Abstract
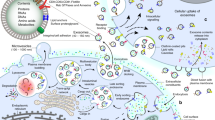
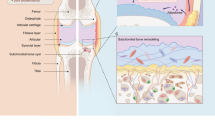
Cell-derived extracellular vesicles (EVs), present in synovial fluid and cartilage extracellular matrix (ECM), are involved in joint development and in the regulation of joint homeostasis. Although the exact function of EVs in these processes remains incompletely defined, the knowledge already acquired in this field suggests a role for these EVs as biomarkers of joint disease, and as a new tool to restore joint homeostasis and enhance articular tissue regeneration. In addition to direct injection of therapeutic EVs into the target site, surface coating of scaffolds and embedding of EVs in hydrogels might also lead to novel therapeutic possibilities. Based on the existing literature of EVs in synovial fluid and articular tissues, and investigation of the molecular factors (including microRNAs) active in joint homeostasis (or during its disturbance), we postulate novel perspectives for the implementation of EVs as a regenerative medicine approach in joint repair.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Witwer, K. W. et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles http://dx.doi.org/10.3402/jev.v2i0.20360 (2013).
Berckmans, R. J. et al. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 46, 2857–2866 (2002).
Thery, C., Zitvogel, L. & Amigorena, S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 (2002).
Thery, C., Ostrowski, M. & Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593 (2009).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Yanez-Mo, M. et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066 (2015).
Zomer, A. et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161, 1046–1057 (2015).
Ridder, K. et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 12, e1001874 (2014).
Anderson, H. C. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J. Cell Biol. 41, 59–72 (1969).
Anderson, H. C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 5, 222–226 (2003).
Nahar, N. N., Missana, L. R., Garimella, R., Tague, S. E. & Anderson, H. C. Matrix vesicles are carriers of bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF), and noncollagenous matrix proteins. J. Bone Miner. Metab. 26, 514–519 (2008).
Fourcade, O. et al. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell 80, 919–927 (1995).
Buzas, E. I., Gyorgy, B., Nagy, G., Falus, A. & Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 10, 356–364 (2014).
Goldring, M. B. & Marcu, K. B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 11, 224 (2009).
Berckmans, R. J. et al. Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Arthritis Res. Ther. 7, R536–R544 (2005).
Messer, L. et al. Microparticle-induced release of B-lymphocyte regulators by rheumatoid synoviocytes. Arthritis Res. Ther. 11, R40 (2009).
Reich, N. et al. Microparticles stimulate angiogenesis by inducing ELR+ CXC-chemokines in synovial fibroblasts. J. Cell. Mol. Med. 15, 756–762 (2011).
Kato, T. et al. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 16, R163 (2014).
Jungel, A. et al. Microparticles stimulate the synthesis of prostaglandin E2 via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum. 56, 3564–3574 (2007).
Distler, J. H. et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc. Natl Acad. Sci. USA 102, 2892–2897 (2005).
Gyorgy, B. et al. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS ONE 7, e49726 (2012).
Zhang, H. et al. A membrane form of TNF-α presented by exosomes delays T cell activation-induced cell death. J. Immunol. 176, 7385–7393 (2006).
Boilard, E. et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327, 580–583 (2010).
Duchez, A. C. et al. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc. Natl Acad. Sci. USA 112, E3564–E3573 (2015).
Cloutier, N. et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol. Med. 5, 235–249 (2013).
Skriner, K., Adolph, K., Jungblut, P. R. & Burmester, G. R. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 54, 3809–3814 (2006).
Mor-Vaknin, N. et al. The DEK nuclear autoantigen is a secreted chemotactic factor. Mol. Cell. Biol. 26, 9484–9496 (2006).
Sillat, T. et al. Toll-like receptors in human chondrocytes and osteoarthritic cartilage. Acta Orthop. 84, 585–592 (2013).
Hu, F. et al. Toll-like receptors expressed by synovial fibroblasts perpetuate Th1 and Th17 cell responses in rheumatoid arthritis. PLoS ONE 9, e100266 (2014).
Gomez, R., Villalvilla, A., Largo, R., Gualillo, O. & Herrero-Beaumont, G. TLR4 signalling in osteoarthritis — finding targets for candidate DMOADs. Nat. Rev. Rheumatol. 11, 159–170 (2015).
Bretz, N. P. et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J. Biol. Chem. 288, 36691–36702 (2013).
Kim, H. A. et al. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 54, 2152–2163 (2006).
Liu-Bryan, R. & Terkeltaub, R. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous Toll-like receptor 2/Toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum. 62, 2004–2012 (2010).
Lo Cicero, A., Majkowska, I., Nagase, H., Di Liegro, I. & Troeberg, L. Microvesicles shed by oligodendroglioma cells and rheumatoid synovial fibroblasts contain aggrecanase activity. Matrix Biol. 31, 229–233 (2012).
Pasztoi, M. et al. The recently identified hexosaminidase D enzyme substantially contributes to the elevated hexosaminidase activity in rheumatoid arthritis. Immunol. Lett. 149, 71–76 (2013).
