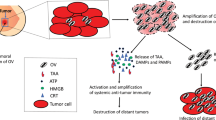
Key Points
-
Oncolytic virotherapy is a cancer treatment that employs replication-competent viruses, which specifically infect, replicate in and lyse malignant tumour cells, while minimizing harm to normal cells
-
Several oncolytic agents have been investigated in clinical trials in patients with urological cancers, demonstrating a number of challenges to their effectiveness and a need to improve their safety
-
Innate and adaptive virus-targeted immune responses, loss of viruses in tumour- associated tissues and suboptimal virus replication hamper the effectiveness of virotherapy
-
Current developments take into account distinctive features of urological tumours, for example by exploiting genetic alterations, such as changed expression levels of oncogenes, tumour suppressor genes and receptors
-
The strategies employed to improve oncolytic effectiveness and safety include alteration of virus tropism, regulating viral gene expression, arming viruses with immunostimulatory factors and combination with chemotherapy and radiotherapy
Abstract
Oncolytic virotherapy is a cancer treatment in which replication-competent viruses are used that specifically infect, replicate in and lyse malignant tumour cells, while minimizing harm to normal cells. Anecdotal evidence of the effectiveness of this strategy has existed since the late nineteenth century, but advances and innovations in biotechnological methods in the 1980s and 1990s led to a renewed interest in this type of therapy. Multiple clinical trials investigating the use of agents constructed from a wide range of viruses have since been performed, and several of these enrolled patients with urological malignancies. Data from these clinical trials and from preclinical studies revealed a number of challenges to the effectiveness of oncolytic virotherapy that have prompted the development of further sophisticated strategies. Urological cancers have a range of distinctive features, such as specific genetic mutations and cell surface markers, which enable improving both effectiveness and safety of oncolytic virus treatments. The strategies employed in creating advanced oncolytic agents include alteration of the virus tropism, regulating transcription and translation of viral genes, combination with chemotherapy, radiotherapy or gene therapy, arming viruses with factors that stimulate the immune response against tumour cells and delivery technologies to ensure that the viral agent reaches its target tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kelly, E. & Russell, S. J. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 15, 651–659 (2007).
Dock, G. The influence of complicating diseases upon leukemia. Am. J. Med. Sci. 127, 563–592 (1904).
De Pace, N. G. Sulla scomparsa di un enorme cancro vegetante del callo dell'utero senza cura chirurgica [Italian]. Ginecologia 9, 82–88 (1912).
Bierman, H. R. et al. Remissions in leukemia of childhood following acute infectious disease: staphylococcus and streptococcus, varicella, and feline panleukopenia. Cancer 6, 591–605 (1953).
Hoster, H. A., Zanes, R. P. Jr & Von Haam, E. Studies in Hodgkin's syndrome; the association of viral hepatitis and Hodgkin's disease; a preliminary report. Cancer Res. 9, 473–480 (1949).
Alemany, R. Viruses in cancer treatment. Clin. Transl. Oncol. 15, 182–188 (2013).
Benencia, F. & Coukos, G. Biological therapy with oncolytic herpesvirus. Adv. Exp. Med. Biol. 622, 221–233 (2008).
Alain, T. et al. The oncolytic effect in vivo of reovirus on tumour cells that have survived reovirus cell killing in vitro. Br. J. Cancer 95, 1020–1027 (2006).
Breitbach, C. J. et al. Targeted inflammation during oncolytic virus therapy severely compromises tumour blood flow. Mol. Ther. 15, 1686–1693 (2007).
Aghi, M. K., Liu, T. C., Rabkin, S. & Martuza, R. L. Hypoxia enhances the replication of oncolytic herpes simplex virus. Mol. Ther. 17, 51–56 (2009).
Hotte, S. J. et al. An optimized clinical regimen for the oncolytic virus PV701. Clin. Cancer Res. 13, 977–985 (2007).
Allen, C. et al. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol. Ther. 16, 1556–1564 (2008).
Smith, B. F. et al. Administration of a conditionally replicative oncolytic canine adenovirus in normal dogs. Cancer Biother. Radiopharm. 21, 601–606 (2006).
Alonso, M. M. et al. Combination of the oncolytic adenovirus ICOVIR-5 with chemotherapy provides enhanced anti-glioma effect in vivo. Cancer Gene Ther. 14, 756–761 (2007).
Carpenter, A. et al. Effects of ammonium tetrathiomolybdate, an oncolytic/angiolytic drug on the viability and proliferation of endothelial and tumour cells. Inflamm. Res. 56, 515–519 (2007).
Wodarz, D. Use of oncolytic viruses for the eradication of drug-resistant cancer cells. J. R. Soc. Interface 6, 179–186 (2009).
Cripe, T. P., Wang, P. Y., Marcato, P., Mahller, Y. Y. & Lee, P. W. Targeting cancer-initiating cells with oncolytic viruses. Mol. Ther. 17, 1677–1682 (2009).
Norman, K. L., Farassati, F. & Lee, P. W. Oncolytic viruses and cancer therapy. Cytokine Growth Factor Rev. 12, 271–282 (2001).
Lilley, C. E., Carson, C. T., Muotri, A. R., Gage, F. H. & Weitzman, M. D. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc. Natl Acad. Sci. USA 102, 5844–5849 (2005).
Morton, E. R. & Blaho, J. A. Herpes simplex virus blocks Fas-mediated apoptosis independent of viral activation of NF-κB in human epithelial HEp-2 cells. J. Interferon Cytokine Res. 27, 365–376 (2007).
Esfandiarei, M. et al. Protein kinase B/Akt regulates coxsackievirus B3 replication through a mechanism which is not caspase dependent. J. Virol. 78, 4289–4298 (2004).
Gregory, D., Hargett, D., Holmes, D., Money, E. & Bachenheimer, S. L. Efficient replication by herpes simplex virus type 1 involves activation of the IκB kinase–IκB–p65 pathway. J. Virol. 78, 13582–13590 (2004).
Breitbach, C. J. et al. Oncolytic vaccinia virus disrupts tumour-associated vasculature in humans. Cancer Res. 73, 1265–1275 (2013).
Kottke, T. et al. Precise scheduling of chemotherapy primes VEGF-producing tumours for successful systemic oncolytic virotherapy. Mol. Ther. 19, 1802–1812 (2011).
Benencia, F., Courreges, M. C., Fraser, N. W. & Coukos, G. Herpes virus oncolytic therapy reverses tumour immune dysfunction and facilitates tumour antigen presentation. Cancer Biol. Ther. 7, 1194–1205 (2008).
Schulz, O. et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433, 887–892 (2005).
International Bladder Cancer Nomogram Consortium, Bochner, B. H., Kattan, M. W. & Vora, K. C. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J. Clin. Oncol. 24, 3967–3972 (2006).
Cohen, H. T. & McGovern, F. J. Renal-cell carcinoma. N. Engl. J. Med. 353, 2477–2490 (2005).
Katsogiannou, M. et al. The hallmarks of castration-resistant prostate cancers. 41, 588–597 (2015).
Russell, S. J., Peng, K. W. & Bell, J. C. Oncolytic virotherapy. Nat. Biotechnol. 30, 658–670 (2012).
Altomonte, J. & Ebert, O. Sorting out Pandora's box: discerning the dynamic roles of liver microenvironment in oncolytic virus therapy for hepatocellular carcinoma. Front. Oncol. 4, 85 (2014).
Fukuhara, H., Homma, Y. & Todo, T. Oncolytic virus therapy for prostate cancer. Int. J. Urol. 17, 20–30 (2010).
Potts, K. G., Hitt, M. M. & Moore, R. B. Oncolytic viruses in the treatment of bladder cancer. Adv. Urol. 2012, 404581 (2012).
Lawson, K. A. & Morris, D. G. Oncolytic virotherapy for renal cell carcinoma: a novel treatment paradigm? Expert Opin. Biol. Ther. 12, 891–903 (2012).
