« Prev Next »

CDK
Multiple checkpoints in the eukaryotic cell cycle ensure that division occurs only after sufficient growth and faithful DNA replication, and only when favorable conditions exist. At each checkpoint, numerous proteins engage in a series of carefully coordinated biochemical reactions. This complexity allows for precise regulation of all steps in the cell cycle — and it is essential to preventing the devastating consequences of cell division gone awry (Figure 1).
What Are Cyclin-Dependent Kinases?
Of the many proteins involved in cell cycle control, cyclin-dependent kinases (CDKs) are among the most important. CDKs are a family of multifunctional enzymes that can modify various protein substrates involved in cell cycle progression. Specifically, CDKs phosphorylate their substrates by transferring phosphate groups from ATP to specific stretches of amino acids in the substrates. Different types of eukaryotic cells contain different types and numbers of CDKs. For example, yeast have only a single CDK, whereas vertebrates have four different ones.
As their name suggests, CDKs require the presence of cyclins to become active. Cyclins are a family of proteins that have no enzymatic activity of their own but activate CDKs by binding to them. CDKs must also be in a particular phosphorylation state — with some sites phosphorylated and others dephosphorylated — in order for activation to occur. Correct phosphorylation depends on the action of other kinases and a second class of enzymes called phosphatases that are responsible for removing phosphate groups from proteins.
How Do CDKs Control the Cell Cycle?
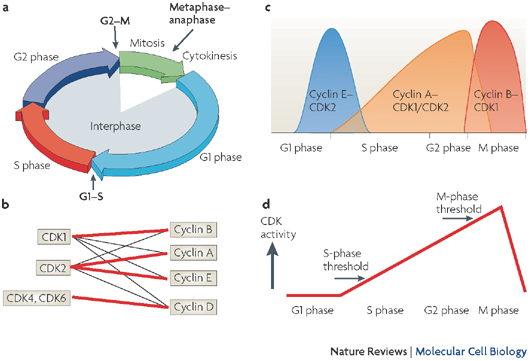
All eukaryotes have multiple cyclins, each of which acts during a specific stage of the cell cycle. (In organisms with multiple CDKs, each CDK is paired with a specific cyclin.) All cyclins are named according to the stage at which they assemble with CDKs. Common classes of cyclins include G1-phase cyclins, G1/S-phase cyclins, S-phase cyclins, and M-phase cyclins. M-phase cyclins form M-CDK complexes and drive the cell's entry into mitosis; G1 cyclins form G1-CDK complexes and guide the cell's progress through the G1 phase; and so on.
All CDKs exist in similar amounts throughout the entire cell cycle. In contrast, cyclin manufacture and breakdown varies by stage — with cell cycle progression dependent on the synthesis of new cyclin molecules. Accordingly, cells synthesize G1- and G1/S-cyclins at different times during the G1 phase, and they produce M-cyclin molecules during the G2 phase (Figure 2). Cyclin degradation is equally important for progression through the cell cycle. Specific enzymes break down cyclins at defined times in the cell cycle. When cyclin levels decrease, the corresponding CDKs become inactive. Cell cycle arrest can occur if cyclins fail to degrade.
Which Proteins Do CDKs Modify?
Conclusion
eBooks
This page appears in the following eBook





















