« Prev Next »
No matter how complex eventually, every eukaryotic organism arises from a single cell that undergoes numerous cell divisions. In eukaryotes, the organism can grow quite large, and develop many differentiated tissues types. For example, adult humans are composed of about 1013 (10 trillion) cells that all arise from a single fertilized egg. During our life span, the cells in our body may undergo about 1016 (10 thousand trillion) divisions. Even as life depends on these precise cell divisions, errors in cell division routinely occur. In complex organisms such as mice and humans, some errors in the cell division cycle result in cancer formation. Understanding how cells faithfully replicate themselves is a prerequisite for the prevention and/or treatment of cancer.
What Is the Cell Division Cycle?
The cell division cycle is a fundamental feature of living organisms, and is responsible for maintaining and/or increasing both cell size and number. There are four successive phases to a standard eukaryotic cell cycle: G1 (growth phase 1), S (synthesis phase), G2 (growth phase 2), and M phase (mitotic phase; Figure 1). During interphase (the combination of G1, S, and G2), a cell grows continuously, and during M phase it divides. DNA replication occurs during S phase.
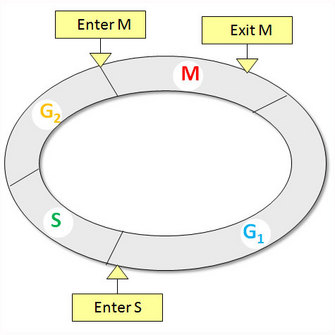
Cell cycle fidelity is the concept that each phase transition occurs at the right time in the right order, and is stringently monitored. What is the molecular nature of these phase transitions? How do we decipher this circuit of cell cycle regulation? Before the early genomics era of the 1990s, cell cycle regulation was studied using two principal model systems: genetic manipulations in yeasts, and biochemical analyses of early embryonic cell division in frog eggs. These different technical approaches led to two seemingly distinct models: the domino model in yeasts, and the clock model in frog eggs. Recently identified proteins that have crucial functions common to both models allow us to reconcile the two. This commonality also highlights fundamental principles of cell cycle regulation that have been conserved throughout evolution in all eukaryotic organisms.
Genetic Isolation of Cell-Division Cycle (CDC) Mutants in Yeasts
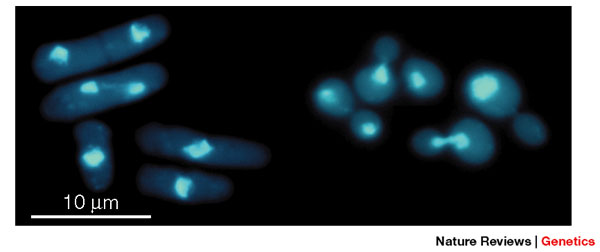
How do we study the cell-division cycle? Yeasts are unicellular fungi, and comprise a large heterogeneous group of eukaryotic organisms. They are favored among cell cycle biologists who want to study a simple model organism among eukaryotes. Scientists focus primarily on two yeast model organisms: budding yeast, Saccharomyces cerevisiae, and fission yeast, Schizosaccharomyces pombe. Each have distinct cell shapes and patterns of division, and both are genetically tractable, as we can easily identify and clone the genes involved in their cellular processes (Figure 2).
In the 1970s, Paul Nurse and Leland Hartwell pioneered the search for mutations that affected the cell cycle in both of these yeast species. They identified mutants by their arrest at distinct stages of the cell cycle, and dubbed these cell-division-cycle (cdc) mutants, or depending on their cell size at the time of arrest, dubbed these the wee mutants. Interestingly, both species of yeast helped scientists identify temperature-sensitive (ts) mutantin cell cycle. This observation may immediately pose the question: why are most yeast mutants temperature-sensitive?
Since cell division is essential for life, without a functional cdc gene the cell cannot propagate. To infer the function of the gene by observing the phenotype of a cdc gene, scientists have isolated and maintained cdc mutants as conditional mutants. In a conditional mutant, a gene product is inactive under one condition, but not another. In fact, a ts mutant is one of the most common conditional mutants in yeasts. How do ts mutants exhibit their phenotype? At the permissive temperature (usually 25 ºC), the gene mutated in a ts mutant behaves similarly to that in the wild type (wt), but at the restrictive temperature (35-37 ºC) it is rendered inactive. We can therefore use this temperature sensitivity to determine the function of a specific gene in the cell-division cycle by inactivating the gene throughout a population of yeast using temperature control. The mutant phenotype only appears at the restrictive temperature.