Megison, M. L. et al. Preclinical evaluation of engineered oncolytic herpes simplex virus for the treatment of pediatric solid tumours. PLoS ONE 9, e86843 (2014).
Ch'ng, W. C., Stanbridge, E. J., Yusoff, K. & Shafee, N. The oncolytic activity of Newcastle disease virus in clear cell renal carcinoma cells in normoxic and hypoxic conditions: the interplay between von Hippel–Lindau and interferon-β signaling. J. Interferon Cytokine Res. 33, 346–354 (2013).
Buijs, P. R., Verhagen, J. H., van Eijck, C. H. & van den Hoogen, B. G. Oncolytic viruses: from bench to bedside with a focus on safety. Hum. Vaccin. Immunother. 11, 1573–1584 (2015).
Pol, J. et al. Trial watch: oncolytic viruses for cancer therapy. Oncoimmunology 3, e28694 (2014).
Johnson, D. B., Puzanov, I. & Kelley, M. C. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy 7, 611–619 (2015).
Burke, J. M. et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J. Urol. 188, 2391–2397 (2012).
Vidal, L. et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin. Cancer Res. 14, 7127–7137 (2008).
Black, A. J. & Morris, D. G. Clinical trials involving the oncolytic virus, reovirus: ready for prime time? Expert Rev. Clin. Pharmacol. 5, 517–520 (2012).
Stoeckel, J. & Hay, J. G. Drug evaluation: reolysin — wild-type reovirus as a cancer therapeutic. Curr. Opin. Mol. Ther. 8, 249–260 (2006).
Gomella, L. G. et al. Phase I study of intravesical vaccinia virus as a vector for gene therapy of bladder cancer. J. Urol. 166, 1291–1295 (2001).
Burke, J. Virus therapy for bladder cancer. Cytokine Growth Factor Rev. 21, 99–102 (2010).
Shi, Y. et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell Res. 16, 126–133 (2006).
Mach, N. & Dranoff, G. Cytokine-secreting tumour cell vaccines. Curr. Opin. Immunol. 12, 571–575 (2000).
Bradley, S. et al. Applications of coxsackievirus A21 in oncology. Oncolytic Virother. 3, 47–55 (2014).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02316171 (2016).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02043665 (2016).
Johansson, E. S., Xing, L., Cheng, R. H. & Shafren, D. R. Enhanced cellular receptor usage by a bioselected variant of coxsackievirus A21. J. Virol. 78, 12603–12612 (2004).
Shafren, D. R., Dorahy, D. J., Ingham, R. A., Burns, G. F. & Barry, R. D. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J. Virol. 71, 4736–4743 (1997).
Pandha, H. et al. Oncolytic immunotherapy for the treatment of non-muscle invasive bladder cancer using intravesical Coxsackievirus A21: Phase I/II CANON study. Presented at the 9th International conference on oncolytic virus therapeutics 2015, P-5 (2015).
DeWeese, T. L. et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 61, 7464–7472 (2001).
Small, E. J. et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol. Ther. 14, 107–117 (2006).
Freytag, S. O. et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 62, 4968–4976 (2002).
Freytag, S. O. et al. Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 89, 268–276 (2014).
Comins, C. et al. REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin. Cancer Res. 16, 5564–5572 (2010).
Heinemann, L. et al. Synergistic effects of oncolytic reovirus and docetaxel chemotherapy in prostate cancer. BMC Cancer 11, 221 (2011).
US National Library of Science. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01619813 (2016).
US National Library of Science. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT00636558 (2012).
Pandha, H. et al. Phase I/II storm study: Intravenous delivery of a novel oncolytic immunotherapy agent, Coxsackievirus A21, in advanced cancer patients. J. Immunother. Cancer 3 (Suppl. 2), 341 (2015).
Li, J. et al. A phase I trial of intratumoural administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumour patients. Gene Ther. 16, 376–382 (2009).
Calderwood, S. K., Stevenson, M. A. & Murshid, A. Heat shock proteins, autoimmunity, and cancer treatment. Autoimmune Dis. 2012, 486069 (2012).
Böttger, E., Multhoff, G., Kun, J. F. & Esen, M. Plasmodium falciparum-infected erythrocytes induce granzyme B by NK cells through expression of host-Hsp70. PLoS ONE 7, e33774 (2012).
US National Library of Science. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT00625456 (2015).
US National Library of Science. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01169584 (2016).
US National Library of Science. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02053220(2016).
US National Library of Science. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT00931931 (2015).
Ramesh, N. et al. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor-armed oncolytic adenovirus for the treatment of bladder cancer. Clin. Cancer Res. 12, 305–313 (2006).
Castleton, A. et al. Human mesenchymal stromal cells deliver systemic oncolytic measles virus to treat acute lymphoblastic leukemia in the presence of humoral immunity. Blood 123, 1327–1335 (2014).
Galanis, E. et al. Phase II trial of intravenous administration of Reolysin® (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol. Ther. 20, 1998–2003 (2012).
Rudin, C. M. et al. Phase I clinical study of Seneca Valley Virus (SVV-001), a replication-competent picornavirus, in advanced solid tumours with neuroendocrine features. Clin. Cancer Res. 17, 888–895 (2011).
Msaouel, P., Opyrchal, M., Domingo Musibay, E. & Galanis, E. Oncolytic measles virus strains as novel anticancer agents. Expert Opin. Biol. Ther. 13, 483–502 (2013).
Msaouel, P., Iankov, I. D., Dispenzieri, A. & Galanis, E. Attenuated oncolytic measles virus strains as cancer therapeutics. Curr. Pharm. Biotechnol. 13, 1732–1741 (2012).
Fulci, G. et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc. Natl Acad. Sci. USA 103, 12873–12878 (2006).
Waldhauer, I. & Steinle, A. NK cells and cancer immunosurveillance. Oncogene 27, 5932–5943 (2008).
Prestwich, R. J. et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum. Gene Ther. 20, 1119–1132 (2009).
Shafren, D. et al. Combination of a novel oncolytic immunotherapeutic agent, CAVATAK (coxsackievirus A21) and immune-checkpoint blockade significantly reduces tumour growth and improves survival in an immune competent mouse melanoma model. J. Immunother. Cancer 2, 125 (2014).
de Gruijl, T. D., Janssen, A. B. & van Beusechem, V. W. Arming oncolytic viruses to leverage antitumour immunity. Expert Opin. Biol. Ther. 15, 959–971 (2015).
Woller, N., Gurlevik, E., Ureche, C. I., Schumacher, A. & Kuhnel, F. Oncolytic viruses as anticancer vaccines. Front. Oncol. 4, 188 (2014).
Zamarin, D. et al. Localized oncolytic virotherapy overcomes systemic tumour resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 6, 226ra32 (2014).
Jiang, H. et al. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS ONE 9, e97407 (2014).
Thirukkumaran, C. M. et al. Oncolytic viral therapy for prostate cancer: efficacy of reovirus as a biological therapeutic. Cancer Res. 70, 2435–2444 (2010).
Hodge, J. W. et al. A recombinant vaccinia virus expressing human prostate-specific antigen (PSA): safety and immunogenicity in a non-human primate. Int. J. Cancer 63, 231–237 (1995).
Singh, P., Pal, S. K., Alex, A. & Agarwal, N. Development of PROSTVAC immunotherapy in prostate cancer. Future Oncol. 11, 2137–2148 (2015).
Kantoff, P. W. et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 28, 1099–1105 (2010).
Campbell, C. T. et al. Humoral response to a viral glycan correlates with survival on PROSTVAC-VF. Proc. Natl Acad. Sci. USA 111, E1749–E1758 (2014).
Campbell, C. T. et al. Serum antibodies to blood group A predict survival on PROSTVAC-VF. Clin. Cancer Res. 19, 1290–1299 (2013).
