Abstract
Oncolytic viruses (OVs) have attracted growing awareness in the twenty-first century, as they are generally considered to have direct oncolysis and cancer immune effects. With the progress in genetic engineering technology, OVs have been adopted as versatile platforms for developing novel antitumor strategies, used alone or in combination with other therapies. Recent studies have yielded eye-catching results that delineate the promising clinical outcomes that OVs would bring about in the future. In this review, we summarized the basic principles of OVs in terms of their classifications, as well as the recent advances in OV-modification strategies based on their characteristics, biofunctions, and cancer hallmarks. Candidate OVs are expected to be designed as “qualified soldiers” first by improving target fidelity and safety, and then equipped with “cold weapons” for a proper cytocidal effect, “hot weapons” capable of activating cancer immunotherapy, or “auxiliary weapons” by harnessing tactics such as anti-angiogenesis, reversed metabolic reprogramming and decomposing extracellular matrix around tumors. Combinations with other cancer therapeutic agents have also been elaborated to show encouraging antitumor effects. Robust results from clinical trials using OV as a treatment congruously suggested its significance in future application directions and challenges in developing OVs as novel weapons for tactical decisions in cancer treatment.
Similar content being viewed by others
Introduction
Viruses used to be associated with the evil devil. However, oncolytic viruses (OVs) are comparable to be noble angels, as they can save lives. Oncolytic virotherapy is an emerging novel tumor therapeutic approach that selectively replicates in and destroys tumor cells while leaving normal cells undamaged.1,2 Initially, in the twentieth century, investigations carried out on the oncolytic effects were generally based on wild-type or naturally occurring viruses such as West Nile virus, rabies virus, yellow fever, hepatitis, etc.,3 and the mechanism was simply thought to be their intrinsic lytic characteristics. Approaching the year 2000, it is technically feasible to carry out an array of modifications on wild-type viruses by means of genetic engineering. Modified OVs can be armed with desired exogenous genes that could exert profound antitumor effects via different mechanisms. At first, the main focus of reconstructions was to improve target specificity, selective replication and oncolysis. Soon later, an elicited antigen-specific antitumor immunoreactive response during tumor lysis was appreciated, which is another advantage of OVs as immunotherapy.2 Strategies have, therefore, begun to shift toward developing viral vectors for enhancing immune responses within the tumors, or for adjusting tumor neovascularization, tumor metabolism and other aspects to counteract the malicious tumor microenvironment (TME) in recent years. This process can be graphically described as “soldiers” equipped with a variety of “sophisticated weapons” to cope with different situations. Also, it is equally important to arm a soldier with the right weapons to maximize tumor damage.
The outcomes of OVs are determined by a three-way race among virus replication, immune activation and tumor growth.4 Unlike the theories of conventional chemoradiotherapy, OVs precisely lyse cancer cells by interacting with specific cellular receptors, or taking advantage of tumor-suppressor gene defects, downregulation of the antiviral pathway in tumor cells, or by designing virus vector with specific gene knockout. The benefits regarding different forms of cell death are various due to the characteristics of virus vectors and tumor cell type, and most of them can trigger immunogenic cell death (ICD), releasing tumor-associated antigens and initiating antitumor immune responses. However, it cannot be ignored that antiviral immunity can be triggered at the same time as the infection has been launched. Therefore, the selection and design of virus vectors are diversified and flexible, considering the balance among the viruses, TME and host immunity. Regarding the activation of tumor immunity, OVs seem to outperform the ICIs and other targeted drugs since ICIs specifically target the immune checkpoint, while small molecule drugs only target a certain molecule. In the context of OVs, a broader range of antitumor immunity activities would be aroused to fight against tumors. For example, the release of TAAs during oncolysis, the initiation of immunity, the promoted immune cell infiltration, improved recognition and killing abilities of immune cells, the reversal of the immunosuppressive microenvironment, and others (a detailed comparison of this part is presented in Bommareddy’s review).5 Compared with the limited effect of other treatment methods, OVs can carry out multiple “weapons” to kill tumors systematically and comprehensively in multiple ways. OVs can also help to regulate the abnormalities in TME, such as neovascularization, tumor metabolisms, and the stiff extracellular matrix barrier brought by tumor stromal cells. In short, oncolytic therapy offers various advantages.
To date, scientists have made a number of preclinical attempts and clinical trials of both naturally occurring OVs (e.g., reovirus and vesicular stomatitis virus)6,7,8 and genetically engineered OVs (e.g., adenovirus, vaccinia virus and herpesvirus),9,10,11 with some encouraging data. From H101 for nasopharyngeal carcinoma admitted by China in 2005 to Delytact for malignant glioma approved in Japan in 2021,12,13 a total of four functionally compensatory OV products have been approved for clinical treatment. OV has gradually managed to secure its place as a powerful anticancer agent in cancer treatment options. As most investigators have found that OVs are ideally suitable for combination strategies compared to single modality therapies because of the complexity of mechanisms involved in the progress of OV to take action in the complex environment of tumors. The development of combination methods implementing antitumor drugs yields synergistic or additional antitumor benefits, for which clinical validations through well-designed and statistically sound clinical trials are required.14,15
This review provides a comprehensive overview of oncolytic virotherapy, especially addressing the basic principles of how OVs take effect in the context of complex TME. Furthermore, recent advances in genetic engineering strategies to construct versatile OVs will be discussed in full range. The accurately selected combination options for cancer treatment and the outcomes of ongoing associated clinical trials are especially worthwhile keeping an eye on because the valuable information would provide future directions for the development of more advanced OVs with maximized capabilities.
The dominate types of OVs
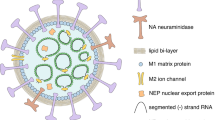
Regarding the antitumor mechanisms, although OVs share properties, different types or subtypes of viruses are being scrutinized for efficacies to cope with various pathological conditions. OVs are derived from single- or double-stranded DNA or RNA viruses according to nucleic acid type. ssRNA and dsDNA viruses are the most prevalent in OVs products, except for reovirus (dsRNA) and parvovirus (ssDNA). dsDNA viruses mainly include adenovirus, vaccinia virus, herpesvirus, etc., while ssRNA viruses are composed of two main categories: positive-sense (coxsackievirus, Seneca Valley virus, poliovirus) and negative-sense (measles virus, Newcastle Disease virus, vesicular stomatitis virus). The genetic information of positive-sense ssRNA viruses is directly translated into protein by ribosomes of host cells, while the nucleic acid of negative-sense ssRNA viruses is complementary to the viral mRNA, which must be transcribed into positive-sense RNA before it can be translated into protein. OVs can also be divided into naturally attenuated viral strains and genetically modified viral vectors according to their structures (Table 1).
Herpesvirus
Herpes simplex virus (HSV), an enveloped virus with dsDNA protected by the nucleocapsid, and surrounded by the tegument, has two specific serotypes (HSV-1 and HSV-2).16 HSV contains a large genome of at least 150 kb and a complex structure, which provides the possibility for the insertion of relatively large fragments and multiple transgenes.17 Four major viral glycoproteins, gB, gD, gH and gL, are expressed on the surface of the HSV envelope enabling the binding with various cellular receptors.18 During infection, the envelope fuses with lipid bilayers of the cell membrane to expose the nucleocapsid to the nuclear membrane.19 The viral genome is then released into the cytosol, and transported into the nucleus where transcription initiates. The viral gene transcription and protein synthesis are strictly regulated by the herpesvirus genome. According to the order of transcription and translation, viral proteins are divided into immediate-early proteins, early proteins and late proteins,20 in which modifying the genes encoded by these proteins is a common method. As a cytolytic virus, HSV can infect multiple types of cancer cells and quickly replicate, spreading the progeny viruses easily within neoplasms.21 In addition, anti-HSV drugs like Acyclovir can be utilized to ensure the safety of oncolytic HSV (oHSV) to counteract virulence.22 Even though more than half of the population possesses neutralizing antibodies against HSV, it can still evade the host immunity through different mechanisms, rendering it a model for an ideal OV vector.23 Currently, HSV-1 is one of the most commonly used strains of OVs. The representative works include T-VEC,24 G20725 and G47Δ.26 The strain HSV-2 is also drawing increasing attention and is under investigation at the moment. An oHSV-2 named OH2 has launched phase I/II clinical trials in solid tumors recently, but its modification strategy is the same as that of T-VEC.27
Adenovirus
Adenovirus is a 90–100 nm naked virus composed of approximately 26–45 kb dsDNA genome wrapped by an icosahedral capsid that is comprised of hexon trimers and penton bases (PB).28 The N-terminal of fiber knobs is attached to PB, and C-terminal is responsible for identifying cellular receptors, which is a desired place to be modified for selective targeting.29 Among a total of 57 different serotypes, Ad2 and Ad5 belong to subgroup C, being the most wildly used as oncolytic adenovirus (oAd).30 Most oAds infect cells by combining coxsackievirus and adenovirus receptor (CAR) except subtype B and some of subtype D that exploit CD46 for infection.31 Upon virus internalization through receptor-mediated endocytosis,32 the viral particles are disassembled and exposed capsids that enter the cytoplasm by lysis of endosomal membrane and are subsequently transported along microtubules to the nuclear envelope, where viral genomes import into the host nucleus.33 E1A and E1B are key early genes that activate the replication and transcription of subsequent viral genes of Ad2 and Ad5.34 The conserved region (CR) 2 of E1A proteins replaces retinoblastoma (Rb) proteins of the E2F transcription factor in infected cells and initiates the cell cycle of the quiescent cell to enter S-phase.35 The E1B-19 kDa protein and E1B-55 kDa protein encoded by the E1B gene prevent post-infection cell death, prolonging the viral replication. Specifically, the E1B-55 kDa protein binds to p53 and induces its degradation, and the E1B-19 kDa protein acts as an antiapoptotic factor.36,37 Adenoviruses are one of the most widely studied viruses because they provide several advantages, such as the feasibility of manufacturing high viral titers, ease of genome manipulation, and inherently potent lytic activity.38 However, adenovirus has an extensive tissue tropism, which addresses the significance of enhancing selective replication in tumor cells of oAds to ensure biosafety. For example, E1A and E1B gene deletion is a common method to generate replication-defective adenoviral vectors.39 Following the success of H101, the first oncolytic agent approved for clinical use in the history of oncolytic virotherapy,40 Onyx-015,41 CG0070,42 etc., has also achieved inspiring results consecutively in clinical trials.
Vaccinia virus
Vaccinia virus (VV) is a dsDNA virus approximately 190 kb, belonging to the orthopoxvirus genus. The virus particle is about 270 × 350 nm in size and appears as brick shaped structure.43 Unlike other dsDNA viruses, intracellular mature virions (IMV), the main particle type of VV, has an asymmetric and complex structure that consists of a nucleoprotein core enclosed by a single lipoprotein membrane.31,44 VV enters host cells either by fusion with the host cell membrane at a neutral pH environment or through receptor-mediated endocytosis under acidic pH.45 The process is assisted by the entry-fusion protein complex consisting of eight viral proteins: A16, A21, A28, G3, G9, H2, J5 and L5,46 but no host cellular receptors have been clearly identified. VV contains enzymes required for initiation of viral post-infection transcription located in the viral core,47 and its replication and progeny assembly occur exclusively in endoplasmic reticulum (ER) surrounded cytoplasmic mini-nuclei.48 Its selective targeting is highly dependent on thymidine kinase (TK) gene, encoding the essential enzyme for viral replication. TK is usually overexpressed in malignant cells but rarely expressed in normal cells. In this way, scientists generated TK-knockout VV strain that only replicates in cancer cells.49 In addition, VV secretes viral proteins to activate EGFR-RAS pathway of host cells to further promote the synthesis of TK.49 Some prominent advantages of VV include fast and efficient spreading of the virus due to high-speed and active life cycle, as well as up to ~40 kd gene insertion capacity and well-studied genome due to the acknowledgment of smallpox.43 The most famous oncolytic VV, JX-594, in particular, shows potential for intravenous injection by resisting the effects of antibodies and complement.50
Reovirus
Reovirus is a naturally occurring non-enveloped dsRNA virus that structurally consists of an outer capsid and an inner core.51 Reovirus enters the host cell primarily through receptor-mediated endocytosis by engaging with junctional adhesion molecule A (JAM-A),52 which served as a receptor for reovirus. Reovirus can be utilized as an OV to target cancer cells since JAM-A is overexpressed in a series of cancers, including breast cancer,53 non-small cell lung cancer,54 diffuse large B-cell lymphoma,55 and multiple myeloma.56 Upon infection, the outer capsid is acid-dependently cleaved in endosomes and the transcriptionally active core is subsequently released.57 The transcription and translation event for the assembly of progeny virus happen in viral inclusions located in the cytoplasm. Throughout the whole life cycle of virion production, maturation and egression, the virus does not enter the host nucleus.58 Another mechanism of reovirus to selectively target tumor cells is via the prevalent mutation of RAS signaling in tumors.59 The modulating RAS in cancer cells is related to PKR inactivation.60 In normal cells, PKR can bind to dsRNA of reovirus and arouse its autophosphorylation and activation, further phosphorylating eIR2 to be inactive, which prevents the translation of viral transcripts.61 Three serotypes have been identified; among them, the type 3 Dearing strain (T3D) has been adopted to manufacture OVs called Reolysin®.62 It shows considerably good adaptability for intravenous injection and potent antitumor effects, exhibiting no dose-limiting toxicity or irritation.63
Other OVs
In addition to the four common types of OVs discussed above, other viruses have also shown efficacy in OV treatment, especially ssRNA viruses that are classified into ss(+)RNA and ss(-)RNA viruses. For ss(+)RNA, they are usually in a smaller size that come from Picornaviridae family as the name suggests, including coxsackievirus, Seneca Valley Virus (SVV) and poliovirus. These viruses are naked, representing icosahedral capsid in electron microscopy (EM) appearance. They replicated in the cytoplasm to avoid the insertion of foreign genes.64 Mechanically, coxsackievirus binds to the surface molecules, such as DAF and ICAM‑1 for cell entry, which is overexpressed in multiple cancers, like melanoma, multiple myeloma, and breast cancer cells.65 Although coxsackieviruses are facing the challenge of being neutralized by antibodies, different serotypes seem not likely to cross-react. In the case of SVV, mainly SVV-001 strain is nonpathogenic in humans and has been shown to infect neuroendocrine tumor.66 However, previous clinical trial results were not entirely satisfactory.67 Poliovirus is highly pathogenic in human anterior horn motor neurons; therefore, its toxicity must be attenuated. Gromeier et al. replaced the viral internal ribosome entry site (IRES) with an IRES of the related human rhinovirus type 2 (HRV2) to target glioblastoma multiforme (GBM), since the receptor of poliovirus CD155 is overexpressed on glioma cells.68
The ss(-)RNA OVs are unique in some aspects. Measles virus and Newcastle disease virus (NDV), which belong to the Paramyxoviridae family, have a relatively large viral particle size but a relatively short length of RNA. Measles virus utilizes the signaling lymphocytic activation molecule (SLAM) receptor or CD46 as the receptor for cell entry,69 while NDV infects via sialic acid on host cells.70 Upon cell entry, the two viruses exercise their life cycle in the cytoplasm, and propagate infection via cell‑to‑cell fusion, resulting in the formation of multicellular aggregates and cell death.64 However, the measles virus may cause measles through respiratory transmission, and attenuated strains (e.g., Edmonston strain) are recommended for use.71 For NDV, both attenuated and non-attenuated strains would be adopted for OV construction, because it is an avian virus that poses no harm to humans, and MEDI5395 has been studied for oncolytic activity.72 Another OV worth of being discussed would be vesicular stomatitis virus (VSV). VSV glycoproteins (G protein) attach and fuse with host cells via the non-specific expressed low-density lipoprotein (LDL) receptor. Following receptor-mediated endocytosis, internalization occurs within the endosomes at low pH condition.73 Although the infectious receptors for VSV do not appear specifically at the cancer cell surface, selective targeting is achieved due to the defects of the antiviral interferon (IFN) signaling pathway in those cells. Four VSV OVs including VSV-IFNβ-NIS have been evaluated in the clinical trials; however, most of them are in phase I at present.74
The arsenal for OVs: modification strategies
“Boot Camp”: training wild-type viruses into “qualified soldiers”
Improving the tumor-targeting selectivity of OVs
Training wild-type viruses into tumor-specific OVs is the prerequisite step that can be described as training civilians into recruits, which may happen either in the process of infection or replication. The training process needs to be carried out according to the characteristics of the viruses and tumor cells. Different types of viruses show different natural affinity and preferential replication tendencies in different tumor cells, while genetically engineered OVs are designed for enhanced targeting selectivity. There are two main modification strategies for improving the fidelity of OVs in tumor targeting. The first is to increase the affinity and the binding activity of the viruses to the overexpressed receptors at the tumor surface. Alternatively, the target accuracy could be enhanced by utilizing the characteristics (e.g., the abnormalities in the pathways/protein expressions in tumor cells) of the tumor cells to differentially improve the viral replication efficiency75 (Table 2).
Improving the OV infection via tumor cellular receptors
First of all, the characteristics of the affinity of naturally occurring viruses have been perceived by using certain tumor-specific cellular proteins. Due to altered pathways within the tumor cells, these receptors have been upregulated. CD155 is widely overexpressed on the surface of many tumor cells, promoting tumor cell invasion and migration. It happens to be the natural receptor of poliovirus, rendering poliovirus the ability to selectively infect tumor cells.76 Reolysin®, a wild-type variant of reovirus (i.e., T3D strain), has been demonstrated to have oncolytic activity across a spectrum of malignancies depending on RAS signaling.77 HSV gD protein binds to herpesvirus entry mediator (HVEM), which has been reported upregulated in melanoma, gastric cancer and hepatocellular carcinoma (HCC).78
Since some receptors are still expressed in normal cells in a relatively lower amount, OVs are designed to recognize tumor-upregulated receptors, allowing the virus for an enhanced fidelity. For a typical example, subgroup C adenovirus (Ad), a commonly used OV, infects host cells by the combination of the fiber knob of Ad and coxsackie adenovirus receptor. However, the efficacy of targeting virotherapy remains limited for the differential expression levels of coxsackie adenovirus receptors on different tumor cells.79 To circumvent the deficiency, there are some strategies to transform Ad capsid for viral retargeting. The first way is to switch fiber knob serotype by reconstructing the chimeric fibers with knob domains derived from another serotype Ad. Based on the differences in receptor utilization, for example, Yang et al. summarized Ad5/F35 (chimeric Ad consisting of the knob and shaft of Ad35 combined with Ad5) enhancing targeting and oncolytic effects on multiple cancers via CD46, which is highly expressed in most tumor cells.80 Another strategy takes heterologous retargeting ligands that are bispecific in binding to the fiber knob domain and a tumor-associated antigen (TAA). Haisma et al. fused neutralizing anti-adenovirus fiber scFv Ab (S11) to EGF-mediated adenovirus retargeting to EGF receptor-positive cells.81 A similar strategy to identify potential bi-soluble adapters for targeting cognate tumor receptors has been adopted in HSV-1 modified with P-V528LH adapter fused to an EGFR-specific monoclonal antibody consisting of gD ectodomain binding region of nectin-1, which is found overexpressed in breast and colorectal cancer.82 OV can also be modified to accurately target human epidermal growth factor receptor 2 (HER2). Leoni et al. inserted an scFv HER2 into the gD of oHSV to target the primary HER2-Lewis lung carcinoma-1 (HER2-LLC1) tumor.83 CD20 is overexpressed in several hematological malignancies, such as CD20-positive non-Hodgkin’s lymphoma (NHL). A CD20-targeting measles virus (MV)-based vector was constructed to target lymphoma and showed promising tropism.84 The growing emergence of tumor-specific receptors or antigens would provide OVs with more attractive modification approaches for advanced targeting accuracy. To be noted, the sequences for the scFvs to be carried have to be evaluated carefully for optimal binding ability.
