Abstract
Human mesenchymal stem cells (hMSC) can home to tumor sites and promote tumor growth. The effects of hMSC on tumor growth are controversial and involvement of hMSC in tumor immunology has not been adequately addressed. Therefore, we investigated whether injection of hMSC affects tumor appearance, growth and metastasis and anti-tumor immunity in an experimental animal model of metastatic breast cancer. Injection of hMSC in BALB/c mice bearing mammary carcinoma promoted tumor growth and metastasis, which was accompanied by lower cytotoxic activity of splenocytes, NK cells and CD8+ T cells in vitro. Tumor-bearing mice that received hMSC had significantly lower percentages of CD3+NKp46+ NKT-like, higher percentages of CD4+Foxp3+ T cells, increased serum levels of Th2 and decreased serum levels of Th1 cytokines and significantly higher number of CD4+ cells expressing IL-10. These results demonstrate that immunosuppressive environment created by hMSC promoted breast tumor growth and metastasis in mice.
Similar content being viewed by others
Introduction
Mesenchymal stem cells (MSC) have been identified in the bone marrow, connective tissue and peripheral blood as multipotent cells that proliferate in vitro as plastic adherent cells. These cells have the capacity to differentiate into fibroblast, osteoblast and adipocyte lineages1,2. It has been demonstrated that human MSC, (hMSC) maintained under standard culture conditions are non-tumorigenic per se: however, several reports indicate their capability to modulate tumor microenvironment thus having an impact on the tumor behaviour3,4. Lin et al.5 demonstrated that MSC have exerted immune protection, and, in case of breast cancer, they can support cancer growth6. Also MSC can contribute to the tumor growth and progression through several mechanisms including their immunomodulatory effects. These effects depend on their basic characteristics, time and method of administration in relation to pathological conditions7,8. As far as tumor modulation is concerned, MSC can have both, stimulatory9 and suppressive effects10. Most specifically, in an experimental model of breast cancer it has been shown that MSC may have both of the above effects. The intravenous administration of MSC appears to effect tumor growth indirectly by modulating the immune microenvironment before they act by direct interaction with tumor cells. It has been shown that MSC overexpressed IFN-β by affecting STAT3 signaling pathway, however, “normal“ MSC stimulated rather than suppresed 4T1 breast cancer cells growth11.
MSC are generally thought to have immunosuppressive effects, which may be an important mechanism through which MSC promote tumor growth or increase incidence of tumor formation also under in vivo conditions They can impair the function of a variety of immune cells directly or by secreting different growth factors possibly by inhibition of both, innate and adaptive immune cells12,13. However, the immunomodulatory effects of MSC, if any, are not well understood within tumors. Djouad et al.14 reported that the immunosuppressive action of MSC led to a higher incidence of melanoma formation in a mouse model. Evidence for the effect of MSC on tumor immunity also comes from a clinical study15 which could be explained by the creation of immunosuppressive milieu including shifting of immune responses from Th1 to Th216 or induction of generation and proliferation of regulatory T cells17. However, regulatory T cells (Tregs) are mostly immunosuppressive and have been shown to suppress effector T cell functions and autoimmunity18,19. They suppress T-cell responses by acting directly or through the inhibition of antigen-presenting cells involving cell-to-cell contact, FasL/Fas and PD1/B7-H1-dependent processes, or secreted factors such as IL-10, TGF-beta, IL-27 and IL-3520,21,22. As a result, Tregs are considered to play a key role in the escape of cancer cells from antitumor effector T cells23,24. Indeed, suppressing Treg has been shown to be effective in enhancing the immune response of host cells to cancer cells25,26. Although, the role and function of T regulatory cells and induction of immunosuppression is well accepted, there is still no conceivable data to explain the factors and mediators which support the generation of T regulatory cells during the process of malignancy. Olkhanud et al.27 have reported that expansion of Tregs was induced without the involvement of MSC showing that FoxP3+Treg conversion from non-regulatory CD4+ T cells (non-Tregs) is mediated by a unique subset of regulatory B cells, designated tumor-evoked Bregs. On the other hand, Selman et al.17 demonstrated that MSC induce the generation and proliferation of regulatory T cells through the stimulation of IL-10 production and the release of HLA-G5. Also Patel et al.28 have recently reported that hMSC under in vitro conditions can support breast cancer cells through TGF-b1 production and Treg augmentation.
The aim of our study was to understand the mechanisms stimulating tumor growth by the intravenous administration of hMSC population cells derived from human peripheral blood. Here we provide evidence that injection of heterogenous population of hMSC may profundly afect mammary tumor growth by stimulating hosts regulatory T cells and producing immunosupressive cytokines.