Reid, T., Warren, R. & Kirn, D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther. 9, 979–986 (2002).
Doronin, K., Shashkova, E. V., May, S. M., Hofherr, S. E. & Barry, M. A. Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum. Gene Ther. 20, 975–988 (2009).
Green, N. K. et al. Tropism ablation and stealthing of oncolytic adenovirus enhances systemic delivery to tumours and improves virotherapy of cancer. Nanomedicine (Lond.) 7, 1683–1695 (2012).
Shashkova, E. V., Doronin, K., Senac, J. S. & Barry, M. A. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 68, 5896–5904 (2008).
Tesfay, M. Z. et al. PEGylation of vesicular stomatitis virus extends virus persistence in blood circulation of passively immunized mice. J. Virol. 87, 3752–3759 (2013).
Delwar, Z., Wong, J. W. J. & Jia, W. W. Potency of oncolytic herpes virotherapy is hindered by microglia barrier in glioblastoma multiforme in-vitro [poster 65]. Presented at the 7th International Oncolytic Viruses Meeting (2013).
Dinney, C. P. et al. Phase I trial of intravesical recombinant adenovirus mediated interferon-α2b formulated in Syn3 for Bacillus Calmette–Guérin failures in nonmuscle invasive bladder cancer. J. Urol. 190, 850–856 (2013).
Shayakhmetov, D. M., Li, Z. Y., Ni, S. & Lieber, A. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J. Virol. 78, 5368–5381 (2004).
Advani, S. J. et al. Preferential replication of systemically delivered oncolytic vaccinia virus in focally irradiated glioma xenografts. Clin. Cancer Res. 18, 2579–2590 (2012).
Bolyard, C. et al. Doxorubicin synergizes with 34.5ENVE to enhance antitumour efficacy against metastatic ovarian cancer. Clin. Cancer Res. 20, 6479–6494 (2014).
Ottolino-Perry, K., Diallo, J.-S., Lichty, B. D., Bell, J. C. & McCart, J. A. Intelligent design: combination therapy with oncolytic viruses. Mol. Ther. 18, 251–263 (2010).
Aghi, M., Rabkin, S. & Martuza, R. L. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J. Natl Cancer Inst. 98, 38–50 (2006).
Kolb, E. A. et al. A phase I trial and viral clearance study of reovirus (Reolysin) in children with relapsed or refractory extra-cranial solid tumours: a Children's Oncology Group Phase I Consortium report. Pediatr. Blood Cancer 62, 751–758 (2015).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015).
Barbieri, C. E. et al. The mutational landscape of prostate cancer. Eur. Urol. 64, 567–576 (2013).
Grasso, C. S. et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 (2012).
Trotman, L. C. et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 1, E59 (2003).
Barbieri, C. E. Evolution of novel biomarkers for detection of prostate cancer. J. Urol. 6, 1970–1971 (2013).
Sun, X. et al. Genetic alterations in the PI3K pathway in prostate cancer. Anticancer Res. 29, 1739–1743 (2009).
Barbieri, C. E. et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat. Genet. 44, 685–689 (2012).
Chen, M. et al. Identification of PHLPP as a tumour suppressor reveals the role of pathway feedback compensation in PTEN-mutant prostate cancer progression. Cancer Res. 71, 2405–2405 (2011).
Taylor, B. S. et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22 (2010).
Chen, W. et al. Enhanced antitumour efficacy of a novel fiber chimeric oncolytic adenovirus expressing p53 on hepatocellular carcinoma. Cancer Lett. 307, 93–103 (2011).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Kompier, L. C. et al. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS ONE 5, e13821 (2010).
Mitra, A. P. et al. The use of genetic programming in the analysis of quantitative gene expression profiles for identification of nodal status in bladder cancer. BMC Cancer 6, 159 (2006).
Mitra, A. P., Lin, H., Datar, R. H. & Cote, R. J. Molecular biology of bladder cancer: prognostic and clinical implications. Clin. Genitourin. Cancer 5, 67–77 (2006).
Sanchez-Carbayo, M. et al. Molecular profiling of bladder cancer using cDNA microarrays defining histogenesis and biological phenotypes. Cancer Res. 62, 6973–6980 (2002).
Miyamoto, H. et al. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 109, 1716–1726 (2012).
Netto, G. J. Molecular genetics and genomics progress in urothelial bladder cancer. Semin. Diagn. Pathol. 30, 313–320 (2013).
Netto, G. J. Molecular biomarkers in urothelial carcinoma of the bladder: are we there yet? Nat. Rev. Urol. 9, 41–51 (2012).
Hann, B. & Balmain, A. Replication of an E1B 55-kilodalton protein-deficient adenovirus (ONYX-015) is restored by gain-of-function rather than loss-of- function p53 mutants. J. Virol. 77, 11588–11595 (2003).
McCormick, F. Interactions between adenovirus proteins and the p53 pathway: the development of ONYX-015. Semin. Cancer Biol. 10, 453–459 (2000).
O'Shea, C. C. et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumour selectivity. Cancer Cell 6, 611–623 (2004).
Yew, P. R. & Berk, A. J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature 357, 82–85 (1992).
Cherubini, G. et al. The oncolytic adenovirus AdΔΔ enhances selective cancer cell killing in combination with DNA-damaging drugs in pancreatic cancer models. Gene Ther. 18, 1157–1165 (2011).
Liu, T. C. et al. An E1B-19 kDa gene deletion mutant adenovirus demonstrates tumour necrosis factor-enhanced cancer selectivity and enhanced oncolytic potency. Mol. Ther. 9, 786–803 (2004).
Nemunaitis, J. et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 8, 746–759 (2001).
Nemunaitis, J. et al. Selective replication and oncolysis in p53 mutant tumours with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 60, 6359–6366 (2000).
Sze, D. Y. et al. Dr. Gary J. Becker Young Investigator Award: intraarterial adenovirus for metastatic gastrointestinal cancer: activity, radiographic response, and survival. J. Vasc. Interv. Radiol. 14, 279–290 (2003).
Bischoff, J. R. et al. An adenovirus mutant that replicates selectively in p53-deficient human tumour cells. Science 274, 373–376 (1996).
Edwards, S. J. et al. Evidence that replication of the antitumour adenovirus ONYX-015 is not controlled by the p53 and p14ARF tumour suppressor genes. J. Virol. 76, 12483–12490 (2002).
Makower, D. et al. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumours with correlative p53 studies. Clin. Cancer Res. 9, 693–702 (2003).
Egan, C., Bayley, S. T. & Branton, P. E. Binding of the Rb1 protein to E1A products is required for adenovirus transformation. Oncogene 4, 383–388 (1989).
Grand, R. J. et al. Human cells arrest in S phase in response to adenovirus 12 E1A. Virology 244, 330–342 (1998).
Grand, R. J. et al. The expression of the retinoblastoma gene product Rb1 in primary and adenovirus-transformed human cells. Oncogene 4, 1291–1298 (1989).
Ding, M. et al. Prostate cancer-specific and potent antitumour effect of a DD3-controlled oncolytic virus harboring the PTEN gene. PLoS ONE 7, e35153 (2012).
Ji, W.-T. & Liu, H. J. PI3K–Akt signaling and viral infection. Recent Pat. Biotechnol. 2, 218–226 (2008).
Dunn, E. F. & Connor, J. H. HijAkt: the PI3K/Akt pathway in virus replication and pathogenesis. Prog. Mol. Biol. Transl. Sci. 106, 223 (2012).
Brown, M. C., Dobrikov, M. I. & Gromeier, M. Mitogen-activated protein kinase-interacting kinase regulates mTOR/AKT signaling and controls the serine/arginine-rich protein kinase-responsive type 1 internal ribosome entry site-mediated translation and viral oncolysis. J. Virol. 88, 13149–13160 (2014).