Enhancing the replication efficiency of OVs in tumor cytoplasm
Improving the replication capability of OVs is an effective approach to developing tumor targeting. Some viruses have their own mechanisms to promote replication. The B18R protein produced by some orthopoxviruses blocks the α subunit of the IFN receptor, inhibiting antiviral responses of the cells, promoting virus replication.85 In the case of Talimogene laherparepvec (T-VEC), a modified oncolytic herpes simplex virus 1 (oHSV-1), has been approved by the Food and Drug Administration (FDA) as the first oncolytic virotherapy for the treatment of melanoma. The mutation in α47 gene gives rise to an early expression of the US11 gene, which was reported to induce viral replication in tumor cells.86
Molecular engineering of viruses also makes it possible to modify viruses to allow their replication to be more efficient specifically in cancer cells. It has been proposed that both loss of the tumor-suppressor genes and dysregulations of signaling pathways in tumor cells would aid in viral replicative selectivity. In Ads, the gene encoding E1B 55kD, which may inactivate the tumor-suppressor p53 by ubiquitination and keep the virus alive in cells, was deleted in many oncolytic Ads such as H101 and ONYX-015.87 E1B 55kD-ablated adenoviruses are more sensitive to p53-induced apoptosis in normal cells versus malignancies where p53 is often mutant that allows high-efficiency viral replication in tumor cells.40,88 However, another study argued that the tumor-specific replication of ONYX-015 was later shown to be due to the loss of E1B-mediated late viral RNA export from nucleus to cytoplasm, rather than p53-inactivation.89 Nevertheless, a similar idea was applied to another oncolytic adenovirus, CG0070, which used the human E2F-1 promoter to drive the viral E1A gene.90 The retinoblastoma tumor-suppressor protein (Rb), commonly mutated in bladder cancer, contributes to transcriptionally active E2F-1 that enables the high-level expression of E1A for CG0070.91 T-VEC deficient in neurovirulence factor (γ34.5) leads to tumor-selective replication.92,93,94 The biofunction of γ34.5 is to block the shut-off of protein synthesis and interferon responses in host cells during virus infection.95 The γ34.5(-) HSV-1 is, therefore, more sensitive to the above antiviral responses in normal cells. Since tumor cells are often deficient in such host response mechanism, the γ34.5-deficient virus such as T-VEC can selectively replicate in cancer cells.96 Alphavirus M1, which belongs to the togavirus family, was isolated from culicine mosquitoes collected from Hainan, China. The expression of ZAP is high in normal cells, which is the mechanism of resisting virus-induced cell death.97 Lin et al. previously reported that M1 virus selectively killed tumor cells lacking zinc-finger antiviral protein (ZAP).
On the other hand, it is noteworthy to mention that some overexpressed genes or proteins yielded from tumor cells may happen to further support the biophysiological activities of OVs. For example, JX-594, a transgene-armed and targeted OV developed with vaccinia virus (VV), was modified by inserting the human granulocyte-macrophage colony-stimulating factor (GM-CSF) gene into the TK gene loci, thus destroying the inherent ability of the virus to transcribe TK.98 TK is required for the replication of JX-594; therefore, replication occurs only in cells that highly express TK, such as most tumor cells.99 Tumor-specific promoters (TSPs) convey high tumor-specific transcriptional activity in an array of cancer types, and thus may serve as genetic engineering sites of OVs for transcriptional targeting. GP73 is a better biomarker for HCC diagnosis than AFP. Taking advantage of this feature, adenovirus GD55, in which endogenous E1A promoter of E1B 55kD-deleted Ad was replaced by GOLPH2 (a Golgi membrane glycoprotein GP73) promoter, has been demonstrated to have more accurate targeting in HCC.100 In cholangiocarcinoma (CCA), Ads with survivin promoter were designed by Zhu et al., exhibiting higher activity. The survivin promoter shows greatly low expression levels in normal cells and indicates strong tumor specificity.101
Nevertheless, it raises the question whether the productivity of virions is the demanding factor influencing the outcomes of oncolytic therapy. According to the review by Davola and Mossman, infected cell protein 0 (ICP0)-defective oHSV-1 and oHSV-2 viruses showed a negative correlation between in vitro replication and in vivo antitumor activity.102,103 In another study, non-replicative VV Ankara (iMVA) was more effective not only in suppressing melanoma tumors but also in the growth of distant tumors than replicating MVA.104 The strong oncolytic efficiency has also been revealed in some oHSV-1 lacking neurovirulence with a much-impaired replication capability.2
Addressing for the issue, some researchers still insist that viral replication is equally important, and have invented a non-attenuated viral skeleton equipped with transcriptional or translational elements that control the regulation of viral essential genes. Knock-in of TSPs in OVs, such as ZD55, GD55, exhibit advanced replication efficiency in tumor cells for their transcriptional characteristics.100 Besides transcriptional regulatory elements, translational switches also provide means to control the replication. The miRNA response elements (MREs) that are able to combine with the corresponding miRNA can be engineered into 3’ UTR region of the essential gene expression of OVs.105 The matching standards of MREs and miRNAs are supposed to obey the following criteria: (1) miRNAs should be downregulated by at least 50% in malignant tissues compared with noncancerous tissues. (2) MREs should bind to miRNAs as little as possible in tumors but as much in normal cells, so that more targeted replication can be carried out. Yao et al. built an oncolytic Ad with MREs controlling E1A gene named OA-4MREs, including MREs of miR-124, miR-128, miR-146b and miR-218, which resulted in increased viral replication and oncolysis in primary glioma cells compared to ONYX-015.106 Similarly, the GC-rich 5’-UTR of genes is often associated with malignancies and metastasis in cancers (e.g., rFGF-2). These regions give rise to a wide range of secondary hairpin structures, thereby inhibiting the translation of downstream mRNA in normal cells. However, such hairpin structures can be untwisted when there is an overexpression of elF4E in cells. Coincidentally, eIF4E is often overexpressed in tumor cells. Therefore, if this type of 5’-UTR is constructed in OV backbone, it can make it difficult for OVs to replicate in normal cells.107 Based on the above discussion, transcriptional/translational dually regulated (TTDR) OVs that combine the advantages of transcriptional and translational elements could be ideal for keeping the balance between replication and oncolysis ability. For example, ICP27, an essential viral gene of oHSV-1, could be regulated by prostate TSP ARR2PB and 5’UTRs of rFGF-2 that enhanced both OV replication and tumor specificity.108 All in all, TTDR-OVs are a promising strategy in OV construction.
Enhance the security of OVs
The viruses have been long perceived as pathogenic microorganisms that may induce pathogenicity. For this consideration, the safety of OVs has been doubted and has attracted much attention from researchers, especially natural OVs, which have been proven to kill tumor cells, were thought to destroy normal cells at the same time, like chemotherapies. For this concern, many studies have demonstrated to transform wild viruses into attenuated OVs with improved targeting fidelity, although there are still concerns related to viral recombination, toxicity, cytotoxic products, and off-target possibilities.109 Therefore, appropriate modifications of OVs for safety improvement are urgent for their clinical applications.
As mentioned above, the improvement of the selectivity and fidelity can enhance the security accordingly. There is no denying that deleting the γ34.5 gene is indispensable in oHSV-1 because this OV would not infect normal neurons; however, the mutation of TK is optional. For example, VG161 is an oHSV-1-based OV-containing TK gene developed by Virogin Biotech Canada Ltd.110 Although keeping the native TK gene partially compromised targeting accuracy of OVs, it helps to keep the sensitivity of VG161 to acyclovir or ganciclovir. In this case, the virulence of VG161 and safety could be effectively controlled in clinical applications.109
Moreover, the off-target effect is another serious concern that leads to organ damage, especially for Ads, which have been reported to be enriched in liver111 and limit the adenoviral transduction in vivo. Based on this, Alba et al. found that Ad5-hexon binding to coagulation factor X (FX) mediated liver transduction. They developed genetically FX-binding-ablated Ad5-hexon vectors to alleviate the symptom.112 The retargeting strategy can also permit CAR-independent infection to prevent liver sequestration as described above.113
Some OVs have shown to present high safety in both animal experiments and clinical trials, such as NDV,114 VSV115 and SVV.116 None of them are human-contagious viruses and are not pathogenic to people. Meanwhile, they have potential natural targeting ability to some tumor cells and are receiving increasing attention in the field of OVs research works. However, the risks generated from environmental shedding and mutation or recombination of oncolytic agents with wildtypes should be noticed and assessed. For example, NDV strains pose a potential risk for animal infections, since birds are more vulnerable to engineered viruses.113 Likewise, the neuroticism-eliminated VSV strains tend to revert into virulent wild-type VSV upon passaging.117 These security issues exist not only in these seemingly secured OVs, but also in almost every other OV. In general, strain screening, enhancement of targeting capability and accuracy, decreased off-target toxic, mutation and recombination probability are inevitable methods for increased safety.
“Cold and ancient weapons” of OVs: an oncolytic spear that pierces the target cells
When the “qualified soldiers” acquire the abilities of precision guidance, selective replication and reliable security, these OV soldiers are called upon take their weapons, that is, to be made express transgenes for further fighting against tumor cells. For the past decades, the antitumor mechanisms of OVs were mainly focused on directly infected cell oncolysis. Type I interferon and other antiviral signaling pathway are widely downregulated in cancers, making cancer cells more vulnerable to OVs that yield offspring through cell lysis.8 During cell lysis, the susceptibility of the cancer cell to the different forms of cell death depends on the types of viral vector and the corresponding transforming elements, which strongly influence the replication and efficacy of viruses.64 In this regard, an increased number of studies have considered the association between the factors influencing cell death and classifications of cell death, including apoptosis, necrosis, pyroptosis and autophagy, during OV development. However, as more is learned about OVs, we realize that this is a necessary but primitive aspect of OV construction, and thus the associated gene to be armed onto OV is termed as the “cold weapon” (Table 3).
Apoptosis
Viral infection modulates cell death via death receptor-mediated pathways, where the death receptors, including Fas, TRAIL-R and TNF-R, form a death-inducing signaling complex (DISC) that mediates apoptosis.118 Viral infection regulates the binding of death receptors to their ligands (e.g., virally encoded proteins), which subsequently triggers caspase cascade and initiates extrinsic apoptosis,119 where tBID is cleaved from BID by Caspase-8 activation and mitochondria-mediated intrinsic apoptosis pathway is activated.120 The regulation of virus on death receptor-mediated apoptosis mainly stems from the overexpression of death receptors or their ligand on the cellular membrane of the infected hosts and the sensitivity increase of this apoptosis signaling.121 Death receptor-mediated apoptosis represents an efficient mechanism for virus-induced cell death and progeny dissemination.122 However, an interesting phenomenon may emerge where apoptosis is rapidly arrested at the onset of oncolysis, and as progression increases, apoptosis is enhanced, and tumor cells continue to divide. In the initial stage, different viruses can manipulate specific abnormal signaling factors within tumor cells to inhibit apoptosis, providing sufficient time and space for viral replication and reproduction. If cancer cells are highly susceptible to apoptosis, the number and the dose of the OV will be limited in the tumor.123 Mansour et al. observed that NDV La Sota strain could stably infect and there is a 2-log increase replicate in targeted cells with an overexpression of the antiapoptotic protein such as Bcl-xL, allowing the OV to propagate and form syncytia required for virus transmission.124 A study by Stanziale et al. also supported the finding that more NV1066 (an engineered oHSV-1) was found to be produced in OCUM-1 cells when exposed to an inhibitor of apoptosis named N-acetylcysteine (NAC) than in untreated cells, and the tumor lysis was also raised correspondingly.125 However, a series of studies confirmed the multiple roles of apoptosis in OV-induced cell death. An H5CmTERT-Ad expressing secretable trimeric tumor necrosis factor-related apoptosis-inducing ligand (H5CmTERT-Ad/TRAIL) was generated by Oh et al. and exhibited a more potent tumor-killing effect in contrast to a cognate control Ad by inducing strong apoptosis.126 Loya et al. armed FusOn-H3 (i.e., engineered oHSV-2) with apoptosis activators Her2-COL-sFasL to increase the caspase activation (especially caspase-3 and -8) in infected cells and bystander killing effect.127 NDV is one of the OVs that has been studied comprehensively in the mechanism of apoptosis. NDV-mediated induction of apoptosis includes the activation of endoplasmic reticulum (ER) stress,128 intrinsic and extrinsic apoptotic pathway.129 All in all, the switch-like modification of apoptosis is a noteworthy direction of OV transformation, which could work along OV replication and lysis in the future.
Necrosis/necroptosis
Necrosis is an irreversible and uncontrolled cell death manifested by rupture of the plasma membrane, swelling of organelles, leakage of intracellular contents and finally cell death.130 Necrosis occurs due to overwhelming deleterious stress from multiple responses, and it is almost always associated with an inflammatory response due to the release of ATP, heat-shock proteins, DNA, uric acid, and nucleoproteins, which lead to cascading inflammasome activation.131 For OV-induced cell death, other forms of death are usually prioritized, and uncontrolled necrosis is more likely to be an endpoint of the post-lytic signal transduction cascade. Modification strategies would not focus on necrosis, but might target downstream substances such as inflammasome release.
However, a form of programmed cell death with a morphology similar to necrosis has been found, termed necroptosis.132 As for a caspase-independent cell death, it requires the activation of the kinases RIPK1 and RIPK3 to assemble into necrosome. The necrosome then phosphorylates and activates mixed-lineage kinase-like protein (MLKL) for trimerization, leading to rapid membrane permeabilization and danger-associated molecular patterns (DAMPs) release.131,133,134 Although necroptosis is a common form of OV-induced cell death, there are few reports of modifications to enhance the effect. Oncolytic VV Lister-dTK was shown to induce necroptosis in ovarian cancer cells.135 The NDV Herts/33 strain triggered necroptosis in vitro.136 MLKL was inserted into VV vectors to induce necroptosis, conferring potent immunity to neoepitopes and antitumor properties.137 Transcriptomic analysis showed that M1 viruses activate necroptosis in triple-negative breast cancer (TNBC), but they amplified this effect not by modification but by binding to doxorubicin.138 A similar strategy was adopted by oHSV-1 + Mitomycin-C, which induced necroptosis to sensitize tumors to ICIs in an osteosarcoma model.139 Viruses also evolved necroptotic inhibited proteins to suppress pathogenesis during infection.140,141 During VV infection, the E3 protein of VV prevents the accumulation of Z-shaped RNA by competing with the N-terminal Zα domain, thereby inhibiting the recruitment of RIPK3 by ZBP1 and reducing necroptosis.142 As for the proper immunogenic death method, more attention should be paid to the modified OV with improved necroptosis.
Pyroptosis
For pyroptosis, OV can be directed to trigger and regulate pyroptosis in cancer cells, leading to tumor shrinkage or remission and eliciting a strong immune response.143,144 Modulation of the inflammatory pyroptotic cell death pathway has been shown to successfully inhibit the proliferation and metastasis of multiple cancer cell types and may become a prospective cancer treatment strategy.143 Faria et al. observed that activation of inflammatory vesicles consisting of NLR or ALR and a bipartite protein called ASC (apoptosis-associated speck-like protein containing caspase activation and recruitment domains), bind to caspase-1 and directly activate the caspase cascade,145 leading to pyroptosis by lysing the gas phase cortex. This then leads to the formation of pores in the cell membrane and membrane rupture, cell rupture, and death.143,146 In addition, they found that pyroptosis releases proinflammatory cytokines, such as IL-1β and IL-18, as well as various DAMPs, which initiate adjuvant antitumor immune responses. Furthermore, it cleaves Gasdermin D (GSDMD) to its active N-terminal fragment, which forms pores in the plasma membrane, leading to a form of inflammatory cell death known as pyroptosis.145,146,147 OVs also induce pyroptosis; for example, an HSV-2 mutant lacking ICP10PK (ΔPK) upregulates the secretion of inflammatory cytokines TNF-α, GM-CSF, and IL-1β through pyroptosis.148 Oncolytic VSV can trigger gasdermin E (GSDME)-mediated pyroptosis, leading to immune switching of the TME by recruiting cytotoxic T lymphocytes in the background and enhancing the efficacy of immune checkpoint therapy.149 The oAds-armed apoptotic protein, encoded by the VP3 gene of chicken anemia virus (CAV), induces pyroptosis by cleaving caspase-3 and GSDME, and significantly inhibits the growth of colorectal tumors.150 Other OVs, such as coxsackievirus B3,151 HSV-1,152 NDV,153 etc., have also adopted the death mode, and these pyroptosis-based anticancer drugs may open up new possibilities for OV therapy in the future.
Autophagy
Unlike apoptosis, autophagy level is continuously increasing in the whole process of oncolysis, and is the strongest during tumor cell lysis, which leads to autophagic cell death.154,155 Some experiments have tried to arm autophagy-related molecules on OVs to improve their effects, such as Beclin-1, the most commonly used protein in modification. Arming with Beclin-1 showed significant therapeutic efficacy of OVs through inducing autophagic cell death in hematological tumor-like leukemia and myeloma.156,157 Other strategies also indicated autophagy played a role in OV therapy. oHSV-1 RH2 strain with γ34.5 gene-deficient induced the formation of autophagosome and autophagic cell death in squamous cell carcinoma.158 SVV-001 could go through blood–brain barrier to eliminate intracerebellar xenografts from medulloblastoma by a subverted autophagy.159 Research aiming at Ads suggested a close relationship with autophagy. Several typical Ads proteins take part in the autophagy regulation, which is promoted by E1A and E1B but suppressed by E4.155 E1A links to the tumor-suppressor Rb to lose the E2F-1 from the Rb-E2F-1 complex. E2F-1 induces autophagy by upregulating autophagy-related proteins like ATG5 and LC3.160,161 On the other hand, E1B interacts with Beclin-1, resulting in the division of the Beclin-1-Bcl-2 complex and the induction of Beclin-1-dependent autophagy.162 Therefore, the transgenic Ads have aimed at these features. Besides the arming of Beclin-1, OBP-301 and its upgraded edition OBP-702 led to autophagic cell death through E2F-1 and downstream microRNAs (miRNAs).163 Nevertheless, autophagy is thought to be secular growth in OV therapy, but this result is more like a patchwork, lacking research into the whole process from the initial stage to the final cracking.
“Hot and modern weapons” of OVs: drawing the magical immune gun
With the in-depth research works on OVs regarding their underlying mechanisms, scientists have increasingly focused on OVs-mediated oncolytic immunogenicity. As soon as tumor cell lysis, the viral progeny is released along with TAAs, pathogen-associated molecular patterns (PAMPs) and DAMPs signals, accompanied by tumor ICD. PAMPs and DAMPs arouse innate immunity by binding toward the receptors such as the Toll-like receptor (TLRs). Furthermore, matured DCs and natural killer (NK) cells are stimulated, which are found to support OV-mediated tumor clearance.164 Specifically, TAAs and tumor neoantigens (TNAs) are caught by antigen-presenting cells (APCs) to set off adaptive immunity. Tumor-specific T cells prime from draining lymph nodes, CD4+ and CD8+ T cells are activated to exert tumor immune effect in the primary site. Meanwhile, OVs themselves or as platforms can stimulate the production of inflammatory factors (e.g., IL-2, IL-12, IL-15, TNF-α)165 and chemokines (e.g., CXCL9, CXCL10, CXCL11)166,167 in TME, where T-cell migration and infiltration is reinforced. Even though this is hinged by stromal barriers (e.g., extracellular matrix, ECM) in some tumors,168 OVs are expected to become a novel weapon to break through the structural barriers. Another difficulty encountered is that the infiltrated immune cells are challenged by immunosuppressive cells (e.g., tumor-associated macrophages; TAMs, myeloid-derived suppressor cells; MDSCs), inhibitory factors (e.g., IL-10, TGF-β) and upregulating immune checkpoints (ICs) on immune cells (e.g., PD-1, CTLA-4) in TME.169 Luckily, the counteract can significantly alter TME by inducing the immune response of proinflammatory T helper 1 (Th1) cell to combat immunosuppression have been proposed170,171 Even in some cases, the counteracts could deplete immunosuppressive cells, for example, to convert M2 macrophages into proinflammatory phenotypes.172 With such approaches, OVs turn the “cold” tumor into the inflamed, immunologically “hot” tumor,1 exerting the function of antitumor immunity. In general, the thought of updated OVs through the strengthening of tumor immunity is attractive, and the arming of related exogenous for these characteristics is worth of putting into efforts. The following sections will introduce the transformation of OVs in immunotherapy (Fig. 1).
Stronger oncolytic immunogenicity of engineered OVs. ① When OVs cleave tumor cells, the viral progeny, TSAs, PAMPs, as well as DAMPs are released simultaneously, triggering ICD. ② Meanwhile, innate immunity is initiated, as DCs and NK cells collaborate for tumor clearance. ③ TSAs ingested by APCs soon migrate into lymph nodes, where T cells are activated, which infiltrate primary and metastatic foci to perform adaptive immunity. ④ In addition, engineered OVs are strengthened with the ability to break through ECM barriers, yielding inflammatory factors and chemokines, even reversing the immunosuppressive characteristic of TME. ⑤ In a collaborative effort, the engineered OVs may transform the immunologically “cold” tumor into “hot” tumor, also exerting an upgraded and more powerful antitumor immunity. Created with BioRender.com
ICD
Most forms of cancer cell death triggered by OVs belong to ICD, which has been regarded as a critical component in both OV development and tumor-specific immune responses in recent years.173 Specifically, cancer cells responding to oncolysis are allowed to mediate the signal of DNA damage responses, ER stress responses, autophagy, and necrotic plasma membrane permeabilization at the premortem stage, DAMPs such as surface-exposed calreticulin (ecto-CRT), heat-shock proteins (HSPs), extracellular ATP and high mobility group box 1 (HMGB1) are released, leading to the maturation of DCs and antigen presentation to T cells in TME.174,175,176 According to Guo et al., the right way of ICD is potent in elevating antitumor immune responses, thereby genetically engineered OVs can be armed with death-pathway modulating-associated genes to skew the infected cancer cells toward ICD as required.177 The majority of OV recombination for ICD promotion involves magnifying a particular form of cell death, some modifications to viral-specific genes may affect the occurrence of ICD.177 The OV dl922-947 is an adenoviral mutant with a 24 bp deletion in the E1A-Conserved region 2, which could induce ICD of malignant pleural mesothelioma (MPM) cells and trigger a cognate antitumor immune response.178 Generally speaking, ICD is like a key to open up oncolytic immunotherapy. However, there is still a lack of specific modifications for ICD recognition characteristics.