Results
hMSC migrated in tumor and promoted breast tumor growth and metastasis in dose dependent manner
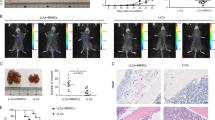
To follow the biodistribution of hMSC, we monitored the engraftment of hMSC by polymerase chain reaction (PCR). Human CYP1A1 gene, which does not show cross-reactivity to mouse DNA, was detected by PCR analysis in tumor, blood, lymph node, spleen, liver and lung samples at 1st and 3rd day of the experiment, which suggested that hMSC had potential to migrate to various murine tissues (Fig. 1a). To explore whether the transplanted hMSC present in tissues of mice at 35th day of the experiment, when the mice were sacrificed, we used an anti-human mitochondria antibody. As shown in Fig. 1c, hMSC could retain for a long period of time in the liver of mice. On the other hand, we did not observe the presence of hMSC in lung tissue (Fig. 1d).
hMSC migrated in tumor and promoted breast tumor growth and metastasis.
(a) Representative samples of PCR analysis showing migration and survival of hMSC. Human CYP1A1 gene, without cross-reactivity with mouse DNA, was detected by PCR analysis in tumor, blood, lymph node, spleen, liver and lung samples at 1st and 3rd day of experiment. Photomicrographs showing the presence human mitochondrial marker in the tissues (b, c). Positive signals were detected in human ESC control group (b) and in the livers of tumor-bearing mice that received hMSC (c), but no compelling evidence of positively stained cells in lung mice from the same group (d). (e) Impact of 4T1: hMSC ratio on tumor growth. All animals received 2 × 104 4T1 cells. The highest incidence of tumor growth (e) and the largest tumor volume (f–g) was seen in tumor-bearing mice that received 1 × 106 hMSC. There is a strong correlation between the number of injected hMSC and tumor volume (Fig. 1h; R2 = 0.865). (i) Semi-quantitative analysis of lung and liver tissue sections. The incidence of lung and liver metastasis was significantly higher in tumor-bearing mice that received hMSC. Combined results of 3 experiments with total of 9 animals per group (mean ± s.e.m.; *P < 0.05).
The 4T1:hMSC ratio had an impact on the incidence of tumor growth (Figs. 1e–h). The highest incidence of tumor growth (Fig. 1e) and the largest tumor volume (Figs. 1f–g) was seen in animals that received 1 × 106 hMSC. Furthermore, as shown in Fig. 1h, linear regression analysis showed a strong correlation (R2 = 0.865) between the number of injected hMSC and tumor volume (Fig. 1g) suggesting that number of injected hMSC in a dose-dependent manner affected tumor growth. It appears that initial immunosuppresive effect of hMSC facilitate the tumor growth increasing tumor load and capacity to metastase.
Semi-quantitative analysis of lung and liver tissue sections showed that the incidence of lung and liver metastasis was significantly (P < 0.05) higher in tumor-bearing mice that received hMSC (Fig. 1i) confirming that hMSC injection promoted both, tumor growth and metastasis.
Injection of hMSC decreased cytotoxic capacity of CD8+ T lymphocytes and NK cells in tumor-bearing mice
There was a significant increase (P < 0.05) in total number of splenocytes in tumor bearing mice compared to mice from control group, but cytotoxic capacity of these cells was significantly lower (P < 0.05) than cytotoxic capacity of splenocytes derived from healthy animals (Fig. 2a).
hMSC decreased cytotoxic capacity of CD8+ T lymphocytes and NK cells in tumor-bearing mice.
(a) There was a significant increase in total number of splenocytes in tumor-bearing mice compared to mice from control group. The cytotoxic capacity of splenocytes derived from tumor-bearing mice was significantly lower than cytotoxic capacity of splenocytes derived from healthy animals. Combined results of 3 experiments with at least 9 animals per group (median ± s.e.m; *P < 0.05). (b) There was no significant difference in total number of CD8+ T lymphocytes in tumor-bearing mice compared to mice from control group. There was significant decrease in cytotoxic capacity of CD8+ T cells derived from spleens of tumor-bearing hMSC treated mice. Combined results of 3 experiments with at least 9 animals per group (median ± s.e.m.; *P < 0.05). (c) There was no significant difference in total number of CD3−NKp46+ NK cells in tumor-bearing mice compared to mice from control group. There was significant decrease in cytotoxic capacity of CD3−NKp46+ NK cells derived from spleens of tumor-bearing hMSC treated mice. Combined results of 3 experiments with at least 9 animals per group (median ± s.e.m.; *P < 0.05).
The total number of CD8+ T lymphocytes (Fig. 2b) and NK cells in the spleen (Fig. 2c) was not decreased after injection of hMSC (representative dotplots that showed percentage of positive cells expressed as mean ± SEM, are provided in the supplemental Figure S1) but there was a significant decrease in cytotoxic capacity of both, CD8+ T cells and CD3−NKp46+ NK cells derived from the spleens of tumor-bearing mice treated with hMSC (P < 0.05 for CD8+ cells and P < 0.01 for NK cells).
The impact of hMSC on cellular make up of the spleen in tumor-bearing mice
There was a significant decrease (P < 0.05) in the percentage of CD3+NKp46+ NKT-like cells (Fig. 3a) in spleens isolated from tumor-bearing hMSC-tretaed animals. Furthermore, hMSC injection significantly (P < 0.05) decreased number of CD3+NKp46+ NKT like cells in the spleen of tumor-bearing animals (Fig. 3b).
hMSC downregulated NKT-like cells and increase number of T regulatory cells.