Kanai, R., Wakimoto, H., Martuza, R. L. & Rabkin, S. D. A novel oncolytic herpes simplex virus that synergizes with phosphoinositide 3-kinase/Akt pathway inhibitors to target glioblastoma stem cells. Clin. Cancer Res. 17, 3686–3696 (2011).
Luo, C. et al. Replication-competent, oncolytic herpes simplex virus type 1 mutants induce a bystander effect following ganciclovir treatment. J. Gene Med. 9, 875–883 (2007).
Veerapong, J. et al. Systemic delivery of γ134.5-deleted herpes simplex virus-1 selectively targets and treats distant human xenograft tumours that express high MEK activity. Cancer Res. 67, 8301–8306 (2007).
Gholami, S. et al. Role of MAPK in oncolytic herpes viral therapy in triple-negative breast cancer. Cancer Gene Ther. 21, 283–289 (2014).
Carver, B. S. Strategies for targeting the androgen receptor axis in prostate cancer. Drug Discov. Today 19, 1493–1497 (2014).
Miyamoto, H. et al. Promotion of bladder cancer development and progression by androgen receptor signals. J. Natl Cancer Inst. 99, 558–568 (2007).
Hu, R. et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 69, 16–22 (2009).
Koivisto, P. et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 57, 314–319 (1997).
Linja, M. J. & Visakorpi, T. Alterations of androgen receptor in prostate cancer. J. Steroid Biochem. Mol. Biol. 92, 255–264 (2004).
Mehra, R. et al. Comprehensive assessment of TMPRSS2 and ETS family gene aberrations in clinically localized prostate cancer. Mod. Pathol. 20, 538–544 (2007).
Tomlins, S. A. et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature 448, 595–599 (2007).
King, J. C. et al. Cooperativity of TMPRSS2–ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat. Genet. 41, 524–526 (2009).
Klezovitch, O. et al. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc. Natl Acad. Sci. USA 105, 2105–2110 (2008).
Boorjian, S. et al. Androgen receptor expression is inversely correlated with pathologic tumour stage in bladder cancer. Urology 64, 383–388 (2004).
Jing, Y. et al. Activated androgen receptor promotes bladder cancer metastasis via Slug mediated epithelial–mesenchymal transition. Cancer Lett. 348, 135–145 (2014).
Mhawech-Fauceglia, P. et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology 50, 472–483 (2007).
Reiter, R. E. et al. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc. Natl Acad. Sci. USA 95, 1735–1740 (1998).
Wu, X. et al. Genetic variation in the prostate stem cell antigen gene PSCA confers susceptibility to urinary bladder cancer. Nat. Genet. 41, 991–995 (2009).
Amara, N. et al. Prostate stem cell antigen is overexpressed in human transitional cell carcinoma. Cancer Res. 61, 4660–4665 (2001).
Bera, T. K. et al. NGEP, a gene encoding a membrane protein detected only in prostate cancer and normal prostate. Proc. Natl Acad. Sci. USA 101, 3059–3064 (2004).
Conrad, F. et al. Human antibodies targeting cell surface antigens overexpressed by the hormone refractory metastatic prostate cancer cells: ICAM-1 is a tumour antigen that mediates prostate cancer cell invasion. J. Mol. Med. 87, 507–514 (2009).
Poovassery, J. S., Kang, J. C., Kim, D., Ober, R. J. & Ward, E. S. Antibody targeting of HER2/HER3 signaling overcomes heregulin-induced resistance to PI3K inhibition in prostate cancer. Int. J. Cancer 137, 267–277 (2015).
Brenner, P. C. et al. TAG-72 expression in primary, metastatic and hormonally treated prostate cancer as defined by monoclonal antibody CC49. J. Urol. 153, 1575–1579 (1995).
Waehler, R., Russell, S. J. & Curiel, D. T. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 8, 573–587 (2007).
Tang, K. et al. Early outcomes of thulium laser versus transurethral resection of the prostate for managing benign prostatic hyperplasia: a systematic review and meta-analysis of comparative studies. J. Endourol. 28, 65–72 (2014).
Logan, C., Brown, M. & Hayne, D. Intravesical therapies for bladder cancer — indications and limitations. BJU Int. 110 (Suppl. 4), 12–21 (2012).
Herr, H. W. & Morales, A. History of bacillus Calmette–Guérin and bladder cancer: an immunotherapy success story. J. Urol. 179, 53–56 (2008).
Choi, I. K. & Yun, C. O. Recent developments in oncolytic adenovirus-based immunotherapeutic agents for use against metastatic cancers. Cancer Gene Ther. 20, 70–76 (2013).
Gujar, S. A., Pan, D. A., Marcato, P., Garant, K. A. & Lee, P. W. Oncolytic virus-initiated protective immunity against prostate cancer. Mol. Ther. 19, 797–804 (2011).
Hersey, P. & Gallagher, S. Intralesional immunotherapy for melanoma. J. Surg. Oncol. 109, 320–326 (2014).
Stojdl, D. F. et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4, 263–275 (2003).
Moussavi, M. et al. Oncolysis of prostate cancers induced by vesicular stomatitis virus in PTEN knockout mice. Cancer Res. 70, 1367–1376 (2010).
Moussavi, M. et al. Targeting and killing of metastatic cells in the transgenic adenocarcinoma of mouse prostate model with vesicular stomatitis virus. Mol. Ther. 21, 842–848 (2013).
Zhang, K. X. et al. Down-regulation of type I interferon receptor sensitizes bladder cancer cells to vesicular stomatitis virus-induced cell death. Int. J. Cancer 127, 830–838 (2010).
Ayala-Breton, C., Barber, G. N., Russell, S. J. & Peng, K. W. Retargeting vesicular stomatitis virus using measles virus envelope glycoproteins. Hum. Gene Ther. 23, 484–491 (2012).
Zhigang, Z. & Wenlu, S. Prostate stem cell antigen (PSCA) mRNA expression in prostatic intraepithelial neoplasia: implications for the development of prostate cancer. Prostate 67, 1143–1151 (2007).
Wen, Y. et al. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 60, 6841–6845 (2000).
Zhang, K. X. et al. Lentiviruses with trastuzumab bound to their envelopes can target and kill prostate cancer cells. Cancer Gene Ther. 16, 820–831 (2009).
Conner, J., Braidwood, L. & Brown, S. M. A strategy for systemic delivery of the oncolytic herpes virus HSV1716: redirected tropism by antibody-binding sites incorporated on the virion surface as a glycoprotein D fusion protein. Gene Ther. 15, 1579–1592 (2008).
Anderson, B. D., Nakamura, T., Russell, S. J. & Peng, K. W. High CD46 receptor density determines preferential killing of tumour cells by oncolytic measles virus. Cancer Res. 64, 4919–4926 (2004).
Sugiyama, T. et al. Measles virus selectively blind to signaling lymphocyte activation molecule as a novel oncolytic virus for breast cancer treatment. Gene Ther. 20, 338–347 (2013).
Noyce, R. S. et al. Tumour cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 7, e1002240 (2011).
Msaouel, P., Iankov, I. D., Allen, C., Russell, S. J. & Galanis, E. Oncolytic measles virus retargeting by ligand display. Methods Mol. Biol. 797, 141–162 (2012).
Jing, Y. et al. Tumour and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor. Cancer Res. 69, 1459–1468 (2009).
Kamiyama, H., Zhou, G. & Roizman, B. Herpes simplex virus 1 recombinant virions exhibiting the amino terminal fragment of urokinase-type plasminogen activator can enter cells via the cognate receptor. Gene Ther. 13, 621–629 (2006).
Kinoh, H. et al. Generation of optimized and urokinase-targeted oncolytic Sendai virus vectors applicable for various human malignancies. Gene Ther. 16, 392–403 (2009).
Dohn, L. H. et al. Urokinase-type plasminogen activator receptor (uPAR) expression is associated with T-stage and survival in urothelial carcinoma of the bladder. Urol. Oncol. 33, 165.e15–165.e24 (2015).