Promoting the function of antitumor immune cells
Proinflammatory cytokines
Cytokines are a large number of soluble proteins or glycoproteins with low molecular mass that regulate cell proliferation, cell differentiation and immune response by cell-to-cell communication. An increasing number of cytokines are perceived to play an active role in eliciting and reinforcing immune responses in tumors, thereby, various proinflammatory cytokines have been wildly studied as the accompanying transgene in OV modification. For example, GM-CSF, which is a hematopoietic growth factor that stimulates the proliferation of macrophages and granulocytes from bone marrow precursor cells,179 has been incorporated into the vectors of T-VEC,180 JX-59498 and other OVs. Such OVs carrying GM-CSF enhanced antigen presentation ability of DCs, thereby inducing the recruitment of NK cells and T cells and strengthening the immune responses.98,180 Interleukins are another type of cytokine that is initially thought to be restricted to leukocytes, but later is found to be produced by a wide variety of cells. IL-2, IL-12, IL-15, IL-23, etc., have been proven to have antitumor effects. Quixabeira et al. engineered an adenovirus coding for a human IL-2 variant (vIL-2) protein (Ad5/3-E2F-d24-vIL-2) aiming to uplift the antitumor response by enhancing the tumor-infiltrating lymphocyte (TIL) cytotoxicity in the context of immunosuppressive solid tumors,181 because vIL-2 can selectively activate CD8+ cytotoxic T cells and CD4+ helper T cells, not affecting Tregs.182 VG161 has been demonstrated to promote T cell and NK cell tumor infiltration by carrying IL-12 and IL-15.110 Oncolytic VV expressing IL-23 variants were generated by homologue recombination, resulting in activated T cells, and transforming the TME to be more conducive to antitumor immunity.183 TNF-α was also designed to be expressed by OV as an immune stimulant. Adenoviruses engineered to express tumor TNF-α and IL-2 were delivered in an anti-PD-1-resistant melanoma model, showing a prolonged survival time, an increased CD8+ T-cell infiltration and a reduced proportion of M2 macrophages and MDSCs.184 IFN-γ functions by promoting immune cell migration and propagation toward TME. The IFN-γ-encoding oncolytic VSV showed a better therapeutic effect in the lung cancer mouse model by droving more secretion of proinflammatory cytokines185 (Table 4).
Chemokines
Chemokines are chemotactic cytokines that generate, recruit, and regulate the migration of immune cells. They coordinate the recruitment of immune cells to build a pro-tumorigenic microenvironment, and guide the cellular migration and interactions within TME for an effective antitumor immune response. Potent T-cell-attracting chemokines (i.e., CXCL9, CXCL10, CXCL11, CX3CL1, CCL2, and CCL5) play an important role in the activation of tumor immune contexture, and were considered to assemble into OVs.186 Eckert et al. engineered VSV to encode CXCL9 to mediate the recruitment of activated CD4+ and CD8+ T cells.187 In Adv-CXCL10, the chemokine CXCL10 is carried by an adenovirus, recruiting more CXCR3+ T cells into the TME to kill colorectal tumor cells via the CXCL10-CXCR3 signaling pathway.188 CXCL11-armed oncolytic poxvirus (vvDD-CXCL11) showed to enhance the infiltration of tumor-specific T cells and increase the number of local CD8+ cytotoxic T lymphocytes (CTLs) as well as granzyme B in TME of murine AB12 mesothelioma model.189 OV-Cmab-CCL5 is produced by expressing oHSV heterodimers consisting of a single-chain fragment variant (scFv) of cetuximab linked to CCL5. In GBM mice, OV-Cmab-CCL5 injections showed tumor shrinkage and prolonged survival due to enhanced migration and activation of NK cells, T cells, and macrophages.190 Other chemokines also contribute to oncolytic virotherapy; for example, Huang et al. constructed a recombinant NDV expressing macrophage inflammatory protein-3 alpha (MIP-3α) (NDV-MIP-3α) to elicit ICD and attract DCs in vitro and in vivo.191 OV-armed chemokine or cytokine strategies are effective in TME due to their short action distance and short half-life compared with cytokines alone, and also avoid the toxicity and risk of cytokine storm caused by high-dose systemic application of cytokines. However, cytokines may also induce stronger antiviral immunity, and whether they affect the subsequent effects of OVs remains to be explored (Table 4).
BiTE or TriTE
Furthermore, the cutting-edge direction of OV immune-related genetic engineering is to combine bi- or tri-specific T cell engager (BiTE or TriTE) with OVs to directly stimulate T-cell immunity without antigen presentation by APCs. BiTE is a recombinant bispecific protein with two linked single-chain fragment variables (scFvs) produced by two individual antibodies, one targeting a TAA and the other targeting a cell-surface molecule (i.e., CD3) on T cells. On this basis, TriTE connects one more on T cell (i.e., CD3 and CD28).192 Like cytokines, these molecules remain drawbacks such as short biological half-life, rapid excretion, poor residence time in TME. Luckily, the problems could be solved when they become a team with OVs. A VV encoding a secretory BiTE, named EphA2-TEA-VV, has been designed to target against EphA2 in lung cancer cells (Fig. 2a). T cell activation, INF-γ and IL-2 secretion, as well as induced bystander killing of non-infected tumor cells were observed.193 Oncolytic Ads armed MUC16-BiTE targets highly glycosylated mucins that are overexpressed in ovarian cancers, leading to the improvement of MHC I antigen presentation, the proliferation and activation of T cells, the cytotoxicity against MUC16+ tumor cells, as well as remodulation of the TME.194 Other strategies such as ICOVIR-15K-cBiTE195 and MV-BiTEs196 have similar effects. The merging of two treatments complements each other, circumventing tumor heterogeneity, poor drug delivery and insufficient T cell infiltration. In a word, these modification strategies for antitumor immune cells are the mainstay, with the BiTE or TriTE technique being especially prospective in OV manipulation (Table 4).
a Schematic diagram showing the mechanism of EphA2-TEA-VV EphA2-TEA-VV has been designed to express BiTE that targets EphA2 expressed on lung cancer cells and CD3 on T cells to stimulate T cells directly without antigen presentation by APCs. b CD19t for improving target identification and tumor control of CD19 CAR-T oVV was designed to express CD19 on the surface of infected tumor cells before oncolysis, which helps CD19 CAR T cells to probe and attack those CD19-marked tumor cells that could not be recognized and targeted essentially. Created with BioRender.com
Fighting against even converting antitumor immunosuppression
Even though antitumor immune cells could be found occasionally infiltrated into the tumor, they still encounter great challenges for the immunosuppressive characteristics of TME. Factors contributing to the immunosuppressive TME, such as low expression of antigen presentation molecules and neoantigens by tumor cells,197 the secretion of immunosuppressive cytokines,198 elevated expressions of ICs, as well as the recruitment and activation of immunosuppressive cells,199 are established via tumor autocrine or paracrine signaling network. Current immunotherapies have been developed to counteract such mechanisms, such as neoantigen vaccines, monoclonal antibody therapy and immune checkpoint blockade (ICB),200 which have been effective to some degree in hematologic cancers and some kinds of solid malignancies. However, tumors are always crafty opponents that adjust the cross-talks between immune and non-immune cells, as well as the ratio and constitution between effecter cells and tumor cells, thereby formatting a new TME that favors tumor growth, and inducing another round of tumor immune evasion and acquired drug resistance. In view of the main issues, OVs and the combination strategies have shown their potential in this field, and could be beneficial to overcome the resistance against immunotherapies to optimize the clinical outcomes of patients.5
ICB therapy has been proven to be a remarkable strategy to restrict immunosuppressive signals and restore antitumor immune responses by targeting checkpoint receptors or ligand checkpoint molecules, such as PD-1/PD-L1 or CTLA-4, LAG-3 and TIGIT.201 In fact, limitations of ICB still exist in different tumors depending on the immunogenicity and components of TME.202 In another aspect, OV single treatment would cause upregulated expression of PD-L1.203 Therefore, the OVs engineered to encode and express ICB have provided a synergistic approach to overcome immunosuppression. VG161 has been manipulated to express PD-L1 blockade that refrains from interactions between PD-L1 and PD-1 expressed on T cells.110 CF-33-hNIS-antiPDL1 is another OV-producing bioactive anti-PD-L1 antibody, which blocked PD-1/PD-L1 interaction and was shown to reduce peritoneal tumor burden and improve the survival of xenograft mice.204 Interestingly, anti-PD-1 single variable heavy chain domain (VHH)-Fc and CTLA-4 monoclonal antibody armed ONCR-177 has been demonstrated potent antitumor activity in multiple immune-competent tumor models, which could be further improved by co-treatment with ICBs (Fig. 3).205 Meanwhile, OVs with other ICBs including OX40L (NCT02705196),206 VISTA207 and ICOS208 are under active investigation.
Three OV-engineered examples of ICIs expressing for reversing immunosuppression ICI-armed OVs infect tumor cells, subsequently releasing ICIs into TME to take effect. ① VG161 expresses PD-L1 blockade. ② CF-33-hNIS-antiPDL1 produces bioactive anti-PD-L1 antibody. ③ ONCR-177 secretes both anti-PD-1 VHH-Fc and anti-CTLA-4 mAbs. These strategies are used to block immune checkpoints and cooperate with OVs to enhance antitumor immunity. Created with BioRender.com
Solid tumors with immune-silent profiles are always accompanied by upregulated expressions of immunosuppressive molecules, causing immunotherapy failure. Preclinical studies have remarkably ameliorated the resistance by using TGF-β inhibitors, which provide inspiration for the modifications of OVs against tumor immunosuppression.209 In some studies, OVs are equipped with genes encoding TGF-β signaling pathway-related molecules to improve anti‐PD‐1 and anti‐CTLA‐4 responses,210,211 addressing the significance of ICB therapy in cancers of immune-desert phenotype. rAd.sT, a transforming TGF-β signaling-targeted oncolytic Ad, was combined with Meso-CAR T cells to treat breast cancer. The OV was found to reduce tumor burden at the initial stage, while CAR T played the role later during treatment.212 Tregs are immunosuppressive subsets of mainly CD4+ T cells that limit the proliferation and survival of T cells through different mechanisms. VV-encoded αCTLA-4 was engineered for CTLA-4+ Treg suppression, and a significant reduction in lung metastases was observed in the VV-αCEA TCE (αCEA BiTE-engineered VV) and VV-αCTLA-4 combination group.213 Oncolytic adenovirus co-expressing IL-12 and decorin reduced Treg expression and circumvent the Treg-mediated immunosuppression in the 4T1 orthotopic breast cancer model.214 TAMs, especially M2 in a narrow sense, are also one of the key cells to orchestrate the immunosuppression in TME. There was also a study that aimed to deplete M2-like macrophage subsets and developed TriTE-armed Ads to recognize M2, T cell and CD206. These were then cultured with DLD-1 tumor cells. Surviving macrophages are characterized by upregulated M1-associated markers and exhibited preferentially decreased M2 markers, suggesting TME repolarization toward a proinflammatory state. Further in vivo experiments of these agents are worthy of thorough exploration.215
Summarizing the above strategies on modified OVs that combat immunosuppression, the core idea is always to transform the “cold tumor” into the “hot tumor”. Plus, the combination of OV and ICB, especially PD-1/PD-L1 blockade, is one of the most frequently adopted approaches and most promising to enter clinical trials that may benefit more patients with “immune desert” tumors (Table 4).
As the main antitumor pathway of OVs, the genetic engineering strategy of OV based on antitumor immunity has been a research hotspot. Several aspects of OV immunotherapy, including ICD, immune stimulation, and immunosuppressive resistance, require multifaceted modifications. Based on the current understanding on TMEs and novel oncology drug development strategies such as bispecific antibodies, different types of transgene combinations are selected for accurate and flexible OV therapy.
Balance between the antiviral and the antitumor immunity
While antitumor immunity is a powerful weapon in OV therapy, the concomitant antiviral immune responses cannot be ignored. The existing theory holds that antiviral responses, including the clearance mediated by the early antiviral activity of NK cells, the viral antigens presentation to CD4+ helper T cells by mature DC, following the neutralizing antibody produced by B cells, and killing effect of CTLs, limit the infection and replication of OVs and then lead to the restriction of oncolytic effect.216 Researchers have attempted to inhibit antiviral immune responses by arming transgenes. Pourchet et al. created BV49.5 (an oHSV-1 with the bovine herpesvirus UL49.5 and US11 genes that replaced γ34.5 genes) to limit antiviral immune recognition of CD8+ T cells by inhibiting the transporter associated with antigen processing (TAP), which has shown significant efficacy in the bladder and breast cancer in murine models.217 OV-CDH1 was engineered to express CDH1, encoding E-cadherin, an inhibitory ligand for KLRG1 that expressed on NK cells, to protect against NK cytotoxicity during the early stage of OV treatment.218 For VV, there have been relatively mature modifications on antigenic epitopes on the viral surface to effectively restrict the cohesion with neutralizing antibodies (NAbs). The common sites that have been identified as major immunogenic proteins including A27L, H3L, L1R and D8L were imbedded into the viral enveloped membranes to reduce the immunogenicity elicited by the virus in vivo (Fig. 4a).219
a OV-modification strategies against antiviral immune responses. ① Expression of TAP-1 inhibitor to limit antiviral CTLs for BV49.5. ② Expression of CDH1 to protect against NK cytotoxicity during the early stage of OV treatment. ③ Modification on enveloped membrane sites of VV (A27L, H3L, L1R and D8L) to restrict NAbs. b The possible mechanism of antitumor immunity benefiting from antiviral immune responses induced by OVs OV-induced antiviral immunological events may create an inflammatory TME. Besides that, IFN-γ produced by antiviral CD4+ T cells approves some DCs to cross-present specific epitopes to CTLs, resulting in ICD and more elicited TSAs that can reinforce antitumor immune responses. Created with BioRender.com
However, some voices asserted that OV-induced antiviral immune responses hold antitumor benefits. If the rationale behind this could be appropriately understood and exploited, a stronger OV-based treatment can be developed.216 Following exposure to OVs, antiviral-related cytokines, chemokines, and signal molecules are activated and aggregated to induce proinflammatory antiviral responses, with a subsequent amplification of downstream cascades.216,220 These antiviral immunological events in TME may turn “cold” tumors into “hot” tumors, and even overturn tumor-associated immunosuppression to establish the basis for antitumor immunity in OVs.216 Besides, DCs exert phagocytic effects to integrate viral antigens.221 Although they present viral antigens to CD4+ T cells leading to the antiviral immune responses, IFN-γ produced by the antiviral CD4+ T cells approve some DCs to cross-present specific epitopes to CD8+ T cells, which in turn attack OV-infected tumor cells by lytic or non-lytic mechanisms,222,223 resulting in OV-induced ICD.152 As a result, it promotes another round of exposure to TSAs and the activation of CTLs in the process of OV transmission.223 Based on the above discussion, we should consider adjusting antiviral immunity in OV modification cautiously to maintain a delicate balance, thereby promoting and prolonging sustainable replication and infection while avoiding adverse effects on the desired anticancer immunity (Fig. 4b).
“Auxiliary weapons” of OVs: anti-angiogenesis, reversing metabolic reprogramming and ECM barrier breakthrough
Anti-angiogenesis
Sustained abnormal angiogenesis is one of the hallmarks characterized by most cancers, driven by the needs for nutrition transport and metabolic exchange.224 While TME resides in a hypoxic condition, “angiogenic switch” remains open and active, causing vessels to continuously sprout and expanding neoplastic growth by the abundance of pro-angiogenic factors such as vascular endothelial growth factor-A (VEGF-A).225 Such vasculature is typically aberrant and immature, characterized twisted and leaky, and accompanied by unstable endothelium, erratic blood flow, and insufficient pericyte coverage, causing a sustained hypoxia-regulated angiogenic vicious loop.226 Thus, this has become a hurdle for antitumor immune therapies, including OVs.227 The kinky neovasculature and pressure limit the delivery of antitumor agents (poor deposition of OVs in TME) and leukocytes including immune cell infiltration. The hypoxic and acidic TME also limits the replication and spread of OVs.228 These realities remind researchers to figure out novel approaches against anti-angiogenesis.
Conventional OV construction armed with anti-angiogenic weapons is dedicated to disseminate nascent vascularization. Some OVs have shown a preference for infecting tumor-associated endothelial cells themselves. For example, JX-594 can selectively infect endothelial cells in tumor-related vascular systems with increased VEGF and FGF-2 signals.229 G207, an oHSV-1, was found to replicate actively in CD31+ endothelial cells and reduced tumor neovasculature in malignant peripheral nerve sheath tumors (MPNSTs) model.230 Breitbach et al. adopted a three-dimensional (3D) reconstruction of infected tumors, revealing the direct infection and damage to the tumor vasculature by VSV.231 Other OVs were modified to target tumor vasculature or receptors of endothelial cells. VB111, an Ad5 containing a modified murine pre-proendothelin promotor (PPE-1-3X), Fas, and human TNF receptor 1, could infect tumor vessels and improve antitumor effects in the thyroid cancer xenograft model.232 In a triple-negative 4T1 breast carcinoma syngeneic mouse model, an oVV expressing CXCR4 antagonist was efficacious in destroying preformed tumor vasculature, inhibiting spontaneous metastasis and increasing overall tumor-free survival rate.233 However, classical anti-angiogenesis OVs may have adverse effects, including the reduction of blood flow inside the tumor, the aggregation of neutrophils in TME, and the accumulation of viruses on tumor rims.234 Although the outcome may be favored by the killed tumors’ starvation and apoptosis, some scientists doubted that the situation might be unfavorable for the infiltration and dissemination of OVs, which is undesired for the continued stage in oncolytic therapy. The loss of intratumoral blood flow was attributed to the recruitment of neutrophils and vascular collapse by OVs attacking endothelial cells, leading to the fibrin deposition and thrombosis, as well as neutrophil extracellular traps (NETs) that capture OVs and prevent them from spreading and delivering of subsequent drugs.228 On the other hand, elevated tumor hypoxia and acidic TME are disadvantaged to the survival and functions of some OVs, especially MVs and HSV that are extremely sensitive to pH changes.235,236 Nevertheless, there have been no long-term studies on OV targeting tumor vasculature so far, and the specific effects in the long run remain to be investigated (Fig. 5).
Different strategies of OV engineering for anti-angiogenesis and the possible induced phenotype of TME. ① Some OVs have been modified to attack tumor-associated endothelial cells, while the immunosuppressive TME provide a perfect niche for “cold” tumor development. ② Normalizing the tumor vasculature may promote immune cell infiltration and OV diffusion, giving rise to “hot” tumors. Created with BioRender.com
In consideration of the possible contradictions mentioned above, researchers never give up seeking for means to normalize tumor vasculature. Anti-angiogenic agents like vascular endothelial cell growth inhibitors have been proven the efficiency, and can reverse the immunosuppressive properties of hypoxia and acidic TME, aiding in increased CTLs infiltration and conversion of TAMs into antitumor M1 phenotype.237 However, there may be several limitations to the anti-angiogenic agents for single use. These agents are cytostatic, not cytotoxic, which means they cannot directly kill the tumor cells and be curative. During the later stage of treatment, acquired or inherent drug resistance possibly follows, accelerating in progression or recurrence. In view of the shortcomings, OVs have been used as engineering platforms or combination agents to combat anti-angiogenesis because of their multifaceted oncolytic properties and plasticity. oHSVs have been investigated to combine tumor vasculature targeting drugs such as trichostatin A238 and bevacizumab (BEV),239 and both showed a VEGF inhibition and antitumor enhancement. Likewise, OVs can be modified to express vasculostatin. G47Δ-mAngio improved tumor lysis, anti-angiogenesis, survive rate, and decreased VEGF expression and BEV-induced invasion markers during BEV combination treatment in mice-bearing human glioblastoma.240 RAMBO, another angiostatin-armed oHSV, has a similar effect to G47Δ-mAngio,241 and also in subcutaneously implanted sarcoma tumors.242 Besides oHSV, Ads have been studied and reformed. Furthermore, VEGI-251 was inserted into a ZD55 Ad and became ZD55-VEGI-251, which inhibited endothelial cell proliferation and increased mitochondria-mediated apoptosis.243 Ad-endo, also known as E10A, encoding secreted human endostatin, has been demonstrated to inhibit tumor growth through anti-angiogenesis, and the phase II clinical trial (NCT00634595) also shown improved outcomes of chemotherapy in advanced nasopharyngeal carcinoma.244 More radically, ICI or ACT treatments with concurrent use of anti-angiogenic OVs may facilitate CTL infiltration and activation via vasculature normalization and well-oxygenated TME (Fig. 5).245,246
Although the strategies targeting tumor vasculature are not the mainstay of OV modification, it can be a promising antitumor weapon if the tumors are greatly affected by abnormal neovascularization. Future research is supposed to be more meticulous and precise on the following perspectives, namely the timing of combination therapies, the dose of therapeutic agents, and the detection of blood perfusion. The core concept of modern anti-angiogenic therapy is to tame neovasculature, rather than terminating.