(a) There was significant decrease in percentage and total number (b) of CD3+NKp46+ NKT-like cells in spleens of tumor-bearing mice that received hMSC. Isotype controls (c) and representative dotplots (d) confirm significant decrease in percentage of NKT cells in spleens of hMSC treated mice. Combined results of 4 experiments with at least 9 animals per group (mean ± s.e.m.; *P < 0.05). (e) There was significant increase in both percentage and total number (f) of Foxp3+ regulatory cells cells in spleens of tumor-bearing mice that received hMSC. Representative histogram (g) confirms significant increase in percentage of Foxp3+ T regulatory cells in spleens of hMSC treated mice. Histograms and mean values presented in the graphs are gated on CD4+ cells. Combined results of 4 experiments with at least 9 animals per group (mean ± s.e.m.; *P < 0.05).
Also, cellular make-up of the spleen showed that there was a significant difference in regulatory T cells. Both, the percentage and total number of CD4+Foxp3+ T regulatory cells (Figs. 3e–f) were significantly higher (P < 0.05) in spleens of tumor-bearing and hMSC-treated animals which suggests that injection of hMSC stimulated proliferation of T regulatory cells in tumor-bearing mice.
Injection of hMSC stimulated production of TGF-beta, IL-4 and IL-10 but inhibited production of IFN-γ in tumor bearing mice
Tumor bearing mice expressed levels of IL-4 that were six times higher than healthy control mice or mice inoculated with hMSC cells alone. When hMSC were injected into tumor-bearing mice the level of IL-4 was even higher reaching levels that were 10-fold greater than that of healthy control mice (P < 0.05). Tumor bearing mice injected with hMSC uniquely expressed high levels of IL-10 that were nearly eight times greater those found in healthy control mice (P < 0.01). Tumour bearing mice injected with hMSC expressed the highest levels of TGF-ß which were nearly 4-fold higher than healthy control mice, also significantly greater than the levels observed in tumor-bearing mice or mice injected with hMSC cells alone (P < 0.01). The high levels of IFN-gamma observed in tumor-bearing mice (P < 0.05), nearly five times higher than seen in healthy mice or those injected with hMSC alone, were halved in tumor-bearing mice injected with hMSC (Fig. 4a).
hMSC stimulated production of Th2 and inhibited production of Th1 cytokines in tumor-bearing mice.
(a) Measurement of cytokines in serum. The level of TGF-β, IL-10 and IL-4 was significantly lower and level of IFN-γ was significantly higher in serum samples of tumor-bearing animals that received hMSC compared to tumor-bearing animals that were not treated with hMSC. Combined results of 4 experiments with at least 9 animals per group (mean ± s.e.m.; *P < 0.05; **P < 0.01). (b) Measurement of intracellular production of cytokines. There was significant difference in total number of IL-10 producing CD4+ splenocytes between hMSC treated and control tumor-bearing or healthy animals but no difference between groups in the total number of IFN-γ producing cells. The ratio between IL-10 and IFN-γ producing CD4+ splenocytes was significantly higher in tumor-bearing animals that received hMSC. Combined results of 4 experiments with 4 animals per group (median ± s.e.m.; *P < 0.05; **P < 0.01).
The CD4+ IL-10+ cells were twice as abundant in tumor bearing mice injected with hMSC than in mice injected with either cell type alone (P < 0.05). The CD4+ IFN-gamma+ cell population levels were not significantly changed either by the presence of a tumor or by injection of hPB-MSC cells (P > 0.05), the ratio between IL-10 and IFN-γ producing CD4+ splenocytes was significantly higher (P < 0.05) in tumor-bearing animals that received hMSC (Fig. 4b).