Lippert, S. et al. Copenhagen uPAR prostate cancer (CuPCa) database: protocol and early results. Biomark. Med. 10, 209–216 (2016).
Li, Y. & Cozzi, P. J. Targeting uPA/uPAR in prostate cancer. Cancer Treat. Rev. 33, 521–527 (2007).
Fuessel, S. et al. Prognostic impact of urokinase-type plasminogen activator system components in clear cell renal cell carcinoma patients without distant metastasis. BMC Cancer 14, 974 (2014).
Suzuki, K. et al. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin. Cancer Res. 7, 120–126 (2001).
Koizumi, N. et al. Fiber-modified adenovirus vectors decrease liver toxicity through reduced IL-6 production. J. Immunol. 178, 1767–1773 (2007).
Hemminki, O. et al. Ad3-hTERT-E1A, a fully serotype 3 oncolytic adenovirus, in patients with chemotherapy refractory cancer. Mol. Ther. 20, 1821–1830 (2012).
Hemminki, O. et al. Oncolytic adenovirus based on serotype 3. Cancer Gene Ther. 18, 288–296 (2011).
Strauss, R. et al. Epithelial phenotype confers resistance of ovarian cancer cells to oncolytic adenoviruses. Cancer Res. 69, 5115–5125 (2009).
Kuhn, I. et al. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS ONE 3, e2409 (2008).
Shobana, R., Samal, S. K. & Elankumaran, S. Prostate-specific antigen-retargeted recombinant newcastle disease virus for prostate cancer virotherapy. J. Virol. 87, 3792–3800 (2013).
Choi, J. W. et al. pH-sensitive oncolytic adenovirus hybrid targeting acidic tumour microenvironment and angiogenesis. J. Control. Release 205, 134–143 (2015).
Lee, C. Y., Bu, L. X., Rennie, P. S. & Jia, W. W. An HSV-1 amplicon system for prostate-specific expression of ICP4 to complement oncolytic viral replication for in vitro and in vivo treatment of prostate cancer cells. Cancer Gene Ther. 14, 652–660 (2007).
Zhai, Z. et al. Antitumour effects of bladder cancer-specific adenovirus carrying E1A-androgen receptor in bladder cancer. Gene Ther. 19, 1065–1074 (2012).
Fan, J. K. et al. Targeting Gene-ViroTherapy for prostate cancer by DD3-driven oncolytic virus-harboring interleukin-24 gene. Int. J. Cancer 127, 707–717 (2010).
Lai, J. et al. PSA/KLK3 AREI promoter polymorphism alters androgen receptor binding and is associated with prostate cancer susceptibility. Carcinogenesis 28, 1032–1039 (2007).
Watt, F. et al. A tissue-specific enhancer of the prostate-specific membrane antigen gene, FOLH1. Genomics 73, 243–254 (2001).
Wolfgang, C. D., Essand, M., Lee, B. & Pastan, I. T-cell receptor γ chain alternate reading frame protein (TARP) expression in prostate cancer cells leads to an increased growth rate and induction of caveolins and amphiregulin. Cancer Res. 61, 8122–8126 (2001).
Yang, C. T. et al. Herpes simplex virus type-1 infection upregulates cellular promoters and telomerase activity in both tumour and nontumour human cells. Gene Ther. 10, 1494–1502 (2003).
Fan, S. et al. Phosphorylated eukaryotic translation initiation factor 4 (eIF4E) is elevated in human cancer tissues. Cancer Biol. Ther. 8, 1463–1469 (2009).
Lee, C. Y. et al. Transcriptional and translational dual-regulated oncolytic herpes simplex virus type 1 for targeting prostate tumours. Mol. Ther. 18, 929–935 (2010).
Yan, Y. et al. Large fragment of the probasin promoter targets high levels of transgene expression to the prostate of transgenic mice. Prostate 32, 129–139 (1997).
Zhang, J., Thomas, T. Z., Kasper, S. & Matusik, R. J. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology 141, 4698–4710 (2000).
Lee, C. Y., Rennie, P. S. & Jia, W. W. MicroRNA regulation of oncolytic herpes simplex virus-1 for selective killing of prostate cancer cells. Clin. Cancer Res. 15, 5126–5135 (2009).
Li, J. M. et al. MicroRNA-145 regulates oncolytic herpes simplex virus-1 for selective killing of human non-small cell lung cancer cells. Virol. J. 10, 241 (2013).
Zhang, Z., Zhang, X., Newman, K., Liu, X. & Seth, P. MicroRNA regulation of oncolytic adenovirus 6 for selective treatment of castration-resistant prostate cancer. Mol. Cancer Ther. 11, 2410–2418 (2012).
Ylosmaki, E. et al. MicroRNA-mediated suppression of oncolytic adenovirus replication in human liver. PLoS ONE 8, e54506 (2013).
Callegari, E. et al. Anti-tumour activity of a miR-199-dependent oncolytic adenovirus. PLoS ONE 8, e73964 (2013).
Leber, M. F. et al. MicroRNA-sensitive oncolytic measles viruses for cancer-specific vector tropism. Mol. Ther. 19, 1097–1106 (2011).
Hikichi, M. et al. MicroRNA regulation of glycoprotein B5R in oncolytic vaccinia virus reduces viral pathogenicity without impairing its antitumour efficacy. Mol. Ther. 19, 1107–1115 (2011).
Bell, J. C. & Kirn, D. MicroRNAs fine-tune oncolytic viruses. Nat. Biotechnol. 26, 1346–1348 (2008).
Kueberuwa, G., Cawood, R., Tedcastle, A. & Seymour, L. W. Tissue-specific attenuation of oncolytic sindbis virus without compromised genetic stability. Hum. Gene Ther. Methods 25, 154–165 (2014).
Nguyen, A., Ho, L. & Wan, Y. Chemotherapy and oncolytic virotherapy: advanced tactics in the war against cancer. Front. Oncol. 4, 145 (2014).
Muthana, M. et al. Macrophage delivery of an oncolytic virus abolishes tumour regrowth and metastasis after chemotherapy or irradiation. Cancer Res. 73, 490–495 (2013).
Kirby, M., Hirst, C. & Crawford, E. Characterising the castration-resistant prostate cancer population: a systematic review. Int. J. Clin. Pract. 65, 1180–1192 (2011).
Bressy, C. & Benihoud, K. Association of oncolytic adenoviruses with chemotherapies: an overview and future directions. Biochem. Pharmacol. 90, 97–106 (2014).
Kanai, R. & Rabkin, S. D. Combinatorial strategies for oncolytic herpes simplex virus therapy of brain tumours. CNS Oncol. 2, 129–142 (2013).
Wennier, S. T., Liu, J. & McFadden, G. Bugs and drugs: oncolytic virotherapy in combination with chemotherapy. Curr. Pharm. Biotechnol. 13, 1817–1833 (2012).
Bazan-Peregrino, M., Arvanitis, C. D., Rifai, B., Seymour, L. W. & Coussios, C. C. Ultrasound-induced cavitation enhances the delivery and therapeutic efficacy of an oncolytic virus in an in vitro model. J. Control. Release 157, 235–242 (2012).
Advani, S. J. et al. Increased oncolytic efficacy for high-grade gliomas by optimal integration of ionizing radiation into the replicative cycle of HSV-1. Gene Ther. 18, 1098–1102 (2011).
Aghi, M., Rabkin, S. & Martuza, R. L. Oncolytic herpes simplex virus mutants exhibit enhanced replication in glioma cells evading temozolomide chemotherapy through deoxyribonucleic acid repair. Clin. Neurosurg. 53, 65–76 (2006).
Lin, S. F. et al. Synergy of a herpes oncolytic virus and paclitaxel for anaplastic thyroid cancer. Clin. Cancer Res. 14, 1519–1528 (2008).