Reversing metabolic reprogramming
Cancer metabolism has become an increasingly popular research issue in recent years. Biological activities are inseparable from metabolism, for which tumors are no exception. Metabolic alterations are closely associated with the occurrence and progression of neoplasms. Since nutrient uptake is directed by oncogenes, the deregulation of those genes causes an abnormal intake of glucose and amino acid, as well as the opportunistic and irregular modes of nutrient acquisition.247 In this regard, it initiates the reprogramming of intracellular metabolism in cancers, where Warburg effect, also known as aerobic glycolysis, was the first and classical to be discovered. Normally, in glycolysis, glucose is usually fermented into lactic acid under hypoxia, but in tumor cells, such fermentation can also happen under oxygen-sufficient conditions.248 Although this effect greatly sacrifices the efficiency of ATP production, it allows glycolysis and TCA cycle intermediate to participate in more biosynthetic physiobiological activities and yield more NADPH.247 Other forms of metabolic reprogramming, including oxidative phosphorylation (OXPHOS),249 glutamine metabolism,250 fatty acid synthesis,251 etc., would induce the alterations of metabolic-driven gene regulation, metabolic interactions with TME, finally resulting in cell behavioral and functional change.247 Interestingly, tumors with highly similar genetic backgrounds but different tissue origins have different metabolic patterns. On the contrary, tumors with different genetic backgrounds but similar TME have similar metabolic patterns.
Metabolisms of tumor cells or other components in TME play a vital role in OV-mediated antitumor effects. At first, OV replication makes use of the host cell metabolic pathway to acquire raw materials such as lipids, amino acids, and nucleotides.252 In some cancer cells, it has been found that glycolysis is upregulated during type I IFN production when infected by OVs.253 Thereby, inhibition of abnormally upregulated glycolysis may be a strategy to enhance the sensitivity and oncolysis for some OVs, such as Ads,254 NDV255 and reovirus.256 In pyruvate metabolism, the phosphorylation of pyruvate dehydrogenase (PDH) suppresses its activation and promotes the production of lactic acid, which is common in TME. The evidence suggested that VV257 and reovirus256 could inhibit PDH activation through upregulated PDH kinases (PDKs) when infecting cells, but these effects coincide with the initiation of antiviral pyruvate metabolism. Based on this, scientists employed dichloroacetate-induced inhibition of PDK to re-activate the activity of PDH and prolong the survival of OVs in tumors. Also, PDH catalyzes pyruvate oxidation to accelerate TCA cycle flux, which has been shown to be beneficial for OV replication and oncolysis.252 Thus, different OVs can better exercise their biological characteristics in tumors by taking advantage of their respective metabolic adaptation.
In addition, the metabolic state in TME partly determines the antitumor immune effects of OV, in which metabolic depletion of immune cells is the major barrier due to the limitation of essential nutrients and the accumulation of immunosuppressive metabolites like lactic acid.165 Tumor-infiltrating T-cell responses are significantly affected by glucose restriction and dysfunction of mitochondria.258 Lactic acid has been shown to polarize TAMs toward M2 phenotype, which made Tregs adapt to low glucose TME.259 There have been a few attempts to utilize OV as a metabolic regulatory platform. VV has been engineered to express adipokine leptin to reprogram tumor-infiltrating T cell metabolism through the persistence of mitochondrial function and a higher OXPHOS in activated CD8+ T cells, resulting in active immune responses and promotion of memory responses to secondary tumor challenge in melanoma-bearing mice.260 Recombinant OVs that express other metabolic modulating proteins, such as insulin or IGF-1, have also been patented and investigated for their role in promoting metabolic reprogramming and immune effects of T cells (WO2019148109). Therefore, it is suggested that a more comprehensive consideration of the effect of metabolic reprogramming on an antitumor immune response would be adopted when designing the optimal OVs.
ECM barrier breakthrough
As the major constituent in the TME, ECM provides the growth niche for most solid tumors. It builds up a physical barrier and plays a key role in cancer initiation, progression, metastasis, and drug resistance. Among them, the immunosuppressive effects exerted by stroma are the main mechanism of tumor progression and treatment failure. The deposition formed by various ECM-secreted components (e.g., collagen and elastic fibers) and ECM remodeling negatively affects immune cell infiltrations.261,262 A classic example is the desmoplastic ECM of pancreatic ductal adenocarcinomas (PDACs), which is called “immune desert”. Traditional chemotherapeutic and molecular targeted therapy can only maintain a few months of median survival time for unresectable PDAC.263 Scientists have realized that the compositions of ECM could serve as promising targets for PDAC and other tumors with similar pathophysiological conditions, like HER2-positive breast cancer,264 high-grade gliomas,265 although no approved ECM-targeting therapeutic is available currently.
In this respect, OV can be a powerful weapon to break down the structural barrier between non-infiltrated immune cells and TME. OV is usually administered and autonomously transmitted intratumorally, which provides advantages for drug delivery and being independent of vein perfusion. The OVs carrying modifiers of ECM-related molecules cause significant changes in TME by producing a series of inflammatory mediators and cytotoxic proteases to facilitate ECM degradation.266 Tedcastle and his colleagues have cloned actin-resistant DNase (aDNAse I) and hyaluronidase (rhPH20) into conditionally replicating group B adenovirus that expresses ECM-degrading enzymes, which enhanced therapeutic efficacy against colorectal adenocarcinoma xenografts.267 In glioblastoma, hyaluronidase-expressing oncolytic Ad, ICOVIR17, combined with PD-1 blockade, successfully induced tumor-associated proinflammatory macrophages and T-cell cytotoxicity locally and systemically.268 For pancreatic cancer, neurotensin peptide (NT)-conjugated polyethylene glycol (PEG) has been armed with oncolytic Ad (oAd/DCN/LRP-PEG-NT), which has the capability of ECM-degrading efficacy by chemically cross-linking to the surface of ECM and disrupting Wnt signaling pathway. This chemical-engineered oAd has exerted reinforced oncolytic efficacy against neoplasms.269 The novel OV-modification strategies focusing on breaking down tough ECM barriers for more efficient drug delivery are worthy of more in-depth research works.
Combination strategies with oncolytic virotherapy in preclinical research works
Single agent-based tumor immunotherapy strategies may lead to drug resistance due to the heterogeneity and complex genetic mutation burdens occurred in tumors, as well as the miscellaneous constitutions in TME; the efficacy of monotherapies including OVs usually fails to reach an optimal antitumor outcome on its own. Luckily, OVs are highly flexible agents that can directly bring the key factors influencing tumor immunity into the TME. These auxiliary agents are considered as potent partners in combination therapies. Indeed, most of the preclinical studies have seen better efficacies of OVs in combination approaches. The other therapies that work collaboratively with OVs, including immune checkpoint inhibitors (ICIs), adoptive cell transfer (ACT) therapies, cytotoxic chemotherapies, or targeted drugs, are summarized, respectively, in Table 5.
Combined with ICIs
PD-1/PD-L1 inhibitors
As one of the most successful ICIs at present, PD-1/PD-L1 inhibitors have made a quantum leap in the treatment of a wide range of tumors. However, for those cancers that develop an immunosuppressive TME, such as PDAC, GBM, patients gain little benefit from the monotherapy. Combination therapies of OVs and PD-1/PD-L1 inhibitors may overcome this dilemma. Mechanically, following the PD-1/PD-L1 blockade, the T cell recruitment and immunity activation in TME would be ideally stimulated by the OVs, because the blockade helped to ameliorate the immunosuppression. In preclinical studies, the promise of this strategy has been adopted. Our previous results demonstrated that VG161, together with anti-PD-1 monoclonal antibody (mAb), provided better therapeutic performance in PDAC humanized mouse model, and that a significant growth of CD8+ T cells and NK cells were observed in the combination group.270 A combination of CF-33 and anti-PD-L1 therapy showed durable antigen-specific antitumor immunity and long-term survival against colon cancer in a syngeneic mouse model.271 Interestingly, Nguyen et al. addressed the significance of the timing of anti-PD-1 mAb to be administered in the combination treatment with OV.272 They have concluded and compared five major drug administration strategies: (i) Anti-PD-1 lead-in → OV; (ii) Concurrent administration of anti-PD-1 and OV; (iii) OV lead-in → anti-PD-1; (iv) Concurrent therapy lead-in → anti-PD-1; and (v) OV lead-in → concurrent therapy. The “OV lead-in → concurrent therapy approach” or the “OV lead-in → anti-PD-1” resulted in significantly improved outcomes compared to the other therapy approaches according to the data from preclinical and clinical trials, which is consistent with the rhythm of treatment-induced cancer-immunity cycle. The latter option may be adapted to cases with little chance of receiving repeated intratumoral injections.
CTLA-4, TIGIT, TIM-3 and LAG-3 inhibitors
In addition to PD-1, there are several other immune checkpoint molecules such as cytotoxic T lymphocyte antigen-4 (CTLA-4), T cell immune receptor with immunoglobulin and ITIM domains (TIGIT), T cell immunoglobulin protein and mucin domain-containing protein-3 (TIM-3) and lymphocyte activation gene 3 (LAG-3) that were also shown to be overexpressed on TILs and can cause the immunosuppression, as well as the exhaustion and depletion of activated CD8+ T cells.273 Intratumoral oHSV G47Δ working with a systemic CTLA-4 antibody-induced T cell recruitment and a broad gene pool associated with T cell activation, as well as restrained the production of Tregs, suggesting a healthy regulation of the TME.274 OVH-aMPD-1 synergizes with anti-TIGIT and showed reinforced immune responses in both MC38 and Hepa1-6 implanted subcutaneous tumor models.275 However, in refractory lung cancer, vvDD monotherapy with anti-PD-1 or anti-TIM-3 mAb showed no apparent therapeutic benefit, even though TIM-3 antibody helped to elevate the PD-1 expression on CD4+ and CD8+ T cells, while dual blocking combined with vvDD can improve the outcome.276 An engineered VV-scfv-TIGIT combined with LAG-3 blockade showed to uplift the complete response rate of CT26-bearing mice by exerting strong antitumor effects.277 It is worth mentioning that some strategies incorporating more than two ICIs often achieve better tumor therapeutic results. However, the side effects and biosafety issues in options of combination treatments require further careful and rigorous considerations.
Combination with targeted drugs
The emergence of targeted drugs indicates that tumor therapy has entered an era of precision medicine. Broadly speaking, both OVs and some of the ICIs are regarded as targeted drugs. Various targeted drugs that have been combined with OVs in clinical use will be discussed in this case. According to different biofunction of the drugs, they are mainly classified as angiogenesis inhibitors that block the formation of new blood vessels (e.g., sorafenib, bevacizumab), monoclonal antibodies that have specific targets on cancer cells (e.g., trastuzumab, cetuximab), proteasome inhibitors (e.g., bortezomib), signal transduction inhibitors (e.g., imatinib), histone deacetylase inhibitors (HDACi, e.g., vorinostat, belinostat), DNA repair inhibitors (e.g., olaparib), etc. Alternatively, they can be divided into small molecular drugs or large molecular drugs based on their molecular weight. Small molecular drugs can enter cells and specifically block or compete for key molecules involved in the targeted signaling pathway to play a therapeutic role. Large molecular drugs usually target cell membrane proteins.
Usually, targeted drugs can be served as assistants of OVs, exerting their respective advantages and synergistically stimulating the antitumor efficacies. Some drugs can antagonize the antiviral immune pathway. Ruxolitinib, a specific JAK-1/2 inhibitor, enhances the replication and activity of VSV-IFNβ by antagonizing antiviral JAK/STAT signaling.278 A similar phenotype was shown in VSV-dM51-treated melanoma, where JAK-1/2 inhibition increased OV sensitivity.279 In VSV-treated glioma cell lines, blockade of IKK/NF-κB signaling by the NF-κB kinase (IKK) inhibitor TPCA-1 has been demonstrated to reduce type I IFN-mediated antiviral responses.280 Other drugs such as bortezomib,281 PI3K inhibitor BKM120,282 MEKi283 have been assessed for the capability to facilitate viral replication in virotherapies. Targeted agents facilitating OVs to activate the immune system or suppress immunosuppressive cytokines and cells have also been discovered. HSV1617 and TGF-β inhibitor A8301 were employed as combination therapy in immunocompetent models bearing murine rhabdomyosarcoma, resulting in the generation of an enhanced antitumor T cell response and significantly prolonged survival compared to the single agent administration.284 The inhibition of Indoleamine-2,3-dioxygenase (IDO), which is related to antiviral function and immune escape mechanism, was explored to improve the oncolytic ability of JD0G in glioblastoma cells.285 Rituximab combined with oncolytic reovirus enhanced NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC) against chronic lymphocytic leukemia.286 Bortezomib united with oHSV strongly induced necroptotic cell death and NK activation.287
Normalization of tumor vasculature prior to the OV administration resulted in systemically enhanced immunotherapy. As a small bioactive recombinant peptide, 3TSR acquires anti-angiogenic properties by binding to the CD36 receptor on endothelial cells to inhibit the proliferation and migration of endothelial cells.288 In epithelial ovarian cancer, the idea had been tested with NDV, leading to tumor regression in preclinical models.289 The VEFGR tyrosine kinase inhibitor (TKI) axitinib combined with G47Δ-mIL12 was associated with a prominent reduction in vascularity, and increased infiltrated macrophage and tumor necrosis in the MGG123 GBM model.290 Anti-VEGF therapy can modulate the immune homeostasis of TME via regulating cytokine expression, such as IL-1β, IL-6, CXCL1 in tumors.291 Sorafenib combined with JX-594 was superior to single agents and showed objective tumor responses in three HCC patients.292 Combination of reovirus with sunitinib, another VEFGR inhibitor, in renal cell carcinoma demonstrated increased IFN-γ produced by tumor-specific CD8+ T and an establishment of protective immunity upon tumor rechallenge.293 Furthermore, the association with bevacizumab has been demonstrated to inhibit angiogenesis, and enhance the viral distribution and survival throughout the infected tissues from an assessed animal in various oncolytic virotherapies.239,240,241,294 However, the trend in combination with targeted drugs is that OVs are engineered to carry small molecule drugs or their derivatives to achieve antitumor effects. The different combination strategies can be adopted for preclinical or mechanistic exploration.
Combination with ACT therapies
There are a considerable number of studies employing combination therapy with modified OVs and ACT.295 ACT is a process that involves transferring a desired amount of qualified and active antitumor lymphocytes that are cultured in vitro to the patients for tumor regression. The therapy includes chimeric antigen receptor (CAR) T/NK cell therapy, T cell receptor engineered T cells (TCR-T) therapy, TIL therapy, etc. Compared to other cancer immunotherapies, ex vivo amplification of active lymphocytes is easier to acquire and more effective in producing a therapeutic effect, because the ex vivo culture is less likely to be influenced by the immune inhibitory factors, and the in vivo initiation of the immune response is rapid.296 The strategy has shown encouraging outcomes in melanoma, lymphoma and certain leukemias; nevertheless, there are limitations in epithelial tumors due to difficulties in target identification, ACT cell infiltration, and tumor heterogeneity.266 Combining OVs with ACT may help overcome the obstacles in solid tumor treatment by reversing TME. Chen et al. incorporated IL-21 into VV Tian Tan strain to create rTTVΔTK-IL-21 and assessed the therapeutic efficacy of OV monotherapy, which works in combination with CAR-T and iNKT in humanized B-NDG mouse model, suggesting that the combination therapies outperformed the monotherapy.297 The two options of the combination strategy can compensate for each other according to their own characteristics.
Priceman’s team came up with an ingenious solution to this problem. They designed an oncolytic VV expressing a truncated CD19 (CD19t) to improve target recognition of CD19 CAR. Targeting the labeled tumor cells further induces local immunity. CAR T cell-mediated killing also resulted in the release of the virus from dying tumor cells, thereby inducing persistent infection of OV19t (Fig. 2b).298 In addition, adoptive cells can also serve as systemic vehicles to deliver OV to tumor sites. Zheng et al. used CAR T and TRP-1 T cells as a high-efficiency carrier to systemically deliver the myxoma virus (MYXV) to homologous antigen-expressing tumors (CAR/TCR-T10%MYXV), inducing specific tumor cell death, autophagy, and showing a potent form of bystander killing that eradicates antigen-negative tumor cells that contribute to tumor elimination and adaptive immunity with suppressed antigen escape.299 Bispecific CAR T cells were also loaded with VSVm-IFNβ or reovirus to treat B16/CT2AEGFRvIII tumor-bearing mice. Compared with unloaded CAR T cells, OV-infected CAR-T allowed further in vivo expansion and reactivation of T cells through homologous enhancement and prolonged survival of mice with subcutaneous melanoma and intracranial glioma tumors.300 Another novel idea was that IL-2-armed oncolytic poxvirus stimulates the accumulation of tumor-specific TILs in hypoimmunogenic tumor tissues. Meanwhile, such tumor-specific TILs are transferred into patients following the ex vivo expansion. These OV-induced TILs lead to colon tumor regression and longer survival in MC38-bearing mice.301
NK cells have some inherent advantages in immunotherapy compared to T cells. NK cells stem from a variety of sources. There is little worry about NK causing graft versus host disease (GvHD) because the recognition is independent of human leukocyte antigen (HLA) matching. The “off-the-shelf” characteristic of NK cells provides an opportunity for large-scale commercial production. However, adoptive NK or CAR-NK cell therapy with OVs is in its fledgling stage. The combination of CCR5-overexpressing NK cells with a CCL5-modified oncolytic VACV showed better efficacy than single agents in a colon cancer model, and greater infiltration of NK cells in the TME compared with the prototype virus.302 OV-expressing human IL-15/IL15Rα (OV-IL15C) and off-the-shelf EGFR-CAR-NK cells have elicited strong antitumor responses in an orthotopic GBM mouse model.303 Taken together, these studies suggest that once OVs are engineered to promote the migration, infiltration, and activation of ACT cells in solid tumors, they can be a powerful tool to break through the bottleneck of ACT therapy.
Combination with chemotherapies
Chemotherapy remains the mainstay of first-line conventional cancer therapies. Therefore, a considerable number of combination research works have incorporated chemotherapy alone, as described in the comprehensively and systematically codified guidelines.291,304 Here, we will discuss the phenomenon of different curative effects resulting from the different orders of chemotherapy and OVs. We believe that effective and sufficient viral replication is the prerequisite for OV to exert full antitumor impact. Successful replication of OVs is dependent on viable tumor cells. The use of early chemotherapy makes it difficult for OVs to obtain an ideal living environment to complete the life cycle, and a large number of tumor cells are killed, resulting in unsatisfactory effects. On the contrary, there is also controversy regarding the administration order between the OV and the chemotherapy, because the antitumor immune cells activated by OV may be killed by chemotherapy drugs. On the other hand, chemotherapeutic drugs may act as antiviral agents and reduce viral replication in TME, largely compromising the efficacy of OV monotherapy or chemotherapy, leading to an impairment in the combination results. In our study, we explored the pharmacodynamics of VG161 combined with gemcitabine + nab-paclitaxel in a mouse model of pancreatic cancer, which produced the best effect in the VG161 post-chemotherapy group, while post-chemotherapy combined with OVs did not show any gain with OVs benefit compared with monotherapy.270 Likewise, treatment with ONYX-015 prior to cisplatin, or adding them concurrently, has been evaluated in earlier studies in the survival of HLaC xenograft tumor models over infection with ONYX-015 following drug treatment.305 However, the specialized mechanisms need to be explored by high-throughput methods such as single-cell RNA sequencing.
Clinical trials of OVs
Currently, a total of four OV products have been approved for marketing: Rigvir (SND005), Oncorine (H101), Imlygic (Talimogene laherparepvec, T-VEC) and Delytact (teserpaturev/G47Δ). Rigvir is an unmodified enteric cytopathic human orphan virus type 7 (ECHO-7), approved for the treatment of melanoma306,307,308,309,310,311 in Latvia in 2004,312 making it the first approved oncolytic drug. Later in 2006, the adenovirus-H101 was approved in China,13 for squamous cell cancer of head and neck or esophagus.313 However, the treatment efficacy of these oncolytic drugs primarily stems from their intrinsic oncolysis characteristics rather than stimulating antitumor immunity. Therefore, the therapeutic effect of single-drug treatment is still limited, and the treatment strategy is more focused on combination therapies.