What Are the Specific Features of CDC Mutants?
While all of the cells in a population of cdc mutants are asynchronous at the permissive temperature, they become synchronously arrested at the restrictive temperature. In a cdc mutant population, cells blocked at a specific phase display similar phenotypes. For example, they exhibit identical lengths in fission yeast, and identical bud size in budding yeast (or no bud at all) (Figure 3). The defective gene in the mutant is known as a cdc gene, and it is a common genetic component that is necessary for a particular phase in cell cycle. In contrast, non-cdc mutants may be deficient in continuous processes, such as ATP production. As ATP is required continuously for biosynthesis and growth during all phases of the cycle, these mutants may halt at any stage, as soon as the ATP reserve runs out.
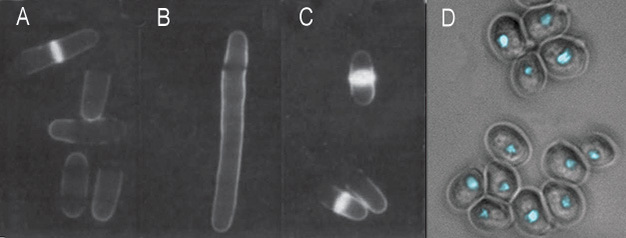
In fission yeast, wee mutants divide at a smaller size than normal wt yeast (Figure 3A, compare panel 3 with panel 1). wee mutants are deficient in a gene product that normally inhibits passage through a phase transition until a standard cell size is attained. Thus wee genes control cell size in response to the availability of nutrients in the environment, and internal cues, such as DNA replication.
Based on the phenotypes of these two types of mutants at the restrictive temperature (the phase at which the mutant is arrested), scientists determined the functions of the normal wt genes in cell cycle regulation. For example, the master protein kinase of cell cycle regulation, Cdc2, is required for the G2/M phase transition in fission yeast. Therefore, ts cdc2 mutant cells are blocked at late G2 at 35 ºC, and exhibit a uniform cell elongation compared with wt cells (Figure 3A, compare panel 2 with panel 1).
The Domino Model of Cell Cycle Regulation
The tight control of yeast gene expression allowed scientists to ask very specific questions about the genetic control of cell cycle progression. For example, the gene that encodes the functional homologue of Cdc2 in budding yeast, called CDC28+, was discovered to be necessary for G1/S transition. Consequently, cdc2 ts mutants cannot pass Start (a restrictive point before entering S phase) at 35 ºC, which results in no cells having buds (Figure 3B). Using this method, scientists elucidated a cast of vital genetic players in the regulation of cell cycle progression, including Cdc2/Cdc28, Cdc25, and Wee1 protein kinases. If the mutants in a population lack the gene necessary for initiating or completing a particular cell cycle phase, that phase becomes restrictive, and it prevents the cell from proceeding to later phases. As each cell cycle phase is dependent on the proper completion of all the prior phases, scientists named this "the domino model" of cell cycle regulation. This sequential order of cell cycle events underlines the concept of checkpoint pathways, which are a series of necessary cell signaling events that ensure proper execution of phase transitions in the cell cycle.
Cell Cycle Phase Transitions: Lessons from Frog Eggs
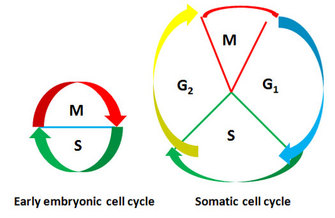
In the African frog (Xenopus laevis), egg cells undergo rapid cleavage, providing a useful system for functionally analyzing and purifying the factors that drive a cell into mitosis. Indeed, most of what we have learned about mitotic progression comes from early studies in frog eggs and frog egg cytoplasmic extracts. What are the distinguishing features of the embryonic cell cycle in the frog? During the rapid cleavages of early frog embryos, no cell growth occurs, so daughter cells from each cell division become halved in size. The cycle time is extraordinarily short, with the first cell cycle lasting only 75 minutes, followed by 11 synchronous cell cycles, each 30 only minutes long. Also, the cycle alternates between S and M phases without the intervening G1 and G2 phases (Figure 4).