Bennett, J. J. et al. Up-regulation of GADD34 mediates the synergistic anticancer activity of mitomycin C and a γ134.5 deleted oncolytic herpes virus (G207). FASEB J. 18, 1001–1003 (2004).
Kanai, R. et al. Effect of γ34.5 deletions on oncolytic herpes simplex virus activity in brain tumours. J. Virol. 86, 4420–4431 (2012).
Sei, S. et al. Synergistic antitumour activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cells. Mol. Cancer 8, 47 (2009).
Siurala, M. et al. Oncolytic adenovirus and doxorubicin-based chemotherapy results in synergistic antitumour activity against soft-tissue sarcoma. Int. J. Cancer 136, 945–954 (2015).
Touchefeu, Y., Vassaux, G. & Harrington, K. J. Oncolytic viruses in radiation oncology. Radiother. Oncol. 99, 262–270 (2011).
Harrington, K. J. et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin. Cancer Res. 16, 4005–4015 (2010).
Harrington, K. J., Melcher, A., Vassaux, G., Pandha, H. S. & Vile, R. G. Exploiting synergies between radiation and oncolytic viruses. Curr. Opin. Mol. Ther. 10, 362–370 (2008).
Uhlman, M. A., Bing, M. T. & Lubaroff, D. M. Prostate cancer vaccines in combination with additional treatment modalities. Immunol. Res. 59, 236–242 (2014).
Roulstone, V. et al. BRAF-and MEK-targeted small molecule inhibitors exert enhanced antimelanoma effects in combination with oncolytic reovirus through ER stress. Mol. Ther. 23, 931–942 (2015).
Tikhmyanova, N., Schultz, D. C., Lee, T., Salvino, J. M. & Lieberman, P. M. Identification of a new class of small molecules that efficiently reactivate latent Epstein–Barr virus. ACS Chem. Biol. 9, 785–795 (2014).
Diallo, J.-S. et al. A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol. Ther. 18, 1123–1129 (2010).
Otsuki, A. et al. Histone deacetylase inhibitors augment antitumour efficacy of herpes-based oncolytic viruses. Mol. Ther. 16, 1546–1555 (2008).
McKenzie, B. A. et al. In vitro screen of a small molecule inhibitor drug library identifies multiple compounds that synergize with oncolytic myxoma virus against human brain tumour-initiating cells. Neuro Oncol. 17, 1086–1094 (2015).
Law, B. K. Rapamycin: an anti-cancer immunosuppressant? Crit. Rev. Oncol. Hematol. 56, 47–60 (2005).
Stanford, M. M., Barrett, J. W., Nazarian, S. H., Werden, S. & McFadden, G. Oncolytic virotherapy synergism with signaling inhibitors: rapamycin increases myxoma virus tropism for human tumour cells. J. Virol. 81, 1251–1260 (2007).
Jimenez, J. A. et al. Antitumour activity of Ad-IU2, a prostate-specific replication-competent adenovirus encoding the apoptosis inducer, TRAIL. Cancer Gene Ther. 17, 180–191 (2010).
Abate-Daga, D. et al. Oncolytic adenoviruses armed with thymidine kinase can be traced by PET imaging and show potent antitumoural effects by ganciclovir dosing. PLoS ONE 6, e26142 (2011).
Cascante, A. et al. GCV modulates the antitumoural efficacy of a replicative adenovirus expressing the Tat8-TK as a late gene in a pancreatic tumour model. Gene Ther. 14, 1471–1480 (2007).
Kirn, D. H. The end of the beginning: oncolytic virotherapy achieves clinical proof-of-concept. Mol. Ther. 13, 237–238 (2006).
Kasuya, H. et al. Selectivity of an oncolytic herpes simplex virus for cells expressing the DF3/MUC1 antigen. Cancer Res. 64, 2561–2567 (2004).
Yang, C. T. et al. Oncolytic herpesvirus with secretable angiostatic proteins in the treatment of human lung cancer cells. Anticancer Res. 25, 2049–2054 (2005).
Zhang, G. et al. Enhanced antitumour efficacy of an oncolytic herpes simplex virus expressing an endostatin–angiostatin fusion gene in human glioblastoma stem cell xenografts. PLoS ONE 9, e95872 (2014).
Chai, L. et al. A novel conditionally replicating adenoviral vector with dual expression of IL-24 and arresten inserted in E1 and the region between E4 and fiber for improved melanoma therapy. Cancer Gene Ther. 19, 247–254 (2012).
Frentzen, A. et al. Anti-VEGF single-chain antibody GLAF-1 encoded by oncolytic vaccinia virus significantly enhances antitumour therapy. Proc. Natl Acad. Sci. USA 106, 12915–12920 (2009).
Choi, I. et al. Oncolytic adenovirus co-expressing IL-12 and IL-18 improves tumour-specific immunity via differentiation of T cells expressing IL-12Rβ2 or IL-18Rα. Gene Ther. 18, 898–909 (2011).
Jin, F., Xie, Z., Kuo, C. J., Chung, L. W. & Hsieh, C. L. Cotargeting tumour and tumour endothelium effectively inhibits the growth of human prostate cancer in adenovirus-mediated antiangiogenesis and oncolysis combination therapy. Cancer Gene Ther. 12, 257–267 (2005).
Thorne, S. H., Tam, B. Y., Kirn, D. H., Contag, C. H. & Kuo, C. J. Selective intratumoural amplification of an antiangiogenic vector by an oncolytic virus produces enhanced antivascular and anti-tumour efficacy. Mol. Ther. 13, 938–946 (2006).
Hu, Z. et al. Systemic delivery of oncolytic adenoviruses targeting transforming growth factor-β inhibits established bone metastasis in a prostate cancer mouse model. Hum. Gene Ther. 23, 871–882 (2012).
Gil, M. et al. CXCL12/CXCR4 blockade by oncolytic virotherapy inhibits ovarian cancer growth by decreasing immunosuppression and targeting cancer-initiating cells. J. Immunol. 193, 5327–5337 (2014).
Andtbacka, R. H. et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33, 2780–2788 (2015).
Heo, J. et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 19, 329–336 (2013).
Sprent, J. Antigen-presenting cells: professionals and amateurs. Curr. Biol. 5, 1095–1097 (1995).
Madan, R. A., Arlen, P. M., Mohebtash, M., Hodge, J. W. & Gulley, J. L. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin. Investig. Drugs 18, 1001–1011 (2009).
Vivier, E., Tomasello, E., Baratin, M., Walzer, T. & Ugolini, S. Functions of natural killer cells. Nat. Immunol. 9, 503–510 (2008).
Pahl, J. & Cerwenka, A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology http://dx.doi.org/10.1016/j.imbio.2015.07.012 (2015).
Janeway, C., Travers, P., Walport, M. & Shlomchik, M. Immunobiology: The Immune System in Health and Disease 6th edn (Garland Science, 2005).
Spurrell, E. L. & Lockley, M. Adaptive immunity in cancer immunology and therapeutics. Ecancermedicalscience 8, 441 (2014).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Sunshine, J. & Taube, J. M. PD-1/PD-L1 inhibitors. Curr. Opin. Pharmacol. 23, 32–38 (2015).
Bracarda, S. et al. Immunologic checkpoints blockade in renal cell, prostate, and urothelial malignancies. Semin. Oncol. 42, 495–505 (2015).
Jochems, C. et al. A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: immune correlates. Cancer Immunol. Immunother. 63, 407–418 (2014).
Ebelt, K. et al. Prostate cancer lesions are surrounded by FOXP3+, PD-1+ and B7-H1+ lymphocyte clusters. Eur. J. Cancer 45, 1664–1672 (2009).
Gevensleben, H. et al. The immune checkpoint regulator PD-L1 is highly expressed in aggressive primary prostate cancer. Clin. Cancer Res. 5, 1969–1977 (2016).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Taube, J. M. et al. Association of PD-1, PD-1 ligands, and other features of the tumour immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 20, 5064–5074 (2014).