In 2015, the U.S. FDA approved T-VEC, an attenuated HSV-1 encoding GM-CSF for the local treatment of unresectable cutaneous, subcutaneous and nodal lesions in patients with recurrent melanoma after the initial surgery.314 It has been shown that GM-CSF may stimulate MDSCs, resulting in diminished innate and adaptive antitumor responses in numerous cancers.234,315,316 An insertion of a sole GM-CSF into the virus seems not an ideal strategy. Results from clinical trials indicated that the administration of T-VEC and ICIs present robust synergistic effects,317,318 suggesting the prospective potentials for the combination use of OVs and ICIs. The recently approved OV, Delytact (G47Δ), showed survival benefits in patients with residual or recurrent glioblastoma, with a good safety profile.26,319 Interestingly, there were no transgenes used as payloads in G47Δ. It thus raised the question whether the modification should be made within the viral vector or the viral genome to be made carry more exogenous regulatory genes would give rise to an optimal effect?
By 2022, there will be a total of 329 OV-related clinical trials registered in ClinicalTrials.gov. The majority of the clinical trials were phase I (n = 171; 52.0%) There were an additional 60 (18.2%) studies that were reported as phase I/II, 84 (25.5%) as phase II, 12 (3.6%) as phase III, and only 2 (0.6%) as phase II/III clinical trials. Details are listed in Supplementary Table 1. The TOP 20 distribution of transgenes and indications are listed in Fig. 6.
Monotherapy of OVs
The significance of monotherapy is undoubtedly greater than that of combination therapy, but its success is also more difficult. At present, the performance of OV monotherapy has demonstrated good safety, but it has not yet shown amazing data in terms of efficacy. In 2015, Andtbacka et al. disclosed important data from OPTiM research,314 distinct performance of T-VEC in patients with advanced melanoma made it approved by FDA. In 2019, they reported the final results of the OPTiM research.320 A total of 436 patients with advanced melanoma were enrolled in the research and were arranged randomly into the group of the T-VEC treatment (n = 295) or the GM-CSF treatment (n = 141). As a result, the overall survival (OS) for the T-VEC group and GM-CSF group was 23.3 and 18.9 months; and durable response rate (DRR) was 19% and 1.4%; objective response rate (ORR) was 31.5% and 6.4%, respectively. Also, in the T-VEC group, 50 patients (16.9%) showed complete response (CR), while only one patient (0.7%) in the GM-CSF group achieved CR. Among patients with CR, 88.5% were estimated to survive at a 5-year landmark analysis. T-VEC also showed satisfying results in melanoma treatment in several other clinical trials.321,322 Andtbacka et al. reported Phase I clinical trial results of a coxsackie virus V937 in advanced melanoma,323 suggesting the DRR of 21.1%, the 12-month progression-free survival (PFS) and 12-month OS hit 32.9% and 75.4%, respectively. In addition, the phase I clinical data of PVSRIPO for patients with unresectable, treatment-refractory melanoma showed that the ORR of patients reached 33%.324 It can be seen that the overall performance of OV monotherapy in melanoma is fairly good, but it is largely because melanoma is highly immunogenic and more sensitive to immunotherapy.
Nevertheless, the efficacy of OV monotherapy in early HCC, which also represents better immunogenicity, is not as satisfactory as in melanoma. For example, JX-594 showed good safety in early clinical trials, and found that the tumor response and patient survival were related to the dose.325 However, the TRAVERSE study, which reported the results of the Phase IIb clinical trial of JX-594 in 2019, showed that there was no significant difference in median OS, overall response rate (RR), and time to progression (TTP) of the experimental group compared with the control group.326 To be noted, we have recently disclosed Phase I clinical data of a new OV VG161 in advanced liver cancer, showing that two patients with HCC in the first and second cohort had prolonged PFS of 3.7 and 11.5 months, respectively. In addition, all patients had received ICIs and had progressed before the enrollment, and significantly prolonged OS was seen in 5 patients who received ICIs after the trial (P = 0.025). It indicates that VG161 helped to re-construct the immunity in the TME of HCC, thus improving the sensitivity to ICIs.327 Furthermore, VG161 carries genes coding for IL-12, IL-15, and IL-15 receptor alpha subunit, along with a peptide fusion protein capable of disrupting PD-1/PD-L1 interactions.110 Compared to the JX-594 that only incorporates GM-CSF, VG161 stimulates a much more powerful antitumor immunity via various signaling pathways. This also implies that arming OVs with appropriate and adequate exogenous transgenes with mutual synergistic capabilities would be a desired option in future development directions.
Several clinical studies have reported the cases of OVs in malignant glioma treatment,26,319,328,329,330 among which the performance of G47Δ and G207 has drawn a lot attention. Todo et al. reported the phase II clinical results of G47Δ in residual or recurrent glioblastoma,26 showing that the 1-year survival rate was 84.2% and the median OS was 20.2 (16.8–23.6) months after G47Δ initiation. According to the report by Friedman et al., the median OS was 12.2 months in 12 patients with pediatric high-grade glioma treated with G207.330 Interestingly, both G47Δ and G207 are oHSV-1, and neither carries any transgene.
In addition to the above-mentioned clinical trials, OV monotherapy has been used in solid tumors,331,332,333,334,335 head and neck cancer,336 pancreatic cancer,337,338,339,340 epithelial cell carcinoma,341 bladder cancer,342 etc. They have shown encouraging outcomes in an array of cancer types. However, in some phase II clinical trials with larger samples, the therapeutic efficacy of OV monotherapy was relatively weak.343,344,345 In terms of safety, the most common treatment-related adverse events in OVs clinical trials are fever, chills, nausea, flu-like symptoms, fatigue and injection site pain. The overall safety is better than other immunotherapy products. In general, OV monotherapy has a certain therapeutic effect against cancers with better immunogenicity, but the overall performance is not as good as expected. Part of the reason is that the reported OVs are mainly products that do not carry or only carry a single transgene. These viruses have a limited ability to stimulate antitumor immunity, while the new generation of OV products that carry multiple immune-stimulating transgenes are expected to augment antitumor efficacy. Most of the new-generation OVs are being evaluated for the efficacy and safety in trials at present, and their ongoing clinical results are worth looking forward to.
Combination therapy of OVs
In this field, the combination of OV and other drugs is one of the key development directions in the future. At present, the most common combination strategy with OV in clinical trials includes chemotherapy, immunotherapy, radiation, and targeted therapy. Chemotherapy was the earliest combination therapy with OV, but compared with others, the efficacy of this combination strategy is more controversial. Although several clinical trials have reported encouraging results,333,346,347,348,349 others with large sample sizes have found the combination strategy to be ineffective.350,351 For example, Eigl et al.350 found that the median survival time of patients with metastatic castration-resistant prostate cancer who received a combination docetaxel plus pelareorep was 19.1 months, while patients receiving docetaxel monotherapy had a median survival time of 21.1 months. It was suggested that the combination of OV and chemotherapy exhibited a “1 + 1 < 1” effect. However, Jonker et al.351 found that the combination of pelareorep with FOLFOX/Bevacizumab was tolerable with an increased ORR, but PFS was inferior.
The combination of OV and immunotherapy is currently the most concerning combination strategy. In 2017, Ribas et al. reported the results of their phase Ib clinical trial. The ORR of T-VEC combined with pembrolizumab in patients with advanced melanoma was as high as 62%, and the CR reached 33%.318 This encouraging result also confirmed that OV combined with ICIs has extremely broad application prospects. The final results of the KEYNOTE-034 study will be announced in 2022.352 Unfortunately, T-VEC with pembrolizumab failed to significantly enhance PFS or OS compared with placebo + pembrolizumab. The ORR was 48.6% for T-VEC + pembrolizumab and 41.3% for placebo + pembrolizumab. In another phase Ib clinical trial of T-VEC combined with ipilimumab in the treatment of melanoma, the combination therapy was also more effective than T-VEC or Ipilimumab monotherapy, with an ORR of 50%.317 In the updated results of the follow-up phase II clinical trial, 39% patients (38/98) in the combination arm and 18% patients (18/100) in the ipilimumab arm had an objective response (P = 0.02).352 In phase II clinical trial of T-VEC combined with pembrolizumab for locally advanced or metastatic sarcoma, the combined therapy also showed positive efficacy, with an overall ORR of 35%.353 Currently, a large number of clinical trials on the combination of OV and various immunotherapies are being carried out around the world, but most of the results are mainly reported in conferences, and the overall performance is encouraging. Among various combination strategies, the prospect of OV combined with immunotherapy is the most promising.
Shirakawa et al.354 reported the results of a phase I trial of OPB-301 combined with radiotherapy in the treatment of oesophageal cancer patients. The ORR reached 91.7%, and the combination strategy showed good safety. In contrast, in phase III clinical trial of JX-594 combined with sorafenib in the treatment of advanced HCC, the ORR of the combination group was only 19.2%, which was lower than the 20.9% of the sorafenib alone group. The study was terminated early because the OS endpoint was not reached. It is suggested that OV combined with targeted therapy has a high risk of failure. Notably, pelareorep combined with atezolizumab and chemotherapy (gemcitabine/nab-paclitaxel) has demonstrated encouraging results as first-line treatment in advanced or metastatic PDAC patients.355 The trial found that the ORR of patients reached 70%, which was almost three times (25%) the average ORR of the historical control, and the results are amazing. However, the trial did not report data on patient survival, such as median PFS, median OS, etc. The follow-up results deserve further attention. Although the overall efficacy of the three-drug combination is acceptable, its potential side effects also call for vigilance.
Concluding remarks and future perspectives
From the accidental discovery of tumor shrinkage after infection with the natural virus to the widespread use of engineered OVs for targeted tumor therapy, OV therapy has gradually shown its powerful and magical antitumor ability, especially in solid tumors. It is not only a demon that can only invade and attack, but rather serve as a powerful tool of a genetically modified vector. It has displayed its targeting fidelity and antitumor immunity in many ways if it is properly harnessed. Our review classifies OVs in terms of variant functionalities, as well as comprehensively elaborates on the genetic engineering transformation of OVs regarding their functions and characteristics. Firstly, transforming the viruses into “qualified soldiers” so that they can selectively and safely destruct tumors. Next, equipping the “cold weapons” onto OVs improves the ability of replication and direct oncolysis. The following strategy focuses on arming the “hot weapons” to enhance the antitumor immunity of OVs, which is the most popular and critical perspective in OV modification. Finally, anti-angiogenesis, reversing metabolic reprogramming and ECM breakthrough can be effective auxiliary and novel weapons to elevate antitumor immune effect. These different engineering methods and ideas have provided references and directions for future research works on OV transformation research. Although a limited therapeutic effect was seen on OV monotherapy in the clinical, we summarized the combination results that incorporated OV treatment. Its collaboration with ICI provides the hope of curing tumors, and the combination with ACT even allows researchers to witness the most cutting-edge, top-notch, and most imaginative antitumor strategies. Finally, we summarize the progress of OV clinical trials, and put forward our thoughts and suggestions on the current trial results.
A perfectly versatile OV is still under inquisitive investigation. For example, capsid modification has been shown to enhance Ads infection but decrease the replication,101 improving HSV-2 replication but impairing the antitumor effect.103 Accordingly, it is expected to consider the balance of their function and the various characteristics of corresponding tumors. If the modification of the backbone enables the virus to replicate in large quantities in a short period of time and rapidly lyse tumor cells, and releasing adequate amount of transgenes while rapidly infecting new tumor cells, it would be an interesting point of OV-modification strategy in the future. Furthermore, a TTDR viral essential gene expression can increase both viral lytic activity and tumor specificity, and this provides a basis for the development of a novel tumor-specific OV for systemic treatment of locally advanced and metastatic prostate cancers.108
Another issue to be addressed is the intratumoral injection of OV, which is the most common delivery method for OV therapy. However, there are certain drawbacks associated with this method, including (1) the need for puncture to achieve intratumoral drug delivery, which poses a risk of bleeding and undesired metastasis at the lesion site; (2) technical difficulties in puncturing deep tumor tissues, which greatly reduces the number of applicable cases; and (3) requirement for skilled and experienced technicians to handle and administer the drug. Even though some naturally occurring OV, such as reovirus356 and alphavirus M1,97,357 are capable of being delivered through intravenous injection, the viruses in the circulation are at risk of being wiped out by the neutralizing antibodies. Also, the viral RNA only provides limited space for modification, making them less ideal for virus vector engineering. Shielding modifications by changing capsid, adding polymer coats,358 or enriching the extracellular envelope of OVs359 may apply to counteract. Another more feasible method is to achieve intravenous injection by encapsulating or loading OV onto special biomaterials. Unfortunately, the volume of nanoparticles is too small for OV encapsulation. In such cases, it is feasible to package the OVs with normal human cells and transfer those cells back to the patients.360 For example, there was a study using mesenchymal stem cells (MSCs) as the carrier for OV delivery, as they not only serve as the carrier, but also provide factories for producing virions, expressing additional transgenes, and modulating the immune system.361,362,363,364,365 In addition, due to the complex distributions of arteries, veins and bile ducts across the liver, underlying risks have to be carefully evaluated prior to intratumoral drug delivery to liver tumors. In this case, transarterial chemoembolization (TACE) is an effective solution.366 OV products are currently liquid preparations, which require high-cost frozen storage and cold chain transportation. Therefore, the development of lyophilized preparations of OVs is also in demand. Last but not least, the preclinical and clinical studies for potentially effective or limited clinical outcome OVs should focus on in-depth mechanisms.
Tumor heterogeneity appears to be an insurmountable obstacle for any existing antitumor treatments, and there is no exception for the OV treatment. Despite the fact that BiTE or TriTE innovatively assembled with OVs are very attractive, the combination medication strategy may be more advantageous in future research and development; however, 2/3 of current clinical studies have used monotherapy. The ideal combinations are not a simple superposition but rather a prudent consideration of the reagent options that functionally compensate for each other, and an accurate selection of targeting strategy by classification according to the type and stage of cancer, as well as the mechanism of each drug. For example, the hNIS gene inserted into CF-33367 promotes the opening of sodium iodide channels and facilitates I129 uptake by tumor cells, making CF-33 a natural coordinating synergist for radiotherapy. In another case, CAR-T/CAR-NK targets are carried by OVs, thereby increasing the tumor tropism of CAR-T/CAR-NK, and finally achieving a synergistic effect. The work of Priceman’s group that we have mentioned above reflects the view well,298 and the combination with CAR-NK is also worth further exploration. “Oncolytic virus-like” drugs that enhance the efficacy of other treatments are also a promising direction. With the advanced understanding of antitumor mechanisms on OVs and the related experiments carried out actively, the weapons depot for OVs will be comprehensively established so that different OV weapons and other antitumor therapies can be selected for individualized treatment. In short, OVs carry our expectations of personalized and precision medicine in the future.
References
Chaurasiya, S., Chen, N. G. & Fong, Y. Oncolytic viruses and immunity. Curr. Opin. Immunol. 51, 83–90 (2018).
Li, Y. et al. Oncolytic virotherapy in hepato-bilio-pancreatic cancer: the key to breaking the log jam? Cancer Med. 9, 2943–2959 (2020).
Kelly, E. & Russell, S. J. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 15, 651–659 (2007).
Wu, J. Analysis of a three-way race between tumor growth, a replication-competent virus and an immune response. Bull. Math. Biol. 66, 605–625 (2004).
Bommareddy, P. K., Shettigar, M. & Kaufman, H. L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 18, 498–513 (2018).
Coffey, M. C., Strong, J. E., Forsyth, P. A. & Lee, P. W. K. Reovirus therapy of tumors with activated Ras pathway. Science 282, 1332–1334 (1998).
Hashiro, G., Loh, P. C. & Yau, J. T. The preferential cytotoxicity of reovirus for certain transformed cell lines. Arch. Virol. 54, 307–315 (1977).
Stojdl, D. F. et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6, 821–825 (2000).
Heise, C. et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 6, 1134–1139 (2000).
McCart, J. A. et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 61, 8751–8757 (2001).
Wong, R. J. et al. Oncolytic herpesvirus effectively treats murine squamous cell carcinoma and spreads by natural lymphatics to treat sites of lymphatic metastases. Hum. Gene Ther. 13, 1213–1223 (2002).
Frampton, J. E. Teserpaturev/G47Δ: first approval. BioDrugs 36, 667–672 (2022).
Liang, M. Oncorine, the world first oncolytic virus medicine and its update in China. Curr. Cancer Drug Targets 18, 171–176 (2018).
Macedo, N., Miller, D. M., Haq, R. & Kaufman, H. L. Clinical landscape of oncolytic virus research in 2020. J. Immunother. Cancer 8, e001486 (2020).
Guo, Z. S. et al. Oncolytic immunotherapy: conceptual evolution, current strategies, and future perspectives. Front. Immunol. 8, 555 (2017).
Yuan, S. et al. Cryo-EM structure of a herpesvirus capsid at 3.1 Å. Science 360, eaao7283 (2018).
Manservigi, R., Argnani, R. & Marconi, P. HSV recombinant vectors for gene therapy. Open Virol. J. 4, 123–156 (2010).
Agelidis, A. M. & Shukla, D. Cell entry mechanisms of HSV: what we have learned in recent years. Future Virol. 10, 1145–1154 (2015).
Weed, D. J. & Nicola, A. V. Herpes simplex virus membrane fusion. Adv. Anat. Embryol. Cell Biol. 223, 29–47 (2017).
Maruzuru, Y. et al. Role of herpes simplex virus 1 immediate early protein ICP22 in viral nuclear egress. J. Virol. 88, 7445–7454 (2014).
Shen, Y. & Nemunaitis, J. Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 13, 975–992 (2006).
Hartkopf, A. D., Fehm, T., Wallwiener, D. & Lauer, U. Oncolytic virotherapy of gynecologic malignancies. Gynecol. Oncol. 120, 302–310 (2011).
Mozzi, A. et al. Simplexviruses successfully adapt to their host by fine-tuning immune responses. Mol. Biol. Evol. 39, msac142 (2022).
Johnson, D. B., Puzanov, I. & Kelley, M. C. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy 7, 611–619 (2015).
Markert, J. M. et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol. Ther. 22, 1048–1055 (2014).
Todo, T. et al. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial. Nat. Med. 28, 1630–1639 (2022).
Zhang, B. et al. Intratumoral OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: a multicenter, phase I/II clinical trial. J. Immunother. Cancer 9, e002224 (2021).
Davison, A. J., Benkő, M. & Harrach, B. Genetic content and evolution of adenoviruses. J. Gen. Virol. 84, 2895–2908 (2003).
Ghebremedhin, B. Human adenovirus: viral pathogen with increasing importance. Eur. J. Microbiol. Immunol. (Bp) 4, 26–33 (2014).
Chu, R. L., Post, D. E., Khuri, F. R. & Van Meir, E. G. Use of replicating oncolytic adenoviruses in combination therapy for cancer. Clin. Cancer Res. 10, 5299–5312 (2004).
Jayawardena, N., Burga, L. N., Poirier, J. T. & Bostina, M. Virus-receptor interactions: structural insights for oncolytic virus development. Oncolytic Virother. 8, 39–56 (2019).
Gaden, F. et al. Gene transduction and cell entry pathway of fiber-modified adenovirus type 5 vectors carrying novel endocytic peptide ligands selected on human tracheal glandular cells. J. Virol. 78, 7227–7247 (2004).
Charman, M., Herrmann, C. & Weitzman, M. D. Viral and cellular interactions during adenovirus DNA replication. FEBS Lett. 593, 3531–3550 (2019).
Schaack, J. et al. E1A and E1B proteins inhibit inflammation induced by adenovirus. Proc. Natl Acad. Sci. USA 101, 3124–3129 (2004).
Abudoureyimu, M. et al. Oncolytic adenovirus–a nova for gene-targeted oncolytic viral therapy in HCC. Front. Oncol. 9, 1182 (2019).
Hidalgo, P., Ip, W. H., Dobner, T. & Gonzalez, R. A. The biology of the adenovirus E1B 55K protein. FEBS Lett. 593, 3504–3517 (2019).
Liu, T. C. et al. An E1B-19 kDa gene deletion mutant adenovirus demonstrates tumor necrosis factor-enhanced cancer selectivity and enhanced oncolytic potency. Mol. Ther. 9, 786–803 (2004).
Niemann, J. & Kühnel, F. Oncolytic viruses: adenoviruses. Virus Genes 53, 700–706 (2017).
Li, S., Ou, M., Wang, G. & Tang, L. Application of conditionally replicating adenoviruses in tumor early diagnosis technology, gene-radiation therapy and chemotherapy. Appl. Microbiol. Biotechnol. 100, 8325–8335 (2016).
Lu, W. et al. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: a pilot phase II clinical trial. World J. Gastroenterol. 10, 3634–3638 (2004).
Makower, D. et al. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin. Cancer Res. 9, 693–702 (2003).
Burke, J. M. et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J. Urol. 188, 2391–2397 (2012).
Guo, Z. S. et al. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. J. Immunother. Cancer 7, 6 (2019).
Ichihashi, Y. & Oie, M. Neutralizing epitope on penetration protein of vaccinia virus. Virology 220, 491–494 (1996).