What is the molecular nature of the switch that operates the transition to M-phase? Early experiments gave a clue. Marc Kirschner's famous "bouncing frog egg" experiment in 1980 suggested a very different mechanism of cell cycle regulation from what was understood from the yeast domino model, and we now know this as the "clock model" of cell cycle progression. How did Kirschner come to observe a bouncing frog egg? In nature, frog eggs need fertilization to initiate cleavage. However, in the laboratory, scientists are able to artificially "activate" frog eggs with biochemical manipulations, to initiate the mitotic cell cycle. This artificial activation mimics fertilization. In his experiments, Kirschner removed the nuclei from Xenopus eggs and observed striking contractions of the enucleated egg: a popping up as mitosis began, and a popping down during interphase.
In subsequent experiments, similar to the bouncing egg ones, scientists split the egg into two parts. They did this by constricting an activated Xenopus egg with a loop of fine human baby hair before it completed the first cell division. One half contained the nucleus, and the other did not. As expected, the part with the nucleus continued the normal program of rapid cleavages. Remarkably, the non-nucleated portion of the egg showed a series of oscillations, repeated cycles of contractions that were in almost perfect synchrony with the cleavages of the nucleated half. This apparent driving force for mitosis underlying these oscillations was later identified as M-phase/maturation promoting factor (MPF). This was because peak activity of MPF overlaps with the onset of mitosis. Scientists followed the patterns of MPF activity, and quickly established that the surge of MPF activity occurred every 30 min in the cleaving Xenopus embryo. It seemed to be some sort of cytoplasmic oscillator, or clock. As seen in the enucleated half of the egg, this clock continues ticking even after the nucleus has been removed. But what is controlling this clock? Pioneering work in frog egg extracts to test MPF demonstrated that, indeed, MPF is the driving force of the mitotic cell cycle, a type of cell cycle engine. This discovery began to grow in relevance beyond the frog, as scientists also found similar MPF activity in mammalian cells.
Reconciliation of the Two Divergent Views
Could any generalizations in cell cycle regulation be drawn? To review, the yeast cdc mutants revealed a fundamental fact about cell cycle control: the execution of later events depends on the prior completion of earlier events. This concept became known as the domino model. In contrast, the rapid contractions of an enucleated Xenopus egg revealed the existence of a cytoplasmic clock that sets the timing of mitotic entry. This concept became known as the clock model. The domino model suggests that cell cycle events are interdependent, whereas the clock model indicates that the timing of the mitosis entry was not influenced by other events. These distinct models begged the question, is cell cycle regulation different in different organisms? In the 1980s, scientists conducting these distinct genetic and biochemical studies were impressed with each others' ideas and the cleverness of approaches when they shared their results, but were unable to find commonality about the cell cycle events they observed among different eukaryotic organisms.
Given that the differences appeared to outweigh the similarities, how could the two divergent views on cell cycle ever converge? The clues about the functional links between the yeast genetic and frog egg biochemical approaches came from further dissection of key molecules involved in cell cycle regulation. Genetic studies in yeast had already identified common central genes in the two yeast model species: cdc2 in fission yeast and CDC28 in budding yeast. David Beach and his colleagues found that the wt CDC28 gene transformed into a cdc2 ts mutant could rescue its elongation phenotype and restore the normal cell cycle (genetic cross-species complementation assay). This established that the Cdc2 and Cdc28 proteins were functionally equivalent in the two yeast species. Cdc2 then became designated the master regulator of the cell cycle in yeast, just like the royal status reserved for MPF in animal cells.