Discussion
It is known that MSC contribute to the maintenance and regeneration of connective tissues29 and after systematic infusion, MSC appear to engraft; this however seem to be dependent on the secretion of paracrine signals in the tissues microenvironment which are not as yet understood30. Solid tumor growth and invasion create a microenvironment similar to that described in wound healing, which also induces MSC to specifically migrate to the wound (or site of inflammation)31,32. The evidence suggests that solid tumors generate a wound-like environment on their periphery as they apply physical and chemical stress to neighboring tissues. Tumors can therefore be regarded as sites of tissue damage or, according to Dvorak, ‘‘wounds that never heal’’31. Therefore, in the present study we examined whether intravenously injection hMSC affects tumor growth in experimental murine model of mammary carcinoma. With regard of MSC ability to migrate, it does not seem that there is a specificity in terms of organ homing as we were able to detect hMSC in tumor, blood, lymph node, spleen, liver and lung samples at 1st and 3rd days of experiment, suggesting the exquisite selectivity of hMSC for tumors. This corroborates with previous reports20,33,34 which have shown that injected MSC engraft into tumor sites and can be used as effective gene delivery vehicles. Also, similar to others, we demonstrated that hMSC may be present for a long period of time after administration35. In the present study, hMSC were persent at 35th day of the experiment in the liver but not in the lung of mice. This finding is similar to previous reports which showed abbreviation of MSC residence in the lung in diferent disease models, accompanied with their persistence in the liver and spleen for long time after administration34,36. After evaluating hMSC tropism for tumor microenvironments, we investigated the impact of hMSC on tumor growth and metastasis. The reported effects of MSC on progression of primary tumors have been both pro- and anti-tumorigenic, but variability in these results, including ours, could be attributable to differences in the sources of the MSC and the type of tumor model used for analysis7,31,37. We demonstrated that injection of hMSC promotes breast tumor growth which was confirmed by the strong correlation between the number of injected hMSC and tumor volume. In addition, hMSC promoted formation metastasis of tumors. By day 35, the incidence of lung and liver metastasis was significantly higher in tumor-bearing and hMSC-treated animals. This is similar to Karnoub et al.32 who reported that MSC treatment can induce a transient prometastatic effect on breast cancer cells but do not corroborate other studies which reported that MSC suppress tumor growth37 and metastasis38. However, MSC-mediated inhibitory effects have only been observed in a few models including Kaposi sarcoma37 and colon carcinoma39. Interestingly, systemic administration of mouse MSC had no effect growth 4T1 tumor40. Pro-tumorigenic effects of MSC described in our study are consistent with other studies14,41,42 and this phenomenon could be explained by the role of direct cell-cell contact and/or by the effects of soluble factors in MSC-mediated stimulation on tumor cell proliferation. Therefore, to investigate potential mechanisms of tumor promotion by MSC, we analyzed the cytotoxic capacity of splenocytes and found that these cells isolated from tumor-bearing and hMSC-treated animals showed decreased cytotoxic activity when compared with cytotoxic activity of splenocytes cells obtained from non-treated tumor-bearing mice. This could be explained by the fact that MSC can directly impair the function of a variety of immune cells, including B and T lymphocytes, dendritic cells and natural killer cells43,44,45,46. In order, to dissect which cell population is responsible for this lower cytotoxicity, we tested CD8+T and NK cytotoxic cells activity in vitro and found a significant decrease in cytotoxic capacity of both cells types in tumor-bearing hMSC-treated animals. NK-cell function is controlled by a variety of mechanisms, some of which are used by MSC to mediate NK-cell inhibition47. With respect to soluble factors, in vitro studies have shown that MSC, without or after stimulation, secrete a wide range of regulating molecules48, including IL-15, TGF-β1 and PGE2 and have the potential to affect NK-cell cytotoxicity and cytokine production49. Also, MSC have a profound inhibitory effect on activation of T cells, which affects both naive and memory T cells and is manifested in antigen-specific proliferation, IFN-γ production and cytotoxic activity43. Therefore, our findings are consistent with other studies43,44 demonstrating that MSC treatment mediates T-cell inhibition, suppression of NK-cell proliferation, cytokine secretion and cytotoxicity.
MSC were shown to exert immune protection and may affect anti-tumor immunity and in case of breast cancer, MSC can support cancer growth5. NKT cells play an important role in anti-tumor immunity. The anti-tumor potential of NKT cells has been demonstrated in numerous models of cancer50 and a selective decrease of number of NKT cells and/or functional activity has been reported in patients with diverse types of cancer51,52. A recent study demonstrated that low levels of circulating NKT cells predict a poor clinical outcome in patients with head and neck squamous cell carcinoma53. In contrary another study showed that MSC confer immune protection of cancer cells trough the generation of FoxP3+ Tregs28. In this regard, we estimated percentage and number of CD3+NKp46+ NKT-like cells and CD4+Foxp3+ T regulatory cells by multicolor cytometry. Tumor-bearing animals that received hMSC have a significantly lower percentage and number of CD3+NKp46+ NKT-like cells but CD4+Foxp3+ T regulatory cells were more numerous in tumor-bearing hMSC-treated animals. Studies suggest that Tregs play a role in the MSC-mediated effects on components of the immune system that normally serve to eliminate cancer cells28,54. The role of Tregs in MSC-induced immunomodulation is controversial16,43. We hypothesize that, despite the short time of hMSC engraftment into tumor, blood, lymph node, spleen and lung samples, but long in the liver, these cells are capable of initiating an active immunomodulating process by interacting with the resident T cell population favoring the generation of CD4+Foxp3+ T regulatory cells similar to that MSC protect breast cancer cells promoting graft tolerance by increased level of Tregs55.
The mechanism of immunosuppression, especialy cytokine involment, is critical to further understanding of the findings of our study. Therefore we quantitated serum levels of IL-4, IL-10, TGF-β and IFN-γ. It is known that MSC are a major source of TGF-β and its immunosuppressive properties generate polarization away from a Th1 phenotype toward to Th2 phenotype28. The observed increase in levels of IL-4, IL-10, TGF-β and decreased level of IFN-γ favors the argument for aberrant activation of the Th2 response. Additionally, we found a clear difference in the number of IL-10 producing CD4+ cells in spleens of hMSC-treated animals mice when compared to tumor-bearing or control group which was not accompanied by different number of IFN-γ producing CD4+ splenocytes cells. Interestingly, when we calculated ratio of IL-10 vs. IFN-γ in individual animals and compared tumor-bearing animals that received hMSC and tumor-bearing or control animals, the ratios were higher in tumor-bearing animals that received hMSC. Recently, Batten et al.56 demonstrated that co-culture of T cells with MSC completely change the cytokine profile of T cells decreasing production of IFN-γ, IL-1α and TNFα increasing Th2 cytokines such as IL-10, IL-5 and IL-13 confirming the fact that MSC create an immunosuppressive environment that promotes tumor growth.
