease.
Similar content being viewed by others
Introduction
Urothelial carcinomas (UCs) rank the fourth most common malignancies and can occur in the lower and upper urinary tract1,2. While bladder cancer (BCa) accounts for 95% of UCs, upper tract UCs (UTUCs) are uncommon and take up 5-10% of UCs1,2,3,4. Unlike the natural history of bladder cancer, 60% of UTUCs are invasive, whilst only 15–20% of bladder tumors are invasive5,6. Due to the lack of effective approaches for early detection and its aggressive nature, the prognosis of UTUCs is usually very poor with <50% of cancer specific survival for pT2/pT3 tumors and <10% for pT4 tumors7,8. Discovery and development of disease markers for UTUCs are therefore potentially beneficial for patients with UTUC. Thus far, there have been several studies investigating tissue-based markers and their prognostic impact9,10,11,12,13. Nonetheless, due to the rarity of the disease, these studies are generally limited by the small sample size. To date, none of the current studies fulfills the clinical and statistical criteria to support daily clinical practice14.
Expressions of p53 and Ki67 are commonly used enzyme labels for urothelial malignancy. P53 is a key tumor suppressor gene involved in the maintenance of genomic stability, response to genotoxic stress and activation of cell cycle apoptosis. Some studies have shown correlations between p53 and tumor stage and grade in urothelial bladder cancer15,16. Likewise, the role of Ki67 in urothelial bladder cancer has been well studied. Ki67 is expressed at G1, S, G2 and M stages of the cell cycle and is an indicator of cell proliferation and a measure of cell growth fraction17. Proliferative activity of tumors determined by Ki67 labeling index has been found to correlate with aggressive behavior in bladder cancer18,19. Recently, a study reported the molecular similarity of UTUC and BC regarding cell cycle and proliferative tissue markers and suggested the extrapolation of the markers in UTUC20.
Taking limitations from previous studies into account, we decide to conduct the current study with a relatively larger sample size, using daily and commonly accessible clinicopathological markers and to investigate their contribution to predict prognosis and metachronous bladder tumor. These markers could, at the very least help designate individualized and closer follow-ups for marker-positive patients.
Results
Population characteristics
In our cohort, there were 59 males (67.05%) and 29 females (32.95%). The average age of the patients was 65.02 years old (40 to 83 years). There were 31 specimens (35.23%) graded as low-grade carcinomas and 57 specimens (64.77%) graded as high-grade carcinomas. There were 36 cases (40.91%) staged as pTa, 14 cases (15.91%) staged as pT1, 16 cases (18.18%) as pT2 and 22 cases (25%) as pT3–pT4. There were 39 patients (44.32%) with renal pelvic tumors and 39 patients (44.32%) with ureteral tumors. The remaining 10 cases (11.36%) had multiple tumors at pelvic and ureter. There were 14 patients (15.91%) with multifocal tumors of upper urinary tract. There were 36 cases with right-sided UTUC and the remaining 52 cases were left-sided. There were 9 patients (10.23%) with concomitant bladder cancer at diagnosis. In addition, 16 patients (18.18%) have ipsilateral non-functioning kidney at the time of diagnosis with UTUC and there were 64 patients (72.73%) with gross hematuria at diagnosis.
p53 and Ki67 expression in relation to clinicopathological parameters
Expressions of Ki67 and p53 were demonstrated in Figure 1 and summarized in Table 1. Ki67 expression was elevated with the progression of tumor grade (P = 0.002) but not stage. Ki67 expression was also significantly higher in female (P = 0.018). In addition, patients with multifocal tumors and ipsilateral non-functioning kidney had elevated Ki67 expression close to statistical significance. P53 expression was significantly higher in patients with right-sided UTUC.
Cancer-specific survival
The mean follow-up was 28.59 months (2 to 82 mo). As of Jan 2013, 73 patients (82.95%) were still alive at the last follow-up with a median follow-up of 28.99 months (2 to 82 mo). Fifteen patients died of UTUC during follow-up, with a median follow-up of 26.50 months (4–69 mo). Multivariate Cox proportional hazards regression analyses were performed, adjusted for gender, gross hematuria, tumor stage, tumor grade, tumor side, tumor location, tumor number, concomitant BCa, non-function kidney and expressions of p53 and Ki67 (Table 2). A history of gross hematuria (present vs. absent, RR 0.060, 95%CI 0.008–0.468), tumor stage and tumor location (ureter vs. renal pelvic, RR 0.037, 95%CI 0.004–0.378) and p53 expression were significant risk factors for cancer-specific survival. Positive correlations were observed between cancer-specific death and tumor stage as well as with p53 expression (Figure 2).