Engeland, C. E. et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol. Ther. 22, 1949–1959 (2014).
Rajani, K. et al. Combination therapy with reovirus and anti-PD-1 blockade controls tumour growth through innate and adaptive immune responses. Mol. Ther. 24, 166–174 (2016).
Au, G., Quah, M., Wong, Y. & Shafren, D. Combination of a novel oncolytic immunotherapeutic agent, CAVATAK™ (Coxsackievirus A21) and immune-checkpoint blockade significantly reduces tumour growth and improves survival in an immune competent mouse melanoma model. Presented at the 9th International conference on oncolytic virus therapeutics 2015, O-34 (2015).
Saha, D., Martuza, R. L. & Rabkin, S. D. Immunovirotherapy in combination with immune checkpoint inhibitors for treating glioblastoma. Presented at the 9th International conference on oncolytic virus therapeutics 2015, P-61 (2015).
Haseley, A. et al. Extracellular matrix protein CCN1 limits oncolytic efficacy in glioma. Cancer Res. 72, 1353–1362 (2012).
Wojton, J. & Kaur, B. Impact of tumour microenvironment on oncolytic viral therapy. Cytokine Growth Factor Rev. 21, 127–134 (2010).
Yaacov, B. et al. Extracellular matrix constituents interfere with Newcastle disease virus spread in solid tissue and diminish its potential oncolytic activity. J. Gen. Virol. 93, 1664–1672 (2012).
Yun, C. O. Overcoming the extracellular matrix barrier to improve intratumoural spread and therapeutic potential of oncolytic virotherapy. Curr. Opin. Mol. Ther. 10, 356–361 (2008).
Power, A. T. & Bell, J. C. Taming the Trojan horse: optimizing dynamic carrier cell/oncolytic virus systems for cancer biotherapy. Gene Ther. 15, 772–779 (2008).
Power, A. T. et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol. Ther. 15, 123–130 (2007).
Willmon, C. et al. Cell carriers for oncolytic viruses: Fed Ex for cancer therapy. Mol. Ther. 17, 1667–1676 (2009).
Ong, H., Hasegawa, K., Dietz, A., Russell, S. & Peng, K. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 14, 324–333 (2007).
Iankov, I. D. et al. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol. Ther. 15, 114–122 (2007).
Ilett, E. J. et al. Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity. Gene Ther. 16, 689–699 (2009).
Peng, K. W. et al. Tumour-associated macrophages infiltrate plasmacytomas and can serve as cell carriers for oncolytic measles virotherapy of disseminated myeloma. Am. J. Hematol. 84, 401–407 (2009).
Thorne, S. H. et al. Targeting localized immune suppression within the tumour through repeat cycles of immune cell-oncolytic virus combination therapy. Mol. Ther. 18, 1698–1705 (2010).
Adair, R. A. et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumour in patients. Sci. Transl. Med. 4, 138ra77 (2012).
Eisenstein, S. et al. Myeloid-derived suppressor cells as a vehicle for tumour-specific oncolytic viral therapy. Cancer Res. 73, 5003–5015 (2013).
Pan, Q. et al. Synergistic induction of tumour cell death by combining cisplatin with an oncolytic adenovirus carrying TRAIL. Mol. Cell. Biochem. 304, 315–323 (2007).
Komarova, S., Kawakami, Y., Stoff-Khalili, M. A., Curiel, D. T. & Pereboeva, L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol. Cancer Ther. 5, 755–766 (2006).
Sonabend, A. M. et al. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells 26, 831–841 (2008).
Jevremovic, D. et al. Use of blood outgrowth endothelial cells as virus-producing vectors for gene delivery to tumours. Am. J. Physiol. Heart Circ. Physiol. 287, H494–H500 (2004).
Muthana, M. et al. Use of macrophages to target therapeutic adenovirus to human prostate tumours. Cancer Res. 71, 1805–1815 (2011).
Muthana, M. et al. Directing cell therapy to anatomic target sites in vivo with magnetic resonance targeting. Nat. Commun. 6, 8009 (2015).
Yang, L. et al. Suppression of ovarian cancer growth via systemic administration with liposome-encapsulated adenovirus-encoding endostatin. Cancer Gene Ther. 17, 49–57 (2010).
Mendez, N. et al. Encapsulation of adenovirus serotype 5 in anionic lecithin liposomes using a bead-based immunoprecipitation technique enhances transfection efficiency. Biomaterials 35, 9554–9561 (2014).
Kwon, O. J., Kang, E., Choi, J. W., Kim, S. W. & Yun, C. O. Therapeutic targeting of chitosan–PEG–folate-complexed oncolytic adenovirus for active and systemic cancer gene therapy. J. Control. Release 169, 257–265 (2013).
Choi, J. W. et al. Tuning surface charge and PEGylation of biocompatible polymers for efficient delivery of nucleic acid or adenoviral vector. Bioconjug. Chem. 26, 1818–1829 (2015).
Kim, P. H. et al. Active targeting and safety profile of PEG-modified adenovirus conjugated with herceptin. Biomaterials 32, 2314–2326 (2011).
Vetter, A. et al. Adenoviral vectors coated with PAMAM dendrimer conjugates allow CAR independent virus uptake and targeting to the EGF receptor. Mol. Pharm. 10, 606–618 (2013).
Fisher, K. D. & Seymour, L. W. HPMA copolymers for masking and retargeting of therapeutic viruses. Adv. Drug Deliv. Rev. 62, 240–245 (2010).
Choi, J. W. et al. Hepatoma targeting peptide conjugated bio-reducible polymer complexed with oncolytic adenovirus for cancer gene therapy. J. Control. Release 220, 691–703 (2015).
Kim, J., Nam, H. Y., Choi, J. W., Yun, C. O. & Kim, S. W. Efficient lung orthotopic tumour-growth suppression of oncolytic adenovirus complexed with RGD-targeted bioreducible polymer. Gene Ther. 21, 476–483 (2014).
Carosella, E. D., Ploussard, G., LeMaoult, J. & Desgrandchamps, F. A. Systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur. Urol. 68, 267–279 (2015).
Dong, B., Minze, L. J., Xue, W. & Chen, W. Molecular insights into the development of T cell-based immunotherapy for prostate cancer. Expert Rev. Clin. Immunol. 10, 1547–1557 (2014).
Nemerow, G. R., Stewart, P. L. & Reddy, V. S. Structure of human adenovirus. Curr. Opin. Virol. 2, 115–121 (2012).
Uusi-Kerttula, H., Hulin-Curtis, S., Davies, J. & Parker, A. L. Oncolytic adenovirus: strategies and insights for vector design and immuno-oncolytic applications. Viruses 7, 6009–6042 (2015).
Roizman, B. & Sears, A. (eds) Herpes Simplex Viruses and their replication (New York Raven Press, 1990).
Zhang, S. X. Turning killer into cure — the story of oncolytic herpes simplex viruses. Discov. Med. 20, 303–309 (2015).
Ning, J. & Wakimoto, H. Oncolytic herpes simplex virus-based strategies: toward a breakthrough in glioblastoma therapy. Front. Microbiol. 5, 303 (2014).
Johnson, L., Gupta, A. K., Ghafoor, A., Akin, D. & Bashir, R. Characterization of vaccinia virus particles using microscale silicon cantilever resonators and atomic force microscopy. Sens. Actuators B Chem. 115, 189–197 (2006).
Moss, B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc. Natl Acad. Sci. USA 93, 11341–11348 (1996).
Jefferson, A., Cadet, V. E. & Hielscher, A. The mechanisms of genetically modified vaccinia viruses for the treatment of cancer. Crit. Rev. Oncol. Hematol. 95, 407–416 (2015).
Kim, M. Replicating poxviruses for human cancer therapy. J. Microbiol. 53, 209–218 (2015).