Another giant leap came in 1987. Using the same cross-species complementation assay, Melanie Lee and Paul Nurse discovered the human counterpart of cdc2, aptly named CDC2, because it could entirely replace the function of cdc2 in fission yeast. All three homologous genes appeared to be closely related to protein kinases. Since Cdc2 is evolutionarily conserved from yeast to humans, the next logical question was, how does Cdc2/Cdc28 in yeast relate to MPF in animal cells? Scientists suspected that the MPF they had observed in frog eggs and mammalian cells might also be the master regulator of a protein kinase cascade. After isolating MPF, they were finally able to confirm this. In fact, MPF was further defined as a two-subunit protein complex, with the catalytic subunit being the kinase, at a size of 34 kDa. In cross species experiments, scientists introduced the fission yeast Cdc2 protein into frog egg extracts, and found that the 34 kDa component of frog MPF was actually the same kinase as in the yeast Cdc2 protein! They concluded that yeast and frog egg kinases are homologous proteins with the same function (i.e., proteins encoded by the same gene in the last common ancestor of yeast and frogs).
Putting the Data Together
As further research ensued, scientists continued to put the disparate pieces of evidence together. It turns out, the master kinase that triggers downstream events for mitotic transition consists of two proteins: a cyclin-dependent kinase (Cdk) and a regulatory subunit, cyclin. In a simple scenario, Cdc2 is associated with cyclin B (the large protein subunit of MPF), to form an activated MPF at the G2/M transition. With this new understanding, the two views of cell cycle regulation were finally unified: the same protein kinase was required for entry into mitosis in both yeasts and animals. This conclusion is both satisfying and surprising for two reasons. First, there is a wide evolutionary distance between yeast and vertebrates. And second, the morphological events of mitosis in each type of cell are quite distinct: yeast exhibit a ‘closed' mitosis, without breaking down the nuclear envelope, and mammalian cells exhibit an ‘open' mitosis, with disassembly of the nuclear membrane. This research on the master kinase for mitosis illustrates a fundamental concept of the cell cycle: in many different and evolutionarily divergent biological systems, the main components of cell cycle engine are fundamentally the same. The specific mechanisms governing the functions of these components, as well as the complexity of the regulating signaling pathway, may vary with phase transitions, organisms, developmental stages, and environment, which are fascinating areas for further research. What is the next frontier in cell cycle research? Scientists are now combining genomic and systems level manipulations to decipher the mechanisms of cell cycle regulation.
Summary
Genetic methods in yeast and biochemical studies in frogs initially delineated distinct mechanisms for cell cycle regulation. The isolation of yeast mutants with perturbed cell cycle control phenotypes helped identify genes involved in the sequential execution of cell cycle phase transitions. In early development, the rapid cleavage of frog eggs and similar preparations of frog egg extracts proved useful in biochemically characterizing a key cell cycle regulator, MPF, whose activity oscillated in synchrony with the mitotic cycle. These studies revealed the core machinery that drives cell cycle progression and cell cycle phase transitions, Cdks and cyclins. Historically, there were two divergent models, the domino and clock models: originally proposed for yeast and early embryonic frog cells, respectively. The domino model emphasized the inter-dependence of cell cycle events on phase transitions, whereas the clock model envisioned a cytoplasmic timer controlling the onset of mitosis that was independent of other events in the cell cycle. The divergent views have since been reconciled on the basis of molecular identification of the crucial players that govern phase transitions of a sequential order. Remarkably, these proteins have been exquisitely conserved through evolution from yeast to humans.
References and Recommended Reading
Beach, D., Durkacz, B., et al. Functionally homologous cell cycle control genes in budding and fission yeast. Nature 300, 706–709 (1982).
Dunphy, W. G., Brizuela, L., et al. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell 54, 23–431 (1988).
Kirschner, M. The cell cycle then and now. Trends in Biochemical Sciences 17, 281–285 (1992).
Hartwell, L. H. & Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science 246, 629–634 (1989).
Hartwell, L. H. Twenty-five years of cell cycle genetics. Genetics 129, 975–980 (1991).
Lee, M. G. & Nurse, P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature 327, 31–35 (1987).
Nurse, P. A long twentieth century of the cell cycle and beyond. Cell 100, 71–78(2000).
Russell, P. & Nurse, P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell 49, 559–567 (1987).






