In conclusion, the findings presented herein indicate that hMSC stimulated sufficient production of Th2 cytokines promoting the development of both primary and metastatic tumors by increased CD4+Foxp3+ Tregs cells. We demonstrated that hMSC treatment could play very important roles in tumor progression and therefore further investigations are needed to explore the long-term biological effects of hMSC for the breast cancer patients.
Methods
Experimental animals
Female BALB/c mice (obtained from the Military Medical Academy Belgrade, Serbia) 8–10 weeks old, were used for this study. All of the animal procedures were subjected to review and approval by the Ethics Committee of Medical Faculty, Kragujevac which also complies with the National Institutes of Health (NIH) guidelines for humane treatment of laboratory animals.
Cell lines and cell culture techniques
The mouse breast tumour cells line 4T1 was purchased from American Type Culture Collection (ATCC, Manassas, VA). Briefly, the cells were cultured in 25 mL tissues culture flasks (BD Falcon) in high glucose DMEM (Sigma, UK) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma, UK) at 37°C in humidified atmosphere containing 5% CO2.
Isolation and expansion of human mesenchymal stem cells (hMSC)
Human MSC were isolated from mononuclear peripheral blood cells using the methodology described by Trivanovic et al.57 and under in vitro conditions showing a multipotent capacity to differentiate along mesenchymal lineages (osteogenic, chondrogenic, adipogenic and myogenic). In addition, they were fully characterized and displaying a normal karyotype. hMSC were cultured in DMEM supplemented by 10% FBS, a-MEM and antibiotics (penicillin 100 U/mL, streptomycin 100 μg/mL; Hyclone, Logan, UT) at 37°C in a humidified atmosphere containing 95% air and 5% CO2. The culture medium was changed every 3 days.
Tumour challenge and tumor volume
Mice were divided into four groups. Two groups of BALB/c mice were injected with 50 μl of a single-cell suspension containing 2 × 104 4T1 mammary carcinoma cells in 50 μl of PBS, orthotopically into the fourth mammary fat-pad of mice (direct injection in the same flank on each mouse). One of these groups was transplanted with 4 × 105 hMSC in 100 μl of PBS intravenously by tail vein injection on the same day (4T1 + hMSC group). Group 3 received 4 × 105 hMSC in 100 μl of PBS intravenously by tail vein injection only (hMSC group); and group 4 was used as a control (no any treatment). Tumor volumes were calculated according to the following formula: tumor volume (mm3) = L × W2/2, where L represents the major axis (largest cross sectional diameter) of the tumor while W represents minor axis58. The data were presented as mean ± standard error of mean (mean ± s.e.m.).
Detection of engrafted human MSC: DNA extraction and polymerase chain reaction (PCR) analysis
The biological samples were submitted to DNA extraction and PCR analysis to detect the presence of human genes in mice recipients. Genomic DNA for PCR analysis was prepared from tissues (peripheral blood, lymph node, spleen, liver, lung and tumor) using the KAPA Express Extract (Kapabiosystems, Boston, Massachusetts, USA). Amplifications were performed using the KAPA2G Robust HotStart ReadyMix (Kapabiosystems). Amplification of human CYP1A1 gene was used for detection of human DNA in each sample of mouse tissue after DNA extraction. Endogenous mouse β-actin gene was also amplified, as an internal control. The primers for human CYP1A1 gene were: forward primer 5' GAA CTG CCA CTT CAG CTG TCT 3' and reverse primer: 5' CAG CTG CAT TTG GAA GTG CTC 3'. The primers for β-actin gene were: sense primer 5' GGACCTGACAGACTACC 3' and antisense primer 3'GGCATAGAGGTCTTTACGG. Also, human DNA was isolated from MSC culture and used as positive controls.
Immunocytochemistry
Immunocytochemistry was performed to explore the presence of hMSC in the liver and lung of mice with intravenously administered hMSC. The tissues were routinely fixed in 4% buffered formaldehyde, dehydrated through graded alcohols, cleared in xylene and subsequently embedded in paraffin. The paraffin-embedded tissue samples were sectioned at 4–5 μm, then deparaffinized by two 10 min washes in xylene and rehydrated in a series of 100%, 96%, 70% and 50% alcohol. Immunohistochemical staining was performed by the streptavidin-biotin method. Briefly, sections were deparaffinized and incubated with 3% hydrogen peroxide to block endogenous peroxidase activity. The sections were microwaved for 20 min in 10 mmol/L sodium citrate (pH 6.0) and incubated with mouse monoclonal IgG1 antibody clone 113-1 targeting a 65 kD protein on the surface of intact human mitochondria (MAB-12730, Millipore) 60 minutes, respectively. After the primary antibody, biotinylated secondary antibodies were applied, followed by detection using the ABC (Avidin-Biotin peroxidase Complex) method. Diaminobenzidine was used as the chromogen. Light counterstaining was performed with haematoxylin. Negative controls were obtained using an irrelevant antibody instead of the primary antibody. The slides were examined by conventional light microscopy.
Histopathological analysis
Paraffin-embedded mouse extracted organs sections (sectioned into 4 μm slides ) were prepared by a routine procedure. The sections were stained with hematoxylin-eosin (HE) by standard protocol.
The fixed lungs and livers were examined under low-power (x100) light microscopy (Zeiss Axioskop 40, Jena, Germany) equipped with digital camera. Images were captured in a computer and the surface areas of the metastatic regions were measured using Autodesk AutoCAD 2012 software application for design and drafting. Briefly, the area of whole tissue section was marked as A and the area of each of metastatic areas as B and the surface of each specified areas was measured and presented in Autodesk AutoCAD program as unitless number. The metastatic lesion was assessed in each section as the percentage of tissue parenchyma with metastasis by using the following formula: N = Bt × 100/At where N represents the percentage (%) of metastatic area in the whole tissue section, At (A total) is the sum of areas of the whole tissue section (At = A1 + A2 + ….An), Bt (B total) is the sum of metastatic regions in the whole tissue section (Bt = B1 + B2 + ….Bm).
Estimation of serum levels of IL-4, IL-10, TGF-β and IFN-γ cytokines
The cytokine production level in mouse serum was analyzed for all three inoculated and one control group using peripheral blood. The serum samples were used to quantitate the serum levels of IL-4, IL-10, TGF-β and IFN-γ. Levels of these cytokines were determined by commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems Minneapolis, MN), which were specific for mouse cytokines and used according to the manufacturer’s instructions.
Cellular analysis of spleens
Thirty five days after 4T1 tumor cells injection, mice were sacrificed and single-cell suspensions from spleen were obtained by mechanical dispersion through a cell strainer (BD Pharmingen, USA) in complete growth medium. Additionally, erythrocytes from spleen were removed using lysing solution (BD Pharmingen). After three washes, cells were resuspended in complete growth medium.
Flow cytometric analysis
Single-cell suspensions of spleens were incubated with mAbs specific for mouse CD3, CD4, CD8, CD25 and NKp46 (BD Pharmingen-eBioscience). Appropriate isotype control antibodies were used to assess the level of specific labeling. Dead cells were excluded by gating out propidium iodide-positive cells. The gate used for FACS analysis was the mononuclear cell region in the forward scatter-side scatter (FSC-SSC) plot. For intracellular staining of Foxp3, cells were fixed and permeabilized with permeabilization buffer (BD Pharmingen). Permeabilized cells were stained with anti-mouse Foxp3 mAbs (BD Pharmingen). Stained cells were analyzed subsequently using a FACSAria instrument (BD). Data were analyzed using CELLQUEST software and DiVa (BD).
Intracellular cytokine staining
Single cell suspensions of spleens were stimulated with Phorbol 12-myristate 13-acetate (50 ng/ml) (Sigma, USA) and Ionomycin (Sigma, USA) (500 ng/ml) with GolgyStop (BD Pharmingen) and incubated 4 hours at 37°C, 5% CO2. Cells were stained with anti-CD4 mAb or isotype-matched control. Subsequently, cells were fixed, permeabilized and intracellular staining was done using anti-IL-10 and anti-IFN-γ mAb (BD Pharmingen) and analyzed by flow cytometry.
NK cell and CD8+ FFT cell separation
NK cells were isolated from spleen cells by magnetic cell sorting. Single-cell suspensions of splenocytes were labeled using microBeads conjugated to monoclonal anti-mouse CD49b (DX5) antibodies (Miltenyi Biotec) and positively selected using MidiMACS separator (Miltenyi Biotec). CD49b labeled cells that retained in the magnetic column were highly enriched NK cells and were used in the cytotoxicity study as purified NK cells. In addition, CD8+ T cells were negatively selected from single-cell suspensions of splenocytes using aDynalmouse T cell negative isolation kit (Invitrogen) which depletes B cells, NK cells, monocytes/macrophages, dendritic cells (DCs), granulocytes and erythrocytes using a mixture of rat mAbs for mouse CD45R, CD11b, Ter-119 and CD16/32. Cell suspensions containing CD8 T cells were then used in the cytotoxicity assays.
Cytotoxicity assays
Cytotoxic activity of splenocytes, enriched NK cells and enriched CD8+ T cells was measured using the 4 h MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (Sigma) at various target-effector (T:E) ratios. 4T1 mouse breast tumor cells were used as targets. The percentage of cytotoxity was calculated as: cytotoxity (%) = [1-(experimental group (OD)/control group (OD)] × 100. Data were expressed as the mean of triplicate wells ± SEM. Cytotoxic activity was also presented by lytic units, LU20/107 cells, calculated from means of triplicates percentages of killing obtained in four different T:E ratios59.
Statistical analysis
All data were expressed as mean ± s.e.m. Statistical analysis of the data was performed using statistical package SPSS, version 13. Comparison between groups was made using one-way ANOVA, or nonparametric Mann-Whitney Rank Sum and Kruskal-Wallis tests. P < 0.05 was considered significant and P < 0.01 was highly significant.
References
Meirelles, D. L., Chagastelles, P. C. & Nardi, N. B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 119, 2204–2213 (2006).
Pittenger, M. F. et al. Multilineage potential ofadult human mesenchymal stem cells. Science. 284, 143–147 (1999).
Stagg, J. Mesenchymal stem cells in cancer. Stem Cell Rev. 4, 119–124 (2008).
Roorda, B. D., Ter Elst, A., Kamps, W. A. & de Bont, E. S. Bone marrow-derived cells and tumor growth: contribution of bone marrow-derived cells to tumor micro-environments with special focus on mesenchymal stem cells. Crit Rev Oncol Hematol. 69, 187–198 (2009).
Li, n. S. Y. et al. The isolation of novel mesenchymal stromal cell chemotactic factors from the conditioned medium of tumor cells. Exp Cell Res. 314, 3107–3117 (2008).
Uccelli, A., Moretta, L. & Pistoia, V. Mesenchymal stem cells in health and disease. Nat Rev. 8, 726–736 (2008).
Ramasamy, R., Lam, E. W., Soeiro, I., Tisato, V., Bonnet, D. & Dazzi, F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 21, 304–310 (2007).
Yu, J. M., Jun, E. S. & Bae, Y. C. Mesenchymal stem cells derived from human adipose tissues favour tumor cell growth in vivo. Stem Cells Dev. 17, 463–473 (2008).
Kucerova, L., Matuskova, M., Hlubinova, K., Altanerova, V. & Altaner, C. Tumor cell behaviour modulation by mesenchymal stromal cells. Mol Cancer. 9, 1–15 (2010).
Secchiero, P. et al. Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin’s lymphoma xenografts. Plos One. 16, 5–6 (2010).
Ling, X. et al. Mesenchymal Stem Cells Overexpressing IFN-β Inhibit Breast Cancer Growth and Metastases through Stat3 Signaling in a Syngeneic Tumor Model. Cancer Microenviron. 3, 83–95 (2010).
Rasmusson, I., Ringden, O., Sundberg, B. & Le Blanc, K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 76, 1208–1213 (2003).
Raffaghello, L. et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 26, 151–162 (2008).
Djouad, F. et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 102, 3837–3844 (2003).
Ning, H. et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 22, 593–599 (2008).
Aggarwal, S. & Pittenger, M. F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 105, 1815–1822 (2005).
Selmani, Z. et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4 + CD25 high FOXP3+ regulatory T cells. Stem Cells. 26, 212–222 (2008).
Fernandez, M. A. et al. T regulatory cells contribute to the attenuated primary CD8+ and CD4+ T cell responses to herpes simplex virus type 2 in neonatal mice. J Immunol. 180, 1556–1564 (2008).
Vasir, B. et al. Fusions of dendritic cells with breast carcinoma stimulate the expansion of regulatory T cells while concomitant exposure to IL-12, CpG oligodeoxynucleotides and anti-CD3/CD28 promotes the expansion of activated tumor reactive cells. J Immunol. 181, 808–821 (2008).
Studeny, M. et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 96, 1593–1603 (2004).
Annacker, O. et al. A CD25 + CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 166, 3008–3018 (2001).
Awasthi, A. et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. l8, 1380–1389 (2007).
Ammirante, M., Luo, J. L., Grivennikov, S., Nedospasov, S. & Karin, M. B-cell derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 464, 302–305 (2010).
Rafei, M. et al. A granulocyte-macrophage colony-stimulating factor and interleukin-15 fusokine induces a regulatory B cell population with immune suppressive properties. Nat Med. 15, 1038–1045 (2009).
Carpentier, A. F. & Meng, Y. Recent advances in immunotherapy for human glioma. Curr Opin Oncol. 18, 631–636 (2006).
Lizee, G., Radvanyi, L. G., Overwijk, W. W. & Hwu, P. Improving antitumor immune responses by circumventing immunoregulatory cells and mechanisms. Clin Cancer Res. 12, 4794–4803 (2006).
Olkhanud, P. B. et al. Tumor-Evoked Regulatory B Cells Promote Breast Cancer Metastasis by Converting Resting CD4+ T Cells to T-Regulatory Cells. Cancer Res. 71, 3505–3515 (2011).
Patel, S. A. et al. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol. 184, 5885–5894 (2010).
Oreffo, R. O., Cooper, C., Mason, C. & Clements, M. Mesenchymal stem cells: lineage, plasticity and skeletal therapeutic potential. Stem Cell Rev. 1, 169–178 (2005).
Parekkadan, B. et al. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun. 363, 247–252 (2007).
Dvorak, H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and woundhealing. N Engl J Med. 315, 1650–1659 (1986).
Karnoub, A. E. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 449, 557–563 (2007).
Studeny, M. et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 62, 3603–3608 (2002).
Kidd, S. et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 27, 2614–2623 (2009).