Shmulevitz, M., Marcato, P. & Lee, P. W. Unshackling the links between reovirus oncolysis, Ras signaling, translational control and cancer. Oncogene 24, 7720–7728 (2005).
Coffey, M. C., Strong, J. E., Forsyth, P. A. & Lee, P. W. Reovirus therapy of tumours with activated Ras pathway. Science 282, 1332–1334 (1998).
Lichty, B. D., Power, A. T., Stojdl, D. F. & Bell, J. C. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol. Med. 10, 210–216 (2004).
Balachandran, S. & Barber, G. N. Vesicular stomatitis virus (VSV) therapy of tumours. IUBMB Life 50, 135–138 (2000).
Stojdl, D. F. et al. Exploiting tumour-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6, 821–825 (2000).
Rodriguez, R. et al. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 57, 2559–2563 (1997).
Yu, D. C., Chen, Y., Seng, M., Dilley, J. & Henderson, D. R. The addition of adenovirus type 5 region E3 enables calydon virus 787 to eliminate distant prostate tumour xenografts. Cancer Res. 59, 4200–4203 (1999).
Rogulski, K. R. et al. Double suicide gene therapy augments the antitumour activity of a replication-competent lytic adenovirus through enhanced cytotoxicity and radiosensitization. Hum. Gene Ther. 11, 67–76 (2000).
Neuman, E., Flemington, E. K., Sellers, W. R. & Kaelin, W. G. Jr. Transcription of the E2F-1 gene is rendered cell cycle dependent by E2F DNA-binding sites within its promoter. Mol. Cell. Biol. 15, 4660 (1995).
Dranoff, G. et al. Vaccination with irradiated tumour cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumour immunity. Proc. Natl Acad. Sci. USA 90, 3539–3543 (1993).
Janetzki, S. et al. Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int. J. Cancer 88, 232–238 (2000).
Huang, X. F. et al. A broadly applicable, personalized heat shock protein-mediated oncolytic tumour vaccine. Cancer Res. 63, 7321–7329 (2003).
Strong, J. E. & Lee, P. W. The v-erbB oncogene confers enhanced cellular susceptibility to reovirus infection. J. Virol. 70, 612–616 (1996).
Gong, J. & Mita, M. M. Activated Ras signaling pathways and reovirus oncolysis: an update on the mechanism of preferential reovirus replication in cancer cells. Front. Oncol. 4, 167 (2014).
Bauzon, M., Jin, F., Kretschmer, P. & Hermiston, T. In vitro analysis of cidofovir and genetically engineered TK expression as potential approaches for the intervention of ColoAd1-based treatment of cancer. Gene Ther. 16, 1169–1174 (2009).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02028442 (2015).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02143804 (2015).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02365818 (2016).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00109655 (2008).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01438112 (2015).
Bjørge, L., Jensen, T. S. & Matre, R. Characterisation of the complement-regulatory proteins decay-accelerating factor (DAF, CD55) and membrane cofactor protein (MCP, CD46) on a human colonic adenocarcinoma cell line. Cancer Immunol. Immunother. 42, 185–192 (1996).
Regidor, P., Callies, R., Regidor, M. & Schindler, A. Expression of the cell adhesion molecules ICAM-1 and VCAM-1 in the cytosol of breast cancer tissue, benign breast tissue and corresponding sera. Eur. J. Gynaecol. Oncol. 19, 377–383 (1997).
Kageshita, T. et al. Clinical relevance of ICAM-1 expression in primary lesions and serum of patients with malignant melanoma. Cancer Res. 53, 4927–4932 (1993).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00005916(2015).
Madan, R. A., Arlen, P. M. & Gulley, J. L. PANVAC-VF™: poxviral-based vaccine therapy targeting CEA and MUC1 in carcinoma. Expert Opin. Biol. Ther. 7, 543–554 (2007).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02015104 (2016).
Parato, K. A. et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. 20, 749–758 (2012).
Tamura, Y., Peng, P., Liu, K., Daou, M. & Srivastava, P. K. Immunotherapy of tumours with autologous tumour-derived heat shock protein preparations. Science 278, 117–120 (1997).
Obuchi, M., Fernandez, M. & Barber, G. N. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J. Virol. 77, 8843–8856 (2003).
Biron, C. A. Role of early cytokines, including alpha and beta interferons (IFN-α/β), in innate and adaptive immune responses to viral infections. Sem. Immunol. 10, 383–390 (1998).
Kirn, D. H., Wang, Y., Le Boeuf, F., Bell, J. & Thorne, S. H. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 4, e353 (2007).
Parker, J. N. et al. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumours. Proc. Natl Acad. Sci. USA 97, 2208–2213 (2000).
Passer, B. J. et al. Combination of vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumour and antiangiogenic effects in prostate cancer models. Cancer Gene Ther. 20, 17–24 (2013).
Freytag, S. O., Barton, K. N. & Zhang, Y. Efficacy of oncolytic adenovirus expressing suicide genes and interleukin-12 in preclinical model of prostate cancer. Gene Ther. 20, 1131–1139 (2013).
Beaulieu, A. M., Madera, S. & Sun, J. C. Molecular programming of immunological memory in natural killer cells. Adv. Exp. Med. Biol. 850, 81–91 (2015).
Zundler, S. & Neurath, M. F. Interleukin-12: functional activities and implications for disease. Cytokine Growth Factor Rev. 26, 559–568 (2015).
Zheng, J. N. et al. Potent antitumour efficacy of interleukin-18 delivered by conditionally replicative adenovirus vector in renal cell carcinoma-bearing nude mice via inhibition of angiogenesis. Cancer Biol. Ther. 8, 599–606 (2009).
Fukuhara, H., Ino, Y., Kuroda, T., Martuza, R. L. & Todo, T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Res. 65, 10663–10668 (2005).
Fabbi, M., Carbotti, G. & Ferrini, S. Context- dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP. J. Leukoc. Biol. 97, 665–675 (2015).
Lee, Y. S. et al. Enhanced antitumour effect of oncolytic adenovirus expressing interleukin-12 and B7-1 in an immunocompetent murine model. Clin. Cancer Res. 12, 5859–5868 (2006).
Tong, Y. et al. PI3K inhibitor LY294002 inhibits activation of the Akt/mTOR pathway induced by an oncolytic adenovirus expressing TRAIL and sensitizes multiple myeloma cells to the oncolytic virus. Oncol. Rep. 31, 1581–1588 (2014).
Lee, G. H. et al. The role of CD40 expression in dendritic cells in cancer biology; a systematic review. Curr. Cancer Drug Targets 14, 610–620 (2014).
Hassan, S. B., Sorensen, J. F., Olsen, B. N. & Pedersen, A. E. Anti-CD40-mediated cancer immunotherapy: an update of recent and ongoing clinical trials. Immunopharmacol. Immunotoxicol. 36, 96–104 (2014).
Author information
Authors and Affiliations
Contributions
Z.D., K.Z., P.S.R. and W.J. researched data for the article and made substantial contributions to the discussion of its content. Z.D., K.Z. and W.J. wrote the article. P.S.R. and W.J. reviewed and/or edited the manuscript before submission. Z.D. and K.Z. contributed equally to the preparation of this article.
Corresponding author
Ethics declarations
Competing interests
W.J. is a founder and director of Virogin Biotech Ltd, a company developing oncolytic viruses for cancer treatment. Z.D., K.Z. and P.S.R. declare no competing interests.
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Delwar, Z., Zhang, K., Rennie, P. et al. Oncolytic virotherapy for urological cancers. Nat Rev Urol 13, 334–352 (2016). https://doi.org/10.1038/nrurol.2016.84
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2016.84
This article is cited by
-
Potentiating prostate cancer immunotherapy with oncolytic viruses
Nature Reviews Urology (2018)
-
Oncolytic alphavirus SFV-VA7 efficiently eradicates subcutaneous and orthotopic human prostate tumours in mice
British Journal of Cancer (2017)