Townsley, A. C., Weisberg, A. S., Wagenaar, T. R. & Moss, B. Vaccinia virus entry into cells via a low-pH-dependent endosomal pathway. J. Virol. 80, 8899–8908 (2006).
Senkevich, T. G., Ojeda, S., Townsley, A., Nelson, G. E. & Moss, B. Poxvirus multiprotein entry-fusion complex. Proc. Natl Acad. Sci. USA 102, 18572–18577 (2005).
Broyles, S. S. Vaccinia virus transcription. J. Gen. Virol. 84, 2293–2303 (2003).
Tolonen, N., Doglio, L., Schleich, S. & Krijnse Locker, J. Vaccinia virus DNA replication occurs in endoplasmic reticulum-enclosed cytoplasmic mini-nuclei. Mol. Biol. Cell 12, 2031–2046 (2001).
Parato, K. A. et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. 20, 749–758 (2012).
Park, B. H. et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 9, 533–542 (2008).
Tenorio, R. et al. Function, architecture, and biogenesis of reovirus replication neoorganelles. Viruses 11, 288 (2019).
Antar, A. A. et al. Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus. Cell Host Microbe 5, 59–71 (2009).
McSherry, E. A. et al. JAM-A expression positively correlates with poor prognosis in breast cancer patients. Int J. Cancer 125, 1343–1351 (2009).
Zhang, M. et al. Overexpression of JAM-A in non-small cell lung cancer correlates with tumor progression. PLoS One 8, e79173 (2013).
Xu, P. P. et al. JAM-A overexpression is related to disease progression in diffuse large B-cell lymphoma and downregulated by lenalidomide. Sci. Rep. 7, 7433 (2017).
Kelly, K. R. et al. Junctional adhesion molecule-A is overexpressed in advanced multiple myeloma and determines response to oncolytic reovirus. Oncotarget 6, 41275–41289 (2015).
Sturzenbecker, L. J., Nibert, M., Furlong, D. & Fields, B. N. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J. Virol. 61, 2351–2361 (1987).
Antczak, J. B. & Joklik, W. K. Reovirus genome segment assortment into progeny genomes studied by the use of monoclonal antibodies directed against reovirus proteins. Virology 187, 760–776 (1992).
Marcato, P., Shmulevitz, M., Pan, D., Stoltz, D. & Lee, P. W. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol. Ther. 15, 1522–1530 (2007).
Strong, J. E., Coffey, M. C., Tang, D., Sabinin, P. & Lee, P. W. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 17, 3351–3362 (1998).
Sadler, A. J. & Williams, B. R. Structure and function of the protein kinase R. Curr. Top. Microbiol. Immunol. 316, 253–292 (2007).
Müller, L., Berkeley, R., Barr, T., Ilett, E. & Errington-Mais, F. Past, present and future of oncolytic reovirus. Cancers (Basel) 12, 3219 (2020).
Mahalingam, D. et al. A phase II study of REOLYSIN(®) (pelareorep) in combination with carboplatin and paclitaxel for patients with advanced malignant melanoma. Cancer Chemother. Pharm. 79, 697–703 (2017).
Kaufman, H. L., Kohlhapp, F. J. & Zloza, A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 14, 642–662 (2015).
Shafren, D. R. et al. Systemic therapy of malignant human melanoma tumors by a common cold-producing enterovirus, coxsackievirus a21. Clin. Cancer Res. 10, 53–60 (2004).
Burke, M. J. Oncolytic Seneca Valley Virus: past perspectives and future directions. Oncolytic Virother 5, 81–89 (2016).
Burke, M. J. et al. Phase I trial of Seneca Valley Virus (NTX-010) in children with relapsed/refractory solid tumors: a report of the Children’s Oncology Group. Pediatr. Blood Cancer 62, 743–750 (2015).
Gromeier, M., Lachmann, S., Rosenfeld, M. R., Gutin, P. H. & Wimmer, E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc. Natl Acad. Sci. USA 97, 6803–6808 (2000).
Dhiman, N., Jacobson, R. M. & Poland, G. A. Measles virus receptors: SLAM and CD46. Rev. Med Virol. 14, 217–229 (2004).
Ravindra, P. V., Tiwari, A. K., Sharma, B. & Chauhan, R. S. Newcastle disease virus as an oncolytic agent. Indian J. Med. Res. 130, 507–513 (2009).
Dörig, R. E., Marcil, A., Chopra, A. & Richardson, C. D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75, 295–305 (1993).
Cheng, X. et al. Genetic modification of oncolytic newcastle disease virus for cancer therapy. J. Virol. 90, 5343–5352 (2016).
Melzer, M. K., Lopez-Martinez, A. & Altomonte, J. Oncolytic vesicular stomatitis virus as a viro-immunotherapy: defeating cancer with a “hammer” and “anvil”. Biomedicines 5, 8 (2017).
Zhang, Y. & Nagalo, B. M. Immunovirotherapy based on recombinant vesicular stomatitis virus: where are we? Front. Immunol. 13, 898631 (2022).
Lan, Q. et al. Development of oncolytic virotherapy: from genetic modification to combination therapy. Front. Med. 14, 160–184 (2020).
Gao, J., Zheng, Q., Xin, N., Wang, W. & Zhao, C. CD155, an onco-immunologic molecule in human tumors. Cancer Sci. 108, 1934–1938 (2017).
Gong, J., Sachdev, E., Mita, A. C. & Mita, M. M. Clinical development of reovirus for cancer therapy: an oncolytic virus with immune-mediated antitumor activity. World J. Methodol. 6, 25–42 (2016).
Gopinath, S. C. et al. Analysis of compounds that interfere with herpes simplex virus-host receptor interactions using surface plasmon resonance. Anal. Chem. 85, 10455–10462 (2013).
Mathis, J. M., Stoff-Khalili, M. A. & Curiel, D. T. Oncolytic adenoviruses – selective retargeting to tumor cells. Oncogene 24, 7775–7791 (2005).
Yang, M. et al. A novel fiber chimeric conditionally replicative adenovirus-Ad5/F35 for tumor therapy. Cancer Biol. Ther. 18, 833–840 (2017).
Haisma, H. J. et al. Targeting of adenoviral vectors through a bispecific single-chain antibody. Cancer Gene Ther. 7, 901–904 (2000).
Nakano, K. et al. Herpes simplex virus targeting to the EGF receptor by a gD-specific soluble bridging molecule. Mol. Ther. 11, 617–626 (2005).
Leoni, V. et al. A fully-virulent retargeted oncolytic HSV armed with IL-12 elicits local immunity and vaccine therapy towards distant tumors. PLoS Pathog. 14, e1007209 (2018).
Yaiw, K. C. et al. CD20-targeted measles virus shows high oncolytic specificity in clinical samples from lymphoma patients independent of prior rituximab therapy. Gene Ther. 18, 313–317 (2011).
Colamonici, O. R., Domanski, P., Sweitzer, S. M., Larner, A. & Buller, R. M. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J. Biol. Chem. 270, 15974–15978 (1995).
Poppers, J., Mulvey, M., Khoo, D. & Mohr, I. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J. Virol. 74, 11215–11221 (2000).
Garber, K. China approves world’s first oncolytic virus therapy for cancer treatment. JNCI: J. Natl Cancer Inst. 98, 298–300 (2006).
Cheng, P.-H., Wechman, S., McMasters, K. & Zhou, H. Oncolytic replication of E1b-deleted adenoviruses. Viruses 7, 5767–5779 (2015).
Geoerger, B. et al. Oncolytic activity of the E1B-55 kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xenografts. Cancer Res. 62, 764–772 (2002).
Ramesh, N. et al. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor–armed oncolytic adenovirus for the treatment of bladder cancer. Clin. Cancer Res. 12, 305–313 (2006).
Zwicker, J. & Müller, R. Cell cycle-regulated transcription in mammalian cells. Prog. Cell Cycle Res. 1, 91–99 (1995).
Chou, J. & Roizman, B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc. Natl Acad. Sci. USA 89, 3266–3270 (1992).
He, B. et al. Suppression of the phenotype of gamma(1)34.5- herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the alpha47 gene. J. Virol. 71, 6049–6054 (1997).
Liu, B. L. et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 10, 292–303 (2003).
Markert, J. et al. Oncolytic HSV-1 for the treatment of brain tumours. Herpes 13, 66–71 (2006).
Agarwalla, P. & Aghi, M. Oncolytic herpes simplex virus engineering and preparation. Methods Mol. Biol. 797, 1–19 (2012).
Lin, Y. et al. Identification and characterization of alphavirus M1 as a selective oncolytic virus targeting ZAP-defective human cancers. Proc. Natl Acad. Sci. USA 111, E4504–E4512 (2014).
Kim, J. H. et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 14, 361–370 (2006).
Dave, R. V. et al. Viral warfare! Front-line defence and arming the immune system against cancer using oncolytic vaccinia and other viruses. Surgeon 12, 210–220 (2014).
Ying, C. et al. GOLPH2-regulated oncolytic adenovirus, GD55, exerts strong killing effect on human prostate cancer stem-like cells in vitro and in vivo. Acta Pharm. Sin. 39, 405–414 (2017).
Zhu, Z. B. et al. Survivin promoter-based conditionally replicative adenoviruses target cholangiocarcinoma. Int J. Oncol. 29, 1319–1329 (2006).
Davola, M. E. & Mossman, K. L. Oncolytic viruses: how “lytic” must they be for therapeutic efficacy? Oncoimmunology 8, e1581528 (2019).
Workenhe, S. T. et al. Immunogenic HSV-mediated oncolysis shapes the antitumor immune response and contributes to therapeutic efficacy. Mol. Ther. 22, 123–131 (2014).
Dai, P. et al. Intratumoral delivery of inactivated modified vaccinia virus Ankara (iMVA) induces systemic antitumor immunity via STING and Batf3-dependent dendritic cells. Sci. Immunol. 2, eaal1713 (2017).
Ruiz, A. J. & Russell, S. J. MicroRNAs and oncolytic viruses. Curr. Opin. Virol. 13, 40–48 (2015).
Yao, W. et al. The application of multiple miRNA response elements enables oncolytic adenoviruses to possess specificity to glioma cells. Virology 458-459, 69–82 (2014).
Wong, J., Lee, C., Zhang, K., Rennie, P. S. & Jia, W. Targeted oncolytic herpes simplex viruses for aggressive cancers. Curr. Pharm. Biotechnol. 13, 1786–1794 (2012).
Lee, C. Y. et al. Transcriptional and translational dual-regulated oncolytic herpes simplex virus type 1 for targeting prostate tumors. Mol. Ther. 18, 929–935 (2010).
Lawler, S. E., Speranza, M. C., Cho, C. F. & Chiocca, E. A. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 3, 841–849 (2017).
Chouljenko, D. V. et al. Induction of durable antitumor response by a novel oncolytic herpesvirus expressing multiple immunomodulatory transgenes. Biomedicines 8, 484 (2020).
Smith, T. et al. In vivo hepatic adenoviral gene delivery occurs independently of the coxsackievirus-adenovirus receptor. Mol. Ther. 5, 770–779 (2002).
Alba, R. et al. Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors. Blood 116, 2656–2664 (2010).
Buijs, P. R., Verhagen, J. H., van Eijck, C. H. & van den Hoogen, B. G. Oncolytic viruses: from bench to bedside with a focus on safety. Hum. Vaccin. Immunother. 11, 1573–1584 (2015).
Sinkovics, J. G. & Horvath, J. C. Newcastle disease virus (NDV): brief history of its oncolytic strains. J. Clin. Virol. 16, 1–15 (2000).
Hastie, E. & Grdzelishvili, V. Z. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J. Gen. Virol. 93, 2529–2545 (2012).
Reddy, P. S. et al. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J. Natl Cancer Inst. 99, 1623–1633 (2007).
Stanifer, M. L., Cureton, D. K. & Whelan, S. P. A recombinant vesicular stomatitis virus bearing a lethal mutation in the glycoprotein gene uncovers a second site suppressor that restores fusion. J. Virol. 85, 8105–8115 (2011).
Galluzzi, L. et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 19, 107–120 (2012).
Zhou, X., Jiang, W., Liu, Z., Liu, S. & Liang, X. Virus infection and death receptor-mediated apoptosis. Viruses 9, 316 (2017).
Kantari, C. & Walczak, H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta 1813, 558–563 (2011).
Teodoro, J. G. & Branton, P. E. Regulation of apoptosis by viral gene products. J. Virol. 71, 1739–1746 (1997).
Roulston, A., Marcellus, R. C. & Branton, P. E. Viruses and apoptosis. Annu. Rev. Microbiol. 53, 577–628 (1999).
Zamarin, D. & Palese, P. Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiol. 7, 347–367 (2012).
Mansour, M., Palese, P. & Zamarin, D. Oncolytic specificity of Newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J. Virol. 85, 6015–6023 (2011).
Stanziale, S. F. et al. Infection with oncolytic herpes simplex virus-1 induces apoptosis in neighboring human cancer cells: a potential target to increase anticancer activity. Clin. Cancer Res. 10, 3225–3232 (2004).
Oh, E., Hong, J., Kwon, O. J. & Yun, C. O. A hypoxia- and telomerase-responsive oncolytic adenovirus expressing secretable trimeric TRAIL triggers tumour-specific apoptosis and promotes viral dispersion in TRAIL-resistant glioblastoma. Sci. Rep. 8, 1420 (2018).
Loya, S. M. & Zhang, X. Enhancing the bystander killing effect of an oncolytic HSV by arming it with a secretable apoptosis activator. Gene Ther. 22, 237–246 (2015).
Fábián, Z., Csatary, C. M., Szeberényi, J. & Csatary, L. K. p53-independent endoplasmic reticulum stress-mediated cytotoxicity of a Newcastle disease virus strain in tumor cell lines. J. Virol. 81, 2817–2830 (2007).
Elankumaran, S., Rockemann, D. & Samal, S. K. Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J. Virol. 80, 7522–7534 (2006).
D’Arcy, M. S. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 43, 582–592 (2019).
Festjens, N., Vanden Berghe, T. & Vandenabeele, P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim. Biophys. Acta 1757, 1371–1387 (2006).
Galluzzi, L., Kepp, O., Chan, F. K. & Kroemer, G. Necroptosis: mechanisms and relevance to disease. Annu. Rev. Pathol. 12, 103–130 (2017).
Weinlich, R., Oberst, A., Beere, H. M. & Green, D. R. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 18, 127–136 (2017).
Linkermann, A. & Green, D. R. Necroptosis. N. Engl. J. Med. 370, 455–465 (2014).
Whilding, L. M. et al. Vaccinia virus induces programmed necrosis in ovarian cancer cells. Mol. Ther. 21, 2074–2086 (2013).
Liao, Y. et al. RIP1 is a central signaling protein in regulation of TNF-α/TRAIL mediated apoptosis and necroptosis during Newcastle disease virus infection. Oncotarget 8, 43201–43217 (2017).
Van Hoecke, L., Riederer, S., Saelens, X., Sutter, G. & Rojas, J. J. Recombinant viruses delivering the necroptosis mediator MLKL induce a potent antitumor immunity in mice. Oncoimmunology 9, 1802968 (2020).
Zhang, J. et al. Necroptotic virotherapy of oncolytic alphavirus M1 cooperated with Doxorubicin displays promising therapeutic efficacy in TNBC. Oncogene 40, 4783–4795 (2021).
Workenhe, S. T. et al. De novo necroptosis creates an inflammatory environment mediating tumor susceptibility to immune checkpoint inhibitors. Commun. Biol. 3, 645 (2020).
Petrie, E. J. et al. Viral MLKL homologs subvert necroptotic cell death by sequestering cellular RIPK3. Cell Rep. 28, 3309–3319.e3305 (2019).
Upton, J. W., Kaiser, W. J. & Mocarski, E. S. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7, 302–313 (2010).
Koehler, H. et al. Vaccinia virus E3 prevents sensing of Z-RNA to block ZBP1-dependent necroptosis. Cell Host Microbe 29, 1266–1276.e1265 (2021).
Faria, S. S. et al. Induction of pyroptotic cell death as a potential tool for cancer treatment. J. Inflamm. (Lond.) 19, 19 (2022).
Lu, Y. et al. Strategies to package recombinant Adeno-Associated Virus expressing the N-terminal gasdermin domain for tumor treatment. Nat. Commun. 12, 7155 (2021).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Man, S. M. & Kanneganti, T. D. Regulation of inflammasome activation. Immunol. Rev. 265, 6–21 (2015).
Man, S. M. & Kanneganti, T. D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 16, 7–21 (2016).
Bollino, D., Colunga, A., Li, B. & Aurelian, L. ΔPK oncolytic activity includes modulation of the tumour cell milieu. J. Gen. Virol. 97, 496–508 (2016).
Lin, J. et al. Vesicular stomatitis virus sensitizes immunologically cold tumors to checkpoint blockade by inducing pyroptosis. Biochim. Biophys. Acta Mol. Basis Dis. 1868, 166538 (2022).
Liu, Z. et al. Apoptin induces pyroptosis of colorectal cancer cells via the GSDME-dependent pathway. Int J. Biol. Sci. 18, 717–730 (2022).
Zhang, Y. et al. Coxsackievirus group B3 has oncolytic activity against colon cancer through gasdermin E-mediated pyroptosis. Cancers (Basel) 14, 6206 (2022).
Takasu, A. et al. Immunogenic cell death by oncolytic herpes simplex virus type 1 in squamous cell carcinoma cells. Cancer Gene Ther. 23, 107–113 (2016).
Schirrmacher, V. Molecular mechanisms of anti-neoplastic and immune stimulatory properties of oncolytic newcastle disease virus. Biomedicines 10, 562 (2022).
Jin, K. T., Tao, X. H., Fan, Y. B. & Wang, S. B. Crosstalk between oncolytic viruses and autophagy in cancer therapy. Biomed. Pharmacother. 134, 110932 (2021).
Tazawa, H., Kuroda, S., Hasei, J., Kagawa, S. & Fujiwara, T. Impact of autophagy in oncolytic adenoviral therapy for cancer. Int. J. Mol. Sci. 18, 1479 (2017).
Lei, W. et al. Enhancing therapeutic efficacy of oncolytic vaccinia virus armed with Beclin-1, an autophagic Gene in leukemia and myeloma. Biomed. Pharmacother. 125, 110030 (2020).
Xie, S. et al. Beclin1‑armed oncolytic Vaccinia virus enhances the therapeutic efficacy of R‑CHOP against lymphoma in vitro and in vivo. Oncol. Rep. 45, 987–996 (2021).
Furukawa, Y., Takasu, A. & Yura, Y. Role of autophagy in oncolytic herpes simplex virus type 1-induced cell death in squamous cell carcinoma cells. Cancer Gene Ther. 24, 393–400 (2017).
Yu, L. et al. A single intravenous injection of oncolytic picornavirus SVV-001 eliminates medulloblastomas in primary tumor-based orthotopic xenograft mouse models. Neuro. Oncol. 13, 14–27 (2011).
Bagchi, S., Raychaudhuri, P. & Nevins, J. R. Adenovirus E1A proteins can dissociate heteromeric complexes involving the E2F transcription factor: a novel mechanism for E1A trans-activation. Cell 62, 659–669 (1990).
Polager, S., Ofir, M. & Ginsberg, D. E2F1 regulates autophagy and the transcription of autophagy genes. Oncogene 27, 4860–4864 (2008).
Piya, S. et al. The E1B19K oncoprotein complexes with Beclin 1 to regulate autophagy in adenovirus-infected cells. PLoS One 6, e29467 (2011).
Hasei, J. et al. Dual programmed cell death pathways induced by p53 transactivation overcome resistance to oncolytic adenovirus in human osteosarcoma cells. Mol. Cancer Ther. 12, 314–325 (2013).
Bhat, R. & Rommelaere, J. Emerging role of Natural killer cells in oncolytic virotherapy. Immunotargets Ther. 4, 65–77 (2015).
Muscolini, M., Tassone, E. & Hiscott, J. Oncolytic immunotherapy: can’t start a fire without a spark. Cytokine Growth Factor Rev. 56, 94–101 (2020).
Berghuis, D. et al. Pro-inflammatory chemokine-chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8(+) T-lymphocyte infiltration and affect tumour progression. J. Pathol. 223, 347–357 (2011).
Bronger, H. et al. CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br. J. Cancer 115, 553–563 (2016).
Hartmann, N. et al. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin. Cancer Res. 20, 3422–3433 (2014).
Noy, R. & Pollard, J. W. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49–61 (2014).
Benencia, F., Courrèges, M. C., Fraser, N. W. & Coukos, G. Herpes virus oncolytic therapy reverses tumor immune dysfunction and facilitates tumor antigen presentation. Cancer Biol. Ther. 7, 1194–1205 (2008).
Gyotoku, T., Ono, F. & Aurelian, L. Development of HSV-specific CD4+ Th1 responses and CD8+ cytotoxic T lymphocytes with antiviral activity by vaccination with the HSV-2 mutant ICP10DeltaPK. Vaccine 20, 2796–2807 (2002).