Lin, Y. T. et al. Human mesenchymal stem cells prolong survival and ameliorate motor deficit through trophic support in Huntington's disease mouse models. Plos One. 6, 8–e22924 (2011).
Eggenhofer, E. et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 3, 297 (2012).
Khakoo, A. Y. et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 203, 1235–1247 (2006).
Li, G. C. et al. Human mesenchymal stem cells inhibit metastasis of a hepatocellular carcinoma model using the MHCC97-H cell line. Cancer Sci. 101, 2546–2553 (2010).
Ohlsson, L. B., Varas, L., Kjellman, C., Edvardsen, K. & Lindvall, M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol Pathol. 75, 248–255 (2003).
Wang, H. et al. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells. 27, 1548–1558 (2009).
Djouad, F. et al. Earlier onset of syngeneic tumors in the presence of mesenchymal stem cells. Transplantation. 82, 1060–1066 (2006).
Muehlberg, F. L. et al. Tissue resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 30, 589–597 (2009).
Krampera, M. et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 101, 3722–3729 (2003).
Rutella, S., Danese, S. & Leone, G. Tolerogenic dendritic cells: Cytokine modulation comes of age. Blood. 108, 1435–1440 (2006).
Corcione, A. et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 107, 367–372 (2006).
Sotiropoulou, P. A., Perez, S. A., Gritzapis, A. D., Baxevanis, C. N. & Papamichail, M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 24, 74–85 (2006).
Spaggiari, G. M. et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 111, 1327–1333 (2008).
Minguell, J. J., Erices, A. & Conget, P. Mesenchymal stem cells. Exp Biol Med. 226, 507–520 (2001).
Joshi, P. C., Zhou, X., Cuchens, M. & Jones, Q. Prostaglandin E2 suppressed IL-15-mediated human NK cell function through down-regulation of common γ-chain. J Immunol. 166, 885–891 (2001).
Swann, J., Crowe, N. Y., Hayakawa, Y., Godfrey, D. I. & Smyth, M. J. Regulation of antitumour immunity by CD1d-restricted NKT cells. Immunol Cell Biol. 82, 323–331 (2004).
Yanagisawa, K. et al. Impaired proliferative response of V alpha 24 NKT cells from cancer patients against alpha-galactosylceramide. J Immunol. 168, 6494–6499 (2002).
Dhodapkar, M. V. et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 197, 1667–1676 (2003).
Molling, J. W. et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 25, 862–868 (2007).
Ghiringhelli, F., Ménard, C., Martin, F. & Zitvogel, L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev. 214, 229–238 (2006).
Casiraghi, F. et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 181, 3933–3946 (2008).
Batten, P. et al. Human mesenchymal stem cells induce T cell anergy and downregulate T cell alloresponses via the TH2 pathway: relevance to tissue engineering human heart valves. Tissue Eng. 12, 2263–2273 (2006).
Trivanovic, D. et al. Mesenchymal stem cells isolated from peripheral blood and umbilical cord Wharton’s jelly. Srp Arh Celok Lek. 141, 178–186 (2013).
Carlsson, G., Ekelund, E., Stigsson, L. & Hafstrom, L. Vascularization and tumour volume estimations of solitary liver tumours in rats. Ann Chir Gynaecol. 72, 187–191 (1983).
Jonjic, B. M. et al. Innate direct anticancer effector function of human immature dendritic cells. I. Involvement of an apoptosis-inducing pathway. J Immunol. 168, 1823–1830 (2002).
Acknowledgements
This work was supported by grants from the Serbian Ministry of Science and Technological Development (ON175103 and ON175069), Serbia and Faculty of Medicine, University of Kragujevac, Serbia (Grant no. JP 15/10). We thank Dr. Nada Pejnovic for critical review of the manuscript and Miljan Nedeljkovic for excellent technical assistance.
Author information
Authors and Affiliations
Contributions
N.A., M.L.L., M.S. and B.Lj. conceived the study and designed the experiments; B.Lj., M.M., V.V., B.M., D.B. and S.P. performed the experiments; B.Lj., M.M., V.V., N.A., M.L.L. and M.S. analyzed the data; B.Lj. wrote the manuscript and all authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Ljujic, B., Milovanovic, M., Volarevic, V. et al. Human mesenchymal stem cells creating an immunosuppressive environment and promote breast cancer in mice. Sci Rep 3, 2298 (2013). https://doi.org/10.1038/srep02298
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02298
This article is cited by
-
Safety and Homing of Human Dental Pulp Stromal Cells in Head and Neck Cancer
Stem Cell Reviews and Reports (2021)
-
Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Cutaneous Wound Healing by Enhancing Angiogenesis through Delivering Angiopoietin-2
Stem Cell Reviews and Reports (2021)
-
An update on human periapical cyst-mesenchymal stem cells and their potential applications in regenerative medicine
Molecular Biology Reports (2020)
-
Paradoxical Role of Glypican-1 in Prostate Cancer Cell and Tumor Growth
Scientific Reports (2019)
-
Effects of mesenchymal stem cells on solid tumor metastasis in experimental cancer models: a systematic review and meta-analysis
Journal of Translational Medicine (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.