Pasztoi, M. et al. Gene expression and activity of cartilage degrading glycosidases in human rheumatoid arthritis and osteoarthritis synovial fibroblasts. Arthritis Res. Ther. 11, R68 (2009).
Mu, W., Rana, S. & Zoller, M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia 15, 875–887 (2013).
Smith, M. M. & Ghosh, P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol. Int. 7, 113–122 (1987).
Bellingham, S. A., Coleman, B. M. & Hill, A. F. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 40, 10937–10949 (2012).
Cheng, L., Sun, X., Scicluna, B. J., Coleman, B. M. & Hill, A. F. Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int. 86, 433–444 (2014).
Gernapudi, R. et al. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res. Treat. 150, 685–695 (2015).
Hong, E. & Reddi, A. H. MicroRNAs in chondrogenesis, articular cartilage, and osteoarthritis: implications for tissue engineering. Tissue Eng. Part B. Rev. 18, 445–453 (2012).
Miyaki, S. et al. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 60, 2723–2730 (2009).
Yamasaki, K. et al. Expression of microRNA-146a in osteoarthritis cartilage. Arthritis Rheum. 60, 1035–1041 (2009).
Nakasa, T., Shibuya, H., Nagata, Y., Niimoto, T. & Ochi, M. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheum. 63, 1582–1590 (2011).
Boukouris, S. & Mathivanan, S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin. Appl. 9, 358–367 (2015).
Cheng, L., Sharples, R. A., Scicluna, B. J. & Hill, A. F. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracell. Vesicles 3, 23743 (2014).
Julich, H., Willms, A., Lukacs-Kornek, V. & Kornek, M. Extracellular vesicle profiling and their use as potential disease specific biomarker. Front. Immunol. 5, 413 (2014).
Skog, J. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 (2008).
Vanniasinghe, A. S. et al. Targeting fibroblast-like synovial cells at sites of inflammation with peptide targeted liposomes results in inhibition of experimental arthritis. Clin. Immunol. 151, 43–54 (2014).
Metselaar, J. M. et al. Liposomal targeting of glucocorticoids to synovial lining cells strongly increases therapeutic benefit in collagen type II arthritis. Ann. Rheum. Dis. 63, 348–353 (2004).
Kim, S. H. et al. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J. Immunol. 174, 6440–6448 (2005).
Heldring, N., Mager, I., Wood, M. J., Le Blanc, K. & Andaloussi, S. E. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum. Gene Ther. 26, 506–517 (2015).
Kordelas, L. et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28, 970–973 (2014).
MacDonald, G. I., Augello, A. & De Bari, C. Role of mesenchymal stem cells in reestablishing immunologic tolerance in autoimmune rheumatic diseases. Arthritis Rheum. 63, 2547–2557 (2011).
Robbins, P. D. & Morelli, A. E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14, 195–208 (2014).
De Jong, O. G., Van Balkom, B. W., Schiffelers, R. M., Bouten, C. V. & Verhaar, M. C. Extracellular vesicles: potential roles in regenerative medicine. Front. Immunol. 5, 608 (2014).
Grande, D. A., Schwartz, J. A., Brandel, E., Chahine, N. O. & Sgaglione, N. Articular cartilage repair: where we have been, where we are now, and where we are headed. Cartilage 4, 281–285 (2013).
Savkovic, V. et al. Mesenchymal stem cells in cartilage regeneration. Curr. Stem Cell. Res. Ther. 9, 469–488 (2014).
De Windt, T. S. et al. Concise review: unraveling stem cell cocultures in regenerative medicine: which cell interactions steer cartilage regeneration and how? Stem Cells Transl. Med. 3, 723–733 (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
Caplan, A. I. & Correa, D. The MSC: an injury drugstore. Cell Stem Cell 9, 11–15 (2011).
Camussi, G., Deregibus, M. C., Bruno, S., Cantaluppi, V. & Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 78, 838–848 (2010).
Bruno, S. et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 20, 1053–1067 (2009).
Baglio, S. R., Pegtel, D. M. & Baldini, N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 3, 359 (2012).
Lamichhane, T. N. et al. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng. Part B. Rev. 21, 45–54 (2015).
Vonk, L. A., Kragten, A. H., Dhert, W. J., Saris, D. B. & Creemers, L. B. Overexpression of hsa-miR-148a promotes cartilage production and inhibits cartilage degradation by osteoarthritic chondrocytes. Osteoarthr. Cartil. 22, 145–153 (2014).
Record, M., Carayon, K., Poirot, M. & Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim. Biophys. Acta 1841, 108–120 (2014).
Cloutier, N. et al. Platelets can enhance vascular permeability. Blood 120, 1334–1343 (2012).
Smith, B. D. & Grande, D. A. The current state of scaffolds for musculoskeletal regenerative applications. Nat. Rev. Rheumatol. 11, 213–222 (2015).
Samad, A., Sultana, Y. & Aqil, M. Liposomal drug delivery systems: an update review. Curr. Drug Deliv. 4, 297–305 (2007).
Sawada, S. et al