Tähtinen, S. et al. Adenovirus improves the efficacy of adoptive T-cell therapy by recruiting immune cells to and promoting their activity at the tumor. Cancer Immunol. Res. 3, 915–925 (2015).
Ahmed, A. & Tait, S. W. G. Targeting immunogenic cell death in cancer. Mol. Oncol. 14, 2994–3006 (2020).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu Rev. Immunol. 31, 51–72 (2013).
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007).
Ito, H. et al. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J. Natl Cancer Inst. 98, 625–636 (2006).
Guo, Z. S., Liu, Z. & Bartlett, D. L. Oncolytic immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front. Oncol. 4, 74 (2014).
Di Somma, S. et al. The oncolytic virus dl922-947 triggers immunogenic cell death in mesothelioma and reduces xenograft growth. Front. Oncol. 9, 564 (2019).
Shiomi, A. & Usui, T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediators Inflamm. 2015, 568543 (2015).
Kaufman, H. L. et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 17, 718–730 (2010).
Quixabeira, D. C. A. et al. Oncolytic adenovirus coding for a variant interleukin 2 (vIL-2) cytokine re-programs the tumor microenvironment and confers enhanced tumor control. Front. Immunol. 12, 674400 (2021).
Sun, Z. et al. A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8(+) T-cell response and effective tumor control. Nat. Commun. 10, 3874 (2019).
Chen, L. et al. Intratumoral expression of interleukin 23 variants using oncolytic vaccinia virus elicit potent antitumor effects on multiple tumor models via tumor microenvironment modulation. Theranostics 11, 6668–6681 (2021).
Cervera-Carrascon, V. et al. Adenovirus armed with TNFa and IL2 added to aPD-1 regimen mediates antitumor efficacy in tumors refractory to aPD-1. Front. Immunol. 12, 706517 (2021).
Bourgeois-Daigneault, M. C. et al. Oncolytic vesicular stomatitis virus expressing interferon-γ has enhanced therapeutic activity. Mol. Ther. Oncolytics 3, 16001 (2016).
Ozga, A. J., Chow, M. T. & Luster, A. D. Chemokines and the immune response to cancer. Immunity 54, 859–874 (2021).
Eckert, E. C. et al. Generation of a tumor-specific chemokine gradient using oncolytic vesicular stomatitis virus encoding CXCL9. Mol. Ther. Oncolytics 16, 63–74 (2020).
Li, X. et al. CXCL10-armed oncolytic adenovirus promotes tumor-infiltrating T-cell chemotaxis to enhance anti-PD-1 therapy. Oncoimmunology 11, 2118210 (2022).
Liu, Z. et al. CXCL11-armed oncolytic poxvirus elicits potent antitumor immunity and shows enhanced therapeutic efficacy. Oncoimmunology 5, e1091554 (2016).
Tian, L. et al. Specific targeting of glioblastoma with an oncolytic virus expressing a cetuximab-CCL5 fusion protein via innate and adaptive immunity. Nat. Cancer 3, 1318–1335 (2022).
Huang, F. Y. et al. A recombinant oncolytic Newcastle virus expressing MIP-3α promotes systemic antitumor immunity. J. Immunother. Cancer 8, e000330 (2020).
Guo, Z. S., Lotze, M. T., Zhu, Z., Storkus, W. J. & Song, X. T. Bi- and tri-specific T cell engager-armed oncolytic viruses: next-generation cancer immunotherapy. Biomedicines 8, 204 (2020).
Yu, F. et al. T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy. Mol. Ther. 22, 102–111 (2014).
Wang, Q. et al. Oncolytic adenovirus with MUC16-BiTE shows enhanced antitumor immune response by reversing the tumor microenvironment in PDX model of ovarian cancer. Oncoimmunology 11, 2096362 (2022).
Fajardo, C. A. et al. Oncolytic adenoviral delivery of an EGFR-targeting T-cell engager improves antitumor efficacy. Cancer Res. 77, 2052–2063 (2017).
Freedman, J. D. et al. Oncolytic adenovirus expressing bispecific antibody targets T-cell cytotoxicity in cancer biopsies. EMBO Mol. Med. 9, 1067–1087 (2017).
Rooney, M. S., Shukla, S. A., Wu, C. J., Getz, G. & Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61 (2015).
Taylor, A., Verhagen, J., Blaser, K., Akdis, M. & Akdis, C. A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology 117, 433–442 (2006).
Vinay, D. S. et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 35 Suppl, S185–S198 (2015).
O’Donnell, J. S., Long, G. V., Scolyer, R. A., Teng, M. W. & Smyth, M. J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 52, 71–81 (2017).
Chauvin, J. M. et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J. Clin. Invest. 125, 2046–2058 (2015).
Jacquelot, N. et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat. Commun. 8, 592 (2017).
Liu, Z., Ravindranathan, R., Kalinski, P., Guo, Z. S. & Bartlett, D. L. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 8, 14754 (2017).
Woo, Y. et al. Novel chimeric immuno-oncolytic virus CF33-hNIS-antiPDL1 for the treatment of pancreatic cancer. J. Am. Coll. Surg. 230, 709–717 (2020).
Haines, B. B. et al. ONCR-177, an oncolytic HSV-1 designed to potently activate systemic antitumor immunity. Cancer Immunol. Res. 9, 291–308 (2021).
Eriksson, E. et al. Shaping the tumor stroma and sparking immune activation by CD40 and 4-1BB signaling induced by an armed oncolytic virus. Clin. Cancer Res. 23, 5846–5857 (2017).
Dempke, W. C. M., Fenchel, K., Uciechowski, P. & Dale, S. P. Second- and third-generation drugs for immuno-oncology treatment – the more the better? Eur. J. Cancer 74, 55–72 (2017).
Zamarin, D. et al. Intratumoral modulation of the inducible co-stimulator ICOS by recombinant oncolytic virus promotes systemic anti-tumour immunity. Nat. Commun. 8, 14340 (2017).
Tauriello, D. V. F. et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018).
Xu, W. et al. LyP-1-modified oncolytic adenoviruses targeting transforming growth factor β inhibit tumor growth and metastases and augment immune checkpoint inhibitor therapy in breast cancer mouse models. Hum. Gene Ther. 31, 863–880 (2020).
Yang, Y. et al. An oncolytic adenovirus targeting transforming growth factor β inhibits protumorigenic signals and produces immune activation: a novel approach to enhance anti-PD-1 and anti-CTLA-4 therapy. Hum. Gene Ther. 30, 1117–1132 (2019).
Li, Y. et al. Oncolytic adenovirus targeting TGF-β enhances anti-tumor responses of mesothelin-targeted chimeric antigen receptor T cell therapy against breast cancer. Cell Immunol. 348, 104041 (2020).
Crupi, M. J. F. et al. Oncolytic virus driven T-cell-based combination immunotherapy platform for colorectal cancer. Front. Immunol. 13, 1029269 (2022).
Oh, E., Choi, I. K., Hong, J. & Yun, C. O. Oncolytic adenovirus coexpressing interleukin-12 and decorin overcomes Treg-mediated immunosuppression inducing potent antitumor effects in a weakly immunogenic tumor model. Oncotarget 8, 4730–4746 (2017).
Scott, E. M. et al. Bi- and tri-valent T cell engagers deplete tumour-associated macrophages in cancer patient samples. J. Immunother. Cancer 7, 320 (2019).
Gujar, S., Pol, J. G., Kim, Y., Lee, P. W. & Kroemer, G. Antitumor benefits of antiviral immunity: an underappreciated aspect of oncolytic virotherapies. Trends Immunol. 39, 209–221 (2018).
Pourchet, A. et al. CD8(+) T-cell immune evasion enables oncolytic virus immunotherapy. EBioMedicine 5, 59–67 (2016).
Xu, B. et al. An oncolytic herpesvirus expressing E-cadherin improves survival in mouse models of glioblastoma. Nat. Biotechnol. 37, 45–54 (2019).
Song, K. & Viskovska, M. Design and engineering of deimmunized vaccinia viral vectors. Biomedicines 8, 491 (2020).
Swiecki, M. & Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 15, 471–485 (2015).
van Dongen, H. M., Masoumi, N., Witwer, K. W. & Pegtel, D. M. Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiol. Mol. Biol. Rev. 80, 369–386 (2016).
Brown, M. C. et al. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci. Transl. Med. 9, eaan4220 (2017).
Swain, S. L., McKinstry, K. K. & Strutt, T. M. Expanding roles for CD4+ T cells in immunity to viruses. Nat. Rev. Immunol. 12, 136–148 (2012).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Folkman, J. What is the evidence that tumors are angiogenesis dependent? J. Natl Cancer Inst. 82, 4–6 (1990).
Bellone, M. & Calcinotto, A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front. Oncol. 3, 231 (2013).
Miller, A. et al. Reporter gene imaging identifies intratumoral infection voids as a critical barrier to systemic oncolytic virus efficacy. Mol. Ther. Oncolytics 1, 14005 (2014).
Santry, L. A. et al. Tumour vasculature: friend or foe of oncolytic viruses? Cytokine Growth Factor Rev. 56, 69–82 (2020).
Breitbach, C. J. et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 73, 1265–1275 (2013).
Mahller, Y. Y. et al. Oncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model. Mol. Ther. 15, 279–286 (2007).
Breitbach, C. J. et al. Targeting tumor vasculature with an oncolytic virus. Mol. Ther. 19, 886–894 (2011).
Reddi, H. V. et al. Antitumor activity of VB-111, a novel antiangiogenic virotherapeutic, in thyroid cancer xenograft mouse models. Genes Cancer 2, 993–995 (2011).
Gil, M., Seshadri, M., Komorowski, M. P., Abrams, S. I. & Kozbor, D. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc. Natl Acad. Sci. USA 110, E1291–E1300 (2013).
Breitbach, C. J. et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol. Ther. 15, 1686–1693 (2007).
Weed, D. J., Pritchard, S. M., Gonzalez, F., Aguilar, H. C. & Nicola, A. V. Mildly acidic pH triggers an irreversible conformational change in the fusion domain of herpes simplex virus 1 glycoprotein B and inactivation of viral entry. J. Virol. 91, e02123–e02116 (2017).
Weiss, K. et al. Influence of process conditions on measles virus stability. Am. J. Biochem. Biotechnol. 9, 243–254 (2013).
Huang, Y. et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl Acad. Sci. USA 109, 17561–17566 (2012).
Liu, T. C., Castelo-Branco, P., Rabkin, S. D. & Martuza, R. L. Trichostatin A and oncolytic HSV combination therapy shows enhanced antitumoral and antiangiogenic effects. Mol. Ther. 16, 1041–1047 (2008).
Deguchi, T. et al. Combination of the tumor angiogenesis inhibitor bevacizumab and intratumoral oncolytic herpes virus injections as a treatment strategy for human gastric cancers. Hepatogastroenterology 59, 1844–1850 (2012).
Zhang, W. et al. Bevacizumab with angiostatin-armed oHSV increases antiangiogenesis and decreases bevacizumab-induced invasion in U87 glioma. Mol. Ther. 20, 37–45 (2012).
Tomita, Y. et al. Oncolytic herpes virus armed with vasculostatin in combination with bevacizumab abrogates glioma invasion via the CCN1 and AKT signaling pathways. Mol. Cancer Ther. 18, 1418–1429 (2019).
Nair, M. et al. Enhancing antitumor efficacy of heavily vascularized tumors by RAMBO virus through decreased tumor endothelial cell activation. Cancers (Basel) 12, 1040 (2020).
Xiao, T. et al. VEGI-armed oncolytic adenovirus inhibits tumor neovascularization and directly induces mitochondria-mediated cancer cell apoptosis. Cell Res. 20, 367–378 (2010).
Li, L. X. et al. Antitumor efficacy of a recombinant adenovirus encoding endostatin combined with an E1B55KD-deficient adenovirus in gastric cancer cells. J. Transl. Med. 11, 257 (2013).
Di Tacchio, M. et al. Tumor vessel normalization, immunostimulatory reprogramming, and improved survival in glioblastoma with combined inhibition of PD-1, angiopoietin-2, and VEGF. Cancer Immunol. Res. 7, 1910–1927 (2019).
Shrimali, R. K. et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 70, 6171–6180 (2010).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
DePeaux, K. & Delgoffe, G. M. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 21, 785–797 (2021).
Hirpara, J. et al. Metabolic reprogramming of oncogene-addicted cancer cells to OXPHOS as a mechanism of drug resistance. Redox Biol. 25, 101076 (2019).
Kodama, M. et al. A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer. Nat. Commun. 11, 1320 (2020).
Currie, E., Schulze, A., Zechner, R., Walther, T. C. & Farese, R. V. Jr Cellular fatty acid metabolism and cancer. Cell Metab. 18, 153–161 (2013).
Kennedy, B. E., Sadek, M. & Gujar, S. A. Targeted metabolic reprogramming to improve the efficacy of oncolytic virus therapy. Mol. Ther. 28, 1417–1421 (2020).
Jiang, H. et al. PFKFB3-driven macrophage glycolytic metabolism is a crucial component of innate antiviral defense. J. Immunol. 197, 2880–2890 (2016).
Dyer, A. et al. Antagonism of glycolysis and reductive carboxylation of glutamine potentiates activity of oncolytic adenoviruses in cancer cells. Cancer Res. 79, 331–345 (2019).
Al-Shammari, A. M., Abdullah, A. H., Allami, Z. M. & Yaseen, N. Y. 2-Deoxyglucose and Newcastle disease virus synergize to kill breast cancer cells by inhibition of glycolysis pathway through glyceraldehyde3-phosphate downregulation. Front. Mol. Biosci. 6, 90 (2019).
Kennedy, B. E. et al. Inhibition of pyruvate dehydrogenase kinase enhances the antitumor efficacy of oncolytic reovirus. Cancer Res. 79, 3824–3836 (2019).
Mazzon, M. et al. A mechanism for induction of a hypoxic response by vaccinia virus. Proc. Natl Acad. Sci. USA 110, 12444–12449 (2013).
Chang, C. H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015).
Colegio, O. R. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Rivadeneira, D. B. et al. Oncolytic viruses engineered to enforce leptin expression reprogram tumor-infiltrating T cell metabolism and promote tumor clearance. Immunity 51, 548–560.e544 (2019).
Barrett, R. L. & Puré, E. Cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy. Elife 9, e57243 (2020).
Yamauchi, M. et al. Fibroblast heterogeneity and its impact on extracellular matrix and immune landscape remodeling in cancer. Matrix Biol. 91-92, 8–18 (2020).
Hosein, A. N., Brekken, R. A. & Maitra, A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 17, 487–505 (2020).
Acerbi, I. et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. (Camb.) 7, 1120–1134 (2015).
Arslan, F. et al. The role of versican isoforms V0/V1 in glioma migration mediated by transforming growth factor-beta2. Br. J. Cancer 96, 1560–1568 (2007).
Twumasi-Boateng, K., Pettigrew, J. L., Kwok, Y. Y. E., Bell, J. C. & Nelson, B. H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer 18, 419–432 (2018).
Tedcastle, A., Illingworth, S., Brown, A., Seymour, L. W. & Fisher, K. D. Actin-resistant DNAse I expression from oncolytic adenovirus enadenotucirev enhances its intratumoral spread and reduces tumor growth. Mol. Ther. 24, 796–804 (2016).
Kiyokawa, J. et al. Modification of extracellular matrix enhances oncolytic adenovirus immunotherapy in glioblastoma. Clin. Cancer Res. 27, 889–902 (2021).
Na, Y. et al. Potent antitumor effect of neurotensin receptor-targeted oncolytic adenovirus co-expressing decorin and Wnt antagonist in an orthotopic pancreatic tumor model. J. Control Release 220, 766–782 (2015).
Shen, Y. et al. VG161 activates systemic antitumor immunity in pancreatic cancer models as a novel oncolytic herpesvirus expressing multiple immunomodulatory transgenes. J. Med. Virol. 95, e28108 (2023).
Kim, S. I. et al. Recombinant orthopoxvirus primes colon cancer for checkpoint inhibitor and cross-primes T cells for antitumor and antiviral immunity. Mol. Cancer Ther. 20, 173–182 (2021).
Nguyen, H. M., Bommareddy, P. K., Silk, A. W. & Saha, D. Optimal timing of PD-1 blockade in combination with oncolytic virus therapy. Semin Cancer Biol. 86, 971–980 (2022).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Sugawara, K. et al. Oncolytic herpes virus G47Δ works synergistically with CTLA-4 inhibition via dynamic intratumoral immune modulation. Mol. Ther. Oncolytics 22, 129–142 (2021).
Lin, C. et al. Intratumoral delivery of a PD-1-blocking scFv encoded in oncolytic HSV-1 promotes antitumor immunity and synergizes with TIGIT blockade. Cancer Immunol. Res. 8, 632–647 (2020).
Sun, F. et al. Dual but not single PD-1 or TIM-3 blockade enhances oncolytic virotherapy in refractory lung cancer. J. Immunother. Cancer 8, e000294 (2020).
Zuo, S. et al. An engineered oncolytic vaccinia virus encoding a single-chain variable fragment against TIGIT induces effective antitumor immunity and synergizes with PD-1 or LAG-3 blockade. J. Immunother. Cancer 9, e002843 (2021).
Patel, M. R. et al. JAK/STAT inhibition with ruxolitinib enhances oncolytic virotherapy in non-small cell lung cancer models. Cancer Gene Ther. 26, 411–418 (2019).
Nguyen, T. T. et al. Mutations in the IFNγ-JAK-STAT pathway causing resistance to immune checkpoint inhibitors in melanoma increase sensitivity to oncolytic virus treatment. Clin. Cancer Res. 27, 3432–3442 (2021).
Du, Z. et al. Inhibition of type I interferon-mediated antiviral action in human glioma cells by the IKK inhibitors BMS-345541 and TPCA-1. J. Interferon Cytokine Res. 32, 368–377 (2012).
Yoo, J. Y. et al. Bortezomib-induced unfolded protein response increases oncolytic HSV-1 replication resulting in synergistic antitumor effects. Clin. Cancer Res. 20, 3787–3798 (2014).
Wang, L. et al. Oncolytic herpes simplex virus and PI3K inhibitor BKM120 synergize to promote killing of prostate cancer stem-like cells. Mol. Ther. Oncolytics 13, 58–66 (2019).
Bommareddy, P. K., Aspromonte, S., Zloza, A., Rabkin, S. D. & Kaufman, H. L. MEK inhibition enhances oncolytic virus immunotherapy through increased tumor cell killing and T cell activation. Sci. Transl. Med. 10, eaau0417 (2018).
Hutzen, B. et al. TGF-β inhibition improves oncolytic herpes viroimmunotherapy in murine models of rhabdomyosarcoma. Mol. Ther. Oncolytics 7, 17–26 (2017).
Reinhart, B. et al. Inhibition of indoleamine-2,3-dioxygenase (IDO) in glioblastoma cells by oncolytic herpes simplex virus. Adv. Virol. 2012, 815465 (2012).
Parrish, C. et al. Oncolytic reovirus enhances rituximab-mediated antibody-dependent cellular cytotoxicity against chronic lymphocytic leukaemia. Leukemia 29, 1799–1810 (2015).
Yoo, J. Y. et al. Bortezomib treatment sensitizes oncolytic HSV-1-treated tumors to NK cell immunotherapy. Clin. Cancer Res. 22, 5265–5276 (2016).
Greenaway, J. et al. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1). J. Cell Physiol. 210, 807–818 (2007).
Matuszewska, K. et al. Combining vascular normalization with an oncolytic virus enhances immunotherapy in a preclinical model of advanced-stage ovarian cancer. Clin. Cancer Res. 25, 1624–1638 (2019).
Saha, D. et al. Combinatorial effects of VEGFR kinase inhibitor axitinib and oncolytic virotherapy in mouse and human glioblastoma stem-like cell models. Clin. Cancer Res. 24, 3409–3422 (2018).
Malfitano, A. M., Di Somma, S., Iannuzzi, C. A., Pentimalli, F. & Portella, G. Virotherapy: from single agents to combinatorial treatments. Biochem. Pharm. 177, 113986 (2020).
Heo, J. et al. Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: preclinical and clinical demonstration of combination efficacy. Mol. Ther. 19, 1170–1179 (2011).
Lawson, K. A. et al. Repurposing sunitinib with oncolytic reovirus as a novel immunotherapeutic strategy for renal cell carcinoma. Clin. Cancer Res. 22, 5839–5850 (2016).
Tan, G. et al. Combination therapy of oncolytic herpes simplex virus HF10 and bevacizumab against experimental model of human breast carcinoma xenograft. Int. J. Cancer 136, 1718–1730 (2015).
Perez, C. et al. Permissive expansion and homing of adoptively transferred T cells in tumor-bearing hosts. Int J. Cancer 137, 359–371 (2015).
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62–68 (2015).
Chen, T. et al. IL-21 arming potentiates the anti-tumor activity of an oncolytic vaccinia virus in monotherapy and combination therapy. J. Immunother. Cancer 9, e001647 (2021).