Metachronous bladder urothelial carcinoma
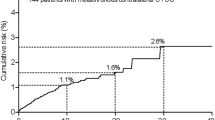
Follow-up data from 85 cases of UTUC undergoing RNU were involved, 3 cases were excluded because of synchronous cystectomy. As of Jan 2013, intravesical recurrence of bladder cancer occurred in 17 patients (20%) at the last follow-up with a median follow-up of 37.65 months (5 to 82 mo). Fifteen cases (88.24%) with BCa were NMIBC while 2 of them were MIBC. We also performed multivariate Cox proportional hazards regression analyses adjusted for gender, gross hematuria, tumor stage, tumor grade, tumor side, tumor location, tumor number, concomitant BCa, non-function kidney, prophylactic chemotherapy and expression of p53 and Ki67 (Table 3). Tumor grade (low grade vs. high grade, RR 5.998, 95%CI 1.359–26.479) and non-functioning kidney (present vs. absent, RR 5.982, 95%CI 1.338–26.750) were significant risk factors for metachronous BCa (Figure 3).
Discussion
In the combat against UTUC, predicative markers for prognosis and recurrence are of great importance. Recently, several nomograms for post-RNU recurrence and survival have been published27,28,29,30. One study using the Surveillance Epidemiology and End Results (SEER) database reveals that age, pathological stage (worse prognosis conferred by >T2), nodal status and grade predicts cancer specific survival28. Other studies incorporate pathological parameters into the prognostic models together with lymphovascular invasion (LVI), tumor necrosis and architecture emerging as strong prognosticators30,31. More recently, a study looking into the predicative value of immunohistochemical staining scores of commonly used markers in UTUCs appears promising32. They have included Cyclin E, p53, p21, Ki67 and p27 and have categorized them as favorable and unfavorable scores, the latter of which is associated with LVI, tumor necrosis and worsened prognosis.
In the current study, we have included relatively large sample size and have investigated various clinicopathological parameters in a multivariate model. In line with previous reports, we find that Ki67 labeling index is associated with advanced tumor grade33,34. Nonetheless, we did not observe an association between Ki67 and tumor stage, which disagrees with other reports. We have not either obtained any association between p53 expression and tumor stage or grade, which once again supports the equivocal role of p53 in UTUC35. More importantly, we have investigated their predictive values for cancer-specific survival and have found 4 significant indicators after adjustment. Presence of gross hematuria is related to better survival, possibly because that a precursor symptom leads to immediate diagnosis and treatment, as delayed treatments are usually accompanied by worsened outcome in bladder cancer patients requiring radical cystectomy36. Nonetheless, there are also reports claiming that time to surgery in UTUC does not affect outcome and prognosis37. We assume that presence of hematuria may represent a very early stage of the tumor in which time to surgery plays a critical role. Next, we have found that tumors located in renal pelvis conferred better prognosis than ureteral tumors, which has also been validated in a series of studies14. Also, pathological staging has been proven to be an independent prognostic factor in our study, a result that is substantially concrete14. As aforementioned, the prognostic value of p53 remains controversial38,39. In our series, however, p53 shows a statistically significant association with worsened survival. Nonetheless, tumors with medium p53 score (score 1, Figure 2D) confer the worst prognosis, which does not seem to be biologically reasonable and leaves an important predicament that warrants further studies.
We have also investigated the metachronous BCa following RNU in the current study. As local recurrence is rare in RNU, the recurrence rate within bladder is not considered as distant metastasis and ranges from 22% to 47%40,41. Therefore, stringent follow-ups for bladder recurrence are mandatory in all UTUC patients. In our cohort, the recurrence rate reached a little bit lower than reports. However, partially consistent with previous studies that tumor grade is associated with bladder cancer, we have not observed an association between tumor location and bladder recurrence42. Some scholars indicate that previous history BCa is the only independent predicative factor for bladder recurrence41. Interestingly, we have found that ipsilateral non-functioning kidney at diagnosis conferred to higher recurrence rate after adjustment to various tumor and clinical parameters. We assume that renal functioning is usually compromised by ureteral carcinoma, which is per se more aggressive than tumors located in renal pelvis or calyces. Moreover, the endogenous subtype of UTUC contributes to a higher odds of seeding in the bladder.
Last but not least, our study has limitations. The sample size, though relatively larger amongst all literature, is still small, limiting the ability to distinguish outcomes further stratified. Further, we lack the nodal and LVI information of the tumor, which are critical prognosticators. More importantly, the retrospective nature of our study renders some of the results biased. For instance, over the long period of time span, the inequity of treatment regimes may bias the outcome. Furthermore, some of our statistically significant results remain hard to explain or interpret. This brings about a broader dispute that whether a P value of <0.05 really confers watershed biological significance. Therefore in our study, we aim to make use of the most commonly used parameters to tease out additional predictive merits so that we may individualize better follow-up and treatment regimes for specific UTUC patients.
Methods
General information
A total of 88 patients with UTUCs who underwent surgery between Dec 2005 and Jan 2013 at Huashan Hospital were included in this study. All patients were confirmed with urothelial malignancy pathologically. All specimens were acquired from radical neprhoureterectomy (RNU). All sections were reviewed independently by two pathologists without knowledge of patient profile. The tumors were graded in accordance with the World Health Organization (WHO)/International Society of Urological Pathology (ISUP) (2004)21. Tumor stage was determined according to the Union for International Cancer Control (UICC) 2009 TNM classification for urothelial cell carcinoma of the upper urinary tract. Nodal and metastatic profiles were not included in this study due to incomplete clinical history22. As for heterogeneity conditions, lesions that involved two grades or stages were classified according to the higher ones identified in the specimen19. Tumor location was defined as either renal pelvic or ureteral23. Tumor multifocality was defined as the synchronous presence of two or more pathologically confirmed tumors in any location of upper urinary tract24. Ipsilateral non-functioning kidney was defined as unilateral kidney glomerular filtration rate (GFR) less than 10 ml/min, which was calculated by SPECT/CT, based on 99mTc-DTPA detection.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue was cut into 4 μm sections and mounted on polylysine-coated glass slides. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 15 min. Before further processing, heat-mediated antigen retrieval was performed by boiling the slides in 0.01 M citrate buffer, pH 6.0, for 20 min in microwave oven. The primary antibodies were diluted, including anti-p53 (clone1B10, Novocastra, Newcastle, UK) at 1:50 and anti-Ki67 (clone MM1, Novocastra, Newcastle, UK) at 1:100. The slides were stained immunohistochemically using the avidin-biotin-complex method for both antibodies. Finally, the slides were dehydrated through graded alcohols to xylene and mounted in mounting medium. For positive controls, we used colon carcinoma for Ki67 and p53. For negative controls, we omitted all primary antibodies.
Assessment of Ki67 and TP53 staining and scoring
Staining and scoring protocols for p53 and Ki67 were previously described (Figure 1)19,25,26. The percentage of Ki67 positive cells was visually counted and the Ki67 value was scored by labeling index. For p53, a semi-quantitative method was used. Each slide was given a value composed of the sum of staining intensity and extensity. The value was scored semi-quantitatively as: 0 for negative, 1 for mild, 2 for moderate and 3 for strong.
Treatment of UTUC and patients follow-up
There were 55 patients with UTUC undergoing open RNU with bladder cuff excision. Twenty-five patients underwent laparoscopic RNU and bladder cuff excision. There were 2 patients undergoing transurethral resection of bladder tumor (TURBt) preoperatively due to concomitant non-invasive bladder cancer (NMIBC). Three patients underwent RNU and radical cystectomy synchronously because of the pathologically confirmed concomitant muscle invasive bladder cancer (MIBC). Adjuvant cisplatin-based systemic chemotherapy was routinely administrated in patients with pT3 or pT4 diseases postoperatively. However, 31.0% of patients had to cease systemic chemotherapy due to poor tolerability. Fifty-five patients (64.71%) received prophylactic intravesical instillation postoperatively with pirarubicin post-operatively. The prophylactic instillation was according to the following regime: weekly administration for 8 weeks, followed by monthly administration for 6 to 12 months.
Patient follow-up after RNU mainly consisted of routine physical examinations, medical imaging examinations and cystoscopy, etc. Cystoscopy was performed in bladder-preserved patients every 3 months during the first year, every 6 months during the second year and once a year from the third year onwards. Medical imaging examinations, which included ultrasonography examinations, enhanced computerized tomography scans (CT) or positron emission tomography/computed tomography scans (PET/CT) were performed in all patients every 6 months until 5 years post-operatively and annually thereafter. All patients were followed retrospectively both through hospital records and by telephone interviews, either to the patients or their close relatives. Informed consents were obtained from all patients and the study was approved by the Huashan institutional review board (HIRB).
Statistical analysis
The SPSS 17.0 for Windows was used for statistical analyses. All data were presented as mean ± standard deviation (SD). The Student's t-test was applied to compare scores of Ki67 and p53 between 2 groups, whilst analysis of variance (ANOVA) was used for comparisons in more than 2 groups. Multivariate Cox proportional regression analysis was performed to determine the independent contribution of clinicopathological factors to cancer specific survival (CSS) and intravesical recurrence-free rates. The end-point variables of interest were cancer specific deaths and intravesical tumor recurrences, respectively. These hazards were estimated with their 95% confidence interval. P value of <0.05 was considered statistically significant.
References
Munoz, J. J. & Ellison, L. M. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol 164, 1523–5 (2000).
Ploeg, M., Aben, K. K. & Kiemeney, L. A. The present and future burden of urinary bladder cancer in the world. World J Urol 27, 289–93 (2009).
Roupret, M. et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol 59, 584–94 (2011).
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2012. CA Cancer J Clin 62, 10–29 (2012).
Babjuk, M. et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 59, 997–1008 (2011).
Margulis, V. et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 115, 1224–33 (2009).
Abouassaly, R. et al. Troubling outcomes from population-level analysis of surgery for upper tract urothelial carcinoma. Urology 76, 895–901 (2010).
Jeldres, C. et al. A population-based assessment of perioperative mortality after nephroureterectomy for upper-tract urothelial carcinoma. Urology 75, 315–20 (2010).
Brien, J. C. et al. Preoperative hydronephrosis, ureteroscopic biopsy grade and urinary cytology can improve prediction of advanced upper tract urothelial carcinoma. J Urol 184, 69–73 (2010).
Eltz, S., Comperat, E., Cussenot, O. & Roupret, M. Molecular and histological markers in urothelial carcinomas of the upper urinary tract. BJU Int 102, 532–5 (2008).
Comperat, E. et al. Prognostic value of MET, RON and histoprognostic factors for urothelial carcinoma in the upper urinary tract. J Urol 179, 868–72; discussion 872 (2008).
Scarpini, S. et al. Impact of the expression of Aurora-A, p53 and MIB-1 on the prognosis of urothelial carcinomas of the upper urinary tract. Urol Oncol 30, 182–7 (2012).
Kosaka, T. et al. Expression of snail in upper urinary tract urothelial carcinoma: prognostic significance and implications for tumor invasion. Clin Cancer Res 16, 5814–23 (2010).
Roupret, M. et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol 63, 1059–71 (2013).
Vet, J. A. et al. p53 mutations have no additional prognostic value over stage in bladder cancer. Br J Cancer 70, 496–500 (1994).
Erill, N. et al. Genetic and immunophenotype analyses of TP53 in bladder cancer: TP53 alterations are associated with tumor progression. Diagn Mol Pathol 13, 217–23 (2004).
Yurakh, A. O. et al. Molecular and immunohistochemical analysis of the prognostic value of cell-cycle regulators in urothelial neoplasms of the bladder. Eur Urol 50, 506–15; discussion 515 (2006).
Wang, L. et al. Ki67 and TP53 expressions predict recurrence of non-muscle-invasive bladder cancer. Tumour Biol. [Epub] 10.1007/s13277-013-1384-9 (2013).
Wang, L. et al. Relationship of TP53 and Ki67 expression in bladder cancer under WHO 2004 classification. J BUON 18, 420–4 (2013).
Krabbe, L. M. et al. Prospective comparison of molecular signatures in urothelial cancer of the bladder and the upper urinary tract: is there evidence for discordant biology? J Urol. [Epub] 10.1016/j.juro.2013.09.031 (2013).
Sauter, G. et al. [Tumours of the urinary system: non-invasive urothelial neoplasias]. WHO classification of classification of tumours of the urinary system and male genital organs [Eble, J. N., Sauter, G., Epstein, J. l., Sesterhenn, I. (eds)]. (IARCC Press, Lyon, 2004).
Sobin, L. et al. [Renal Pelvis and ureter]. TNM classification of malignant tumors. Urological tumors. International Union Against Cancer. (Wiley-Blackwell, New York, 2009).
Ouzzane, A. et al. Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy. Eur Urol 60, 1258–65 (2011).
Chromecki, T. F. et al. The impact of tumor multifocality on outcomes in patients treated with radical nephroureterectomy. Eur Urol 61, 245–53 (2012).
Feng, C.-c. et al. Pigment epithelium-derived factor expression is down-regulated in bladder tumors and correlates with vascular endothelial growth factor and matrix metalloproteinase-9. International urology and nephrology 43, 383–390 (2011).
Feng, C. C. et al. [Urinary BLCA-4 level is useful to detect upper urinary tract urothelial cell carcinoma]. Actas Urol Esp 36, 597–602 (2012).
Cho, D. S., Hong, S. Y., Kim, Y. K., Kim, S. I. & Kim, S. J. Prognostic factors in transitional cell carcinoma of the upper urinary tract after radical nephroureterectomy. Korean J Urol 52, 310–6 (2011).
Jeldres, C. et al. Highly predictive survival nomogram after upper urinary tract urothelial carcinoma. Cancer 116, 3774–84 (2010).
Margulis, V. et al. Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract. J Urol 184, 453–8 (2010).
Verhoest, G. et al. Predictive factors of recurrence and survival of upper tract urothelial carcinomas. World J Urol 29, 495–501 (2011).
Kikuchi, E. et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol 27, 612–8 (2009).
Bagrodia, A. et al. Prospective evaluation of molecular markers for the staging and prognosis of upper tract urothelial carcinoma. Eur Urol 62, e27–9 (2012).
Rey, A., Lara, P. C., Redondo, E., Valdes, E. & Apolinario, R. KI67 proliferation index in tumors of the upper urinary tract as related to established prognostic factors and long-term survival. Arch Esp Urol 51, 204–10 (1998).
Kojima, K., Naruo, S., Kanayama, H. & Kagawa, S. [Evaluation of Ki67 antigen using MIB1 antibody as a prognostic factor in renal pelvic and ureteral cancer]. Nihon Hinyokika Gakkai Zasshi 87, 822–30 (1996).
Kamijima, S. et al. The prognostic value of p53, Ki-67 and matrix metalloproteinases MMP-2 and MMP-9 in transitional cell carcinoma of the renal pelvis and ureter. Int J Urol 12, 941–7 (2005).
Sanchez-Ortiz, R. F. et al. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol 169, 110–5; discussion 115 (2003).
Sundi, D. et al. Upper tract urothelial carcinoma: impact of time to surgery. Urol Oncol 30, 266–72 (2012).
Ku, J. H. et al. The Role of p53 on Survival of Upper Urinary Tract Urothelial Carcinoma: A Systematic Review and Meta-Analysis. Clin Genitourin Cancer 11, 221–8 (2013).
Hashimoto, H., Sue, Y., Saga, Y., Tokumitsu, M. & Yachiku, S. Roles of p53 and MDM2 in tumor proliferation and determination of the prognosis of transitional cell carcinoma of the renal pelvis and ureter. Int J Urol 7, 457–63 (2000).
Xylinas, E. et al. Multifocal carcinoma in situ of the upper tract is associated with high risk of bladder cancer recurrence. Eur Urol 61, 1069–70 (2012).
Novara, G. et al. Independent predictors of metachronous bladder transitional cell carcinoma (TCC) after nephroureterectomy for TCC of the upper urinary tract. BJU Int 101, 1368–74 (2008).
Zigeuner, R. E., Hutterer, G., Chromecki, T., Rehak, P. & Langner, C. Bladder tumour development after urothelial carcinoma of the upper urinary tract is related to primary tumour location. BJU Int 98, 1181–6 (2006).
Author information
Authors and Affiliations
Contributions
C.F., L.W., G.D. and Z.W. wrote the manuscript. C.F., L.W. and G.D. analyzed the data. Z.Z., Q.D. and H.J. prepared all figures. Q.D. and H.J. edited all tables, C.F., L.W. and Z.W. designed the study. All authors reviewed and approved the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Feng, C., Wang, L., Ding, G. et al. Predictive Value of Clinicopathological Markers for the Metachronous Bladder Cancer and Prognosis of Upper Tract Urothelial Carcinoma. Sci Rep 4, 4015 (2014). https://doi.org/10.1038/srep04015
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04015
This article is cited by
-
Prognostic value of Ki67 and p63 expressions in bladder cancer patients who underwent radical cystectomy
International Urology and Nephrology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.