Park, A. K. et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci. Transl. Med. 12, eaaz1863 (2020).
Zheng, N. et al. Induction of tumor cell autosis by myxoma virus-infected CAR-T and TCR-T cells to overcome primary and acquired resistance. Cancer Cell 40, 973–985.e977 (2022).
Evgin, L. et al. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci. Transl. Med. 14, eabn2231 (2022).
Feist, M. et al. Oncolytic virus promotes tumor-reactive infiltrating lymphocytes for adoptive cell therapy. Cancer Gene Ther. 28, 98–111 (2021).
Li, F. et al. CCL5-armed oncolytic virus augments CCR5-engineered NK cell infiltration and antitumor efficiency. J. Immunother. Cancer 8, e000131 (2020).
Ma, R. et al. An oncolytic virus expressing IL15/IL15Rα combined with off-the-shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res. 81, 3635–3648 (2021).
Simpson, G. R., Relph, K., Harrington, K., Melcher, A. & Pandha, H. Cancer immunotherapy via combining oncolytic virotherapy with chemotherapy: recent advances. Oncolytic Virother 5, 1–13 (2016).
Heise, C., Lemmon, M. & Kirn, D. Efficacy with a replication-selective adenovirus plus cisplatin-based chemotherapy: dependence on sequencing but not p53 functional status or route of administration. Clin. Cancer Res. 6, 4908–4914 (2000).
Cema, I. et al. Stage IIA skin melanoma treatment with ECHO-7 oncolytic virus Rigvir. Perm. J. 26, 139–144 (2022).
Donina, S. et al. Adapted ECHO-7 virus Rigvir immunotherapy (oncolytic virotherapy) prolongs survival in melanoma patients after surgical excision of the tumour in a retrospective study. Melanoma Res. 25, 421–426 (2015).
Jaunalksne, I., Brokane, L., Petroska, D., Rasa, A. & Alberts, P. ECHO-7 oncolytic virus Rigvir(R) in an adjuvant setting for stage I uveal melanoma; a retrospective case report. Am. J. Ophthalmol. Case Rep. 17, 100615 (2020).
Sorokins, V. et al. A progressive stage IIIB melanoma treated with oncolytic ECHO-7 virus: a case report. SAGE Open Med. Case Rep. 8, 2050313X20934978 (2020).
Tilgase, A. et al. Effect of oncolytic ECHO-7 virus strain Rigvir on uveal melanoma cell lines. BMC Res. Notes 13, 222 (2020).
Alberts, P. et al. Long-term treatment with the oncolytic ECHO-7 virus Rigvir of a melanoma stage IV M1c patient, a small cell lung cancer stage IIIA patient, and a histiocytic sarcoma stage IV patient-three case reports. APMIS 124, 896–904 (2016).
Alberts, P., Tilgase, A., Rasa, A., Bandere, K. & Venskus, D. The advent of oncolytic virotherapy in oncology: the Rigvir(R) story. Eur. J. Pharm. 837, 117–126 (2018).
Xia, Z. J. et al. [Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus]. Ai Zheng 23, 1666–1670 (2004).
Andtbacka, R. H. et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33, 2780–2788 (2015).
Dranoff, G. et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA 90, 3539–3543 (1993).
Tamadaho, R. S. E., Hoerauf, A. & Layland, L. E. Immunomodulatory effects of myeloid-derived suppressor cells in diseases: role in cancer and infections. Immunobiology 223, 432–442 (2018).
Puzanov, I. et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J. Clin. Oncol. 34, 2619–2626 (2016).
Ribas, A. et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170, 1109–1119.e1110 (2017).
Todo, T., Ino, Y., Ohtsu, H., Shibahara, J. & Tanaka, M. A phase I/II study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma. Nat. Commun. 13, 4119 (2022).
Andtbacka, R. H. I. et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J. Immunother. Cancer 7, 145 (2019).
Malvehy, J. et al. Talimogene laherparepvec upregulates immune-cell populations in non-injected lesions: findings from a phase II, multicenter, open-label study in patients with stage IIIB-IVM1c melanoma. J. Immunother. Cancer 9, e001621 (2021).
Yamazaki, N. et al. A phase I study of the safety and efficacy of talimogene laherparepvec in Japanese patients with advanced melanoma. Cancer Sci. 113, 2798–2806 (2022).
Andtbacka, R. H. I. et al. Clinical responses of oncolytic coxsackievirus A21 (V937) in patients with unresectable melanoma. J. Clin. Oncol. 39, 3829–3838 (2021).
Beasley, G. M. et al. Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. J. Immunother. Cancer 9, e002203 (2021).
Heo, J. et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 19, 329–336 (2013).
Moehler, M. et al. Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: a randomized multicenter Phase IIb trial (TRAVERSE). Oncoimmunology 8, 1615817 (2019).
Shen, Y. et al. 694 An open-label phase I dose-escalation clinical trial to evaluate the safety, tolerability, pharmacokinetic profile and preliminary efficacy of VG161 in patients with advanced primary liver cancer. J. Immunother. Cancer 10, A725–A725 (2022).
Desjardins, A. et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 379, 150–161 (2018).
Fares, J. et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 22, 1103–1114 (2021).
Friedman, G. K. et al. Oncolytic HSV-1 G207 immunovirotherapy for pediatric high-grade gliomas. N. Engl. J. Med. 384, 1613–1622 (2021).
Aroldi, F. et al. 421 Initial results of a phase 1 trial of RP2, a first in class, enhanced potency, anti-CTLA-4 antibody expressing, oncolytic HSV as single agent and combined with nivolumab in patients with solid tumors. J. Immunother. Cancer 8, A256–A257 (2020).
Cripe, T. P. et al. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol. Ther. 23, 602–608 (2015).
Garcia-Carbonero, R. et al. Phase I, multicenter, open-label study of intravenous VCN-01 oncolytic adenovirus with or without nab-paclitaxel plus gemcitabine in patients with advanced solid tumors. J. Immunother. Cancer 10, e003255 (2022).
Kolb, E. A. et al. A phase I trial and viral clearance study of reovirus (Reolysin) in children with relapsed or refractory extra-cranial solid tumors: a Children’s Oncology Group Phase I Consortium report. Pediatr. Blood Cancer 62, 751–758 (2015).
Ranki, T. et al. Phase I study with ONCOS-102 for the treatment of solid tumors – an evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer 4, 17 (2016).
Mell, L. K. et al. Phase I trial of intravenous oncolytic vaccinia virus (GL-ONC1) with cisplatin and radiotherapy in patients with locoregionally advanced head and neck carcinoma. Clin. Cancer Res. 23, 5696–5702 (2017).
Aguilar, L. K. et al. Gene-mediated cytotoxic immunotherapy as adjuvant to surgery or chemoradiation for pancreatic adenocarcinoma. Cancer Immunol. Immunother. 64, 727–736 (2015).
Barton, K. N. et al. Phase I trial of oncolytic adenovirus-mediated cytotoxic and interleukin-12 gene therapy for the treatment of metastatic pancreatic cancer. Mol. Ther. Oncolytics 20, 94–104 (2021).
Hajda, J. et al. Phase 2 trial of oncolytic H-1 parvovirus therapy shows safety and signs of immune system activation in patients with metastatic pancreatic ductal adenocarcinoma. Clin. Cancer Res. 27, 5546–5556 (2021).
Noonan, A. M. et al. Randomized phase 2 trial of the oncolytic virus pelareorep (Reolysin) in upfront treatment of metastatic pancreatic adenocarcinoma. Mol. Ther. 24, 1150–1158 (2016).
Machiels, J. P. et al. A phase 1 dose escalation study of the oncolytic adenovirus enadenotucirev, administered intravenously to patients with epithelial solid tumors (EVOLVE). J. Immunother. Cancer 7, 20 (2019).
Packiam, V. T. et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol. Oncol. 36, 440–447 (2018).
Bradbury, P. A. et al. Canadian Cancer Trials Group (CCTG) IND211: a randomized trial of pelareorep (Reolysin) in patients with previously treated advanced or metastatic non-small cell lung cancer receiving standard salvage therapy. Lung Cancer 120, 142–148 (2018).
Cohn, D. E. et al. Randomized phase IIB evaluation of weekly paclitaxel versus weekly paclitaxel with oncolytic reovirus (Reolysin(R)) in recurrent ovarian, tubal, or peritoneal cancer: an NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 146, 477–483 (2017).
Monga, V. et al. Intratumoral talimogene laherparepvec injection with concurrent preoperative radiation in patients with locally advanced soft-tissue sarcoma of the trunk and extremities: phase IB/II trial. J. Immunother. Cancer 9, e003119 (2021).
Bernstein, V. et al. A randomized phase II study of weekly paclitaxel with or without pelareorep in patients with metastatic breast cancer: final analysis of Canadian Cancer Trials Group IND.213. Breast Cancer Res. Treat. 167, 485–493 (2018).
Lee, J. C. et al. Tolerability and safety of EUS-injected adenovirus-mediated double-suicide gene therapy with chemotherapy in locally advanced pancreatic cancer: a phase 1 trial. Gastrointest. Endosc. 92, 1044–1052. e1041 (2020).
Mahalingam, D. et al. A Phase II Study of Pelareorep (REOLYSIN((R))) in combination with gemcitabine for patients with advanced pancreatic adenocarcinoma. Cancers (Basel) 10, 160 (2018).
Moreno, V. et al. Safety and efficacy of the tumor-selective adenovirus enadenotucirev with or without paclitaxel in platinum-resistant ovarian cancer: a phase 1 clinical trial. J. Immunother. Cancer 9, e003645 (2021).
Eigl, B. J. et al. A randomized phase II study of pelareorep and docetaxel or docetaxel alone in men with metastatic castration resistant prostate cancer: CCTG study IND 209. Oncotarget 9, 8155–8164 (2018).
Jonker, D. J. et al. A randomized phase II study of FOLFOX6/Bevacizumab with or without pelareorep in patients with metastatic colorectal cancer: IND.210, a Canadian Cancer Trials Group Trial. Clin. Colorectal Cancer 17, 231–239.e237 (2018).
Chesney, J. et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 36, 1658–1667 (2018).
Kelly, C. M. et al. Objective response rate among patients with locally advanced or metastatic sarcoma treated with talimogene laherparepvec in combination with pembrolizumab: a phase 2 clinical trial. JAMA Oncol. 6, 402–408 (2020).
Shirakawa, Y. et al. Phase I dose-escalation study of endoscopic intratumoral injection of OBP-301 (Telomelysin) with radiotherapy in oesophageal cancer patients unfit for standard treatments. Eur. J. Cancer 153, 98–108 (2021).
Arnold, D. et al. 650 Pelareorep combined with atezolizumab and chemotherapy demonstrates encouraging results as first-line treatment in advanced or metastatic pancreatic ductal adenocarcinoma (PDAC) patients – interim results from the GOBLET study. J. Immunother. Cancer 10, A681–A681 (2022).
Vidal, L. et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin. Cancer Res. 14, 7127–7137 (2008).
Muhuri, M. & Gao, G. Oncolytic virus alphavirus M1: a new and promising weapon to fight cancer. Hum. Gene Ther. 32, 136–137 (2021).
Sun, Y. et al. Exploring the functions of polymers in adenovirus-mediated gene delivery: Evading immune response and redirecting tropism. Acta Biomater. 97, 93–104 (2019).
Kirn, D. H., Wang, Y., Liang, W., Contag, C. H. & Thorne, S. H. Enhancing poxvirus oncolytic effects through increased spread and immune evasion. Cancer Res. 68, 2071–2075 (2008).
Adair, R. A. et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci. Transl. Med. 4, 138ra177 (2012).
Du, W. et al. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proc. Natl Acad. Sci. USA 114, E6157–E6165 (2017).
Moreno, R. Mesenchymal stem cells and oncolytic viruses: joining forces against cancer. J. Immunother. Cancer 9, e001684 (2021).
Ramirez, M., Garcia-Castro, J., Melen, G. J., Gonzalez-Murillo, A. & Franco-Luzon, L. Patient-derived mesenchymal stem cells as delivery vehicles for oncolytic virotherapy: novel state-of-the-art technology. Oncolytic Virother 4, 149–155 (2015).
Xia, X. et al. Mesenchymal stem cells as carriers and amplifiers in CRAd delivery to tumors. Mol. Cancer 10, 134 (2011).
Yong, R. L. et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 69, 8932–8940 (2009).
He, C. B. & Lin, X. J. Inflammation scores predict the survival of patients with hepatocellular carcinoma who were treated with transarterial chemoembolization and recombinant human type-5 adenovirus H101. PLoS One 12, e0174769 (2017).
Warner, S. G. et al. A novel chimeric poxvirus encoding hNIS is tumor-tropic, imageable, and synergistic with radioiodine to sustain colon cancer regression. Mol. Ther. Oncolytics 13, 82–92 (2019).
Grekova, S. P., Raykov, Z., Zawatzky, R., Rommelaere, J. & Koch, U. Activation of a glioma-specific immune response by oncolytic parvovirus Minute Virus of Mice infection. Cancer Gene Ther. 19, 468–475 (2012).
Ma, J. et al. Characterization of virus-mediated immunogenic cancer cell death and the consequences for oncolytic virus-based immunotherapy of cancer. Cell Death Dis. 11, 48 (2020).
Miyamoto, S. et al. Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma. Cancer Res. 72, 2609–2621 (2012).
Donnelly, O. G. et al. Measles virus causes immunogenic cell death in human melanoma. Gene Ther. 20, 7–15 (2013).
Rajecki, M. et al. Mre11 inhibition by oncolytic adenovirus associates with autophagy and underlies synergy with ionizing radiation. Int. J. Cancer 125, 2441–2449 (2009).
Hu, L. et al. Oncolytic newcastle disease virus triggers cell death of lung cancer spheroids and is enhanced by pharmacological inhibition of autophagy. Am. J. Cancer Res. 5, 3612–3623 (2015).
Qian, M., Tan, H. M., Yu, N., Wang, T. & Zhang, Q. Inactivated Sendai Virus induces ROS-dependent apoptosis and autophagy in human prostate cancer cells. Biomed. Environ. Sci. 31, 280–289 (2018).
Xia, M. et al. Mitophagy enhances oncolytic measles virus replication by mitigating DDX58/RIG-I-like receptor signaling. J. Virol. 88, 5152–5164 (2014).
Post, D. E. et al. Targeted cancer gene therapy using a hypoxia inducible factor dependent oncolytic adenovirus armed with interleukin-4. Cancer Res. 67, 6872–6881 (2007).
Choi, I. K. et al. Oncolytic adenovirus co-expressing IL-12 and IL-18 improves tumor-specific immunity via differentiation of T cells expressing IL-12Rβ2 or IL-18Rα. Gene Ther. 18, 898–909 (2011).
Naik, S., Nace, R., Barber, G. N. & Russell, S. J. Potent systemic therapy of multiple myeloma utilizing oncolytic vesicular stomatitis virus coding for interferon-β. Cancer Gene Ther. 19, 443–450 (2012).
Li, J. et al. Expression of CCL19 from oncolytic vaccinia enhances immunotherapeutic potential while maintaining oncolytic activity. Neoplasia 14, 1115–1121 (2012).
Porter, C. E. et al. Oncolytic adenovirus armed with BiTE, cytokine, and checkpoint inhibitor enables CAR T cells to control the growth of heterogeneous tumors. Mol. Ther. 28, 1251–1262 (2020).
Smith, H. G. et al. PD-1 blockade following isolated limb perfusion with vaccinia virus prevents local and distant relapse of soft-tissue sarcoma. Clin. Cancer Res. 25, 3443–3454 (2019).
Hou, W., Sampath, P., Rojas, J. J. & Thorne, S. H. Oncolytic virus-mediated targeting of PGE2 in the tumor alters the immune status and sensitizes established and resistant tumors to immunotherapy. Cancer Cell 30, 108–119 (2016).
Zamarin, D. et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 6, 226ra232 (2014).
Bridle, B. W. et al. HDAC inhibition suppresses primary immune responses, enhances secondary immune responses, and abrogates autoimmunity during tumor immunotherapy. Mol. Ther. 21, 887–894 (2013).
Yoo, J. Y. et al. Oncolytic HSV therapy increases trametinib access to brain tumors and sensitizes them in vivo. Neuro. Oncol. 21, 1131–1140 (2019).
Roulstone, V. et al. BRAF- and MEK-targeted small molecule inhibitors exert enhanced antimelanoma effects in combination with oncolytic reovirus through ER stress. Mol. Ther. 23, 931–942 (2015).
Iankov, I. D. et al. Inhibition of the Aurora A kinase augments the anti-tumor efficacy of oncolytic measles virotherapy. Cancer Gene Ther. 22, 438–444 (2015).
Wu, Z. et al. Combination of cetuximab and oncolytic virus canerpaturev synergistically inhibits human colorectal cancer growth. Mol. Ther. Oncolytics 13, 107–115 (2019).
Passaro, C. et al. PARP inhibitor olaparib increases the oncolytic activity of dl922-947 in in vitro and in vivo model of anaplastic thyroid carcinoma. Mol. Oncol. 9, 78–92 (2015).
Tanoue, K. et al. Armed oncolytic adenovirus-expressing PD-L1 mini-body enhances antitumor effects of chimeric antigen receptor T cells in solid tumors. Cancer Res. 77, 2040–2051 (2017).
Tang, X. et al. Adenovirus-mediated specific tumor tagging facilitates CAR-T therapy against antigen-mismatched solid tumors. Cancer Lett. 487, 1–9 (2020).
Nishio, N. et al. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 74, 5195–5205 (2014).
Gebremeskel, S. et al. Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis. J. Immunother. Cancer 9, e002096 (2021).
Nounamo, B., Liem, J., Cannon, M. & Liu, J. Myxoma virus optimizes cisplatin for the treatment of ovarian cancer in vitro and in a syngeneic murine dissemination model. Mol. Ther. Oncolytics 6, 90–99 (2017).
Bourgeois-Daigneault, M. C. et al. Combination of Paclitaxel and MG1 oncolytic virus as a successful strategy for breast cancer treatment. Breast Cancer Res. 18, 83 (2016).
Siurala, M. et al. Oncolytic adenovirus and doxorubicin-based chemotherapy results in synergistic antitumor activity against soft-tissue sarcoma. Int J. Cancer 136, 945–954 (2015).
Annels, N. E. et al. Oncolytic immunotherapy for bladder cancer using Coxsackie A21 virus. Mol. Ther. Oncolytics 9, 1–12 (2018).
Bhattacharyya, M., Francis, J., Eddouadi, A., Lemoine, N. R. & Halldén, G. An oncolytic adenovirus defective in pRb-binding (dl922-947) can efficiently eliminate pancreatic cancer cells and tumors in vivo in combination with 5-FU or gemcitabine. Cancer Gene Ther. 18, 734–743 (2011).
Bai, Y. et al. Newcastle disease virus enhances the growth-inhibiting and proapoptotic effects of temozolomide on glioblastoma cells in vitro and in vivo. Sci. Rep. 8, 11470 (2018).
Wang, J. et al. Recombinant oncolytic adenovirus combined with cyclophosphamide induces synergy in the treatment of breast cancer in vitro and in vivo. Cancer Manag Res. 14, 2749–2761 (2022).
Ottolino-Perry, K. et al. Oncolytic vaccinia virus synergizes with irinotecan in colorectal cancer. Mol. Oncol. 9, 1539–1552 (2015).
Acknowledgements
This work was supported by National Key Research and Development Program (2019YFC1316000), National Natural Science Foundation of China (U20A20378 and 82103044), China Postdoctoral Science Foundation (2020M761761 and 2021M702826), Natural Science Foundation of Zhejiang Province/Exploration Project (LY21H160037), and Health Technology Plan of Zhejiang Province/Young Innovative Talents Program (2022RC140). We thank Sida Guo for providing the language refinement, Wei Song, Zifan Yang, Zijun Wang, and Jiaxin Li for the help with the revised version, and biorender.com for the figure creation.
Author information
Authors and Affiliations
Contributions
D.L. and Y.S. conceived and wrote this review. D.L. drew the pictures and made the tables. All authors have read and approved the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, D., Shen, Y. & Liang, T. Oncolytic virotherapy: basic principles, recent advances and future directions. Sig Transduct Target Ther 8, 156 (2023). https://doi.org/10.1038/s41392-023-01407-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-023-01407-6
This article is cited by
-
Enhanced cellular therapy: revolutionizing adoptive cellular therapy
Experimental Hematology & Oncology (2024)
-
SOCS3 inhibiting JAK-STAT pathway enhances oncolytic adenovirus efficacy by potentiating viral replication and T-cell activation
Cancer Gene Therapy (2024)
-
Application of tumor microparticles in tumor prevention and treatment
Cancer Nanotechnology (2023)
-
Antiproliferative effects of mesenchymal stem cells carrying Newcastle disease virus and Lactobacillus Casei extract on CT26 Cell line: synergistic effects in cancer therapy
Infectious Agents and Cancer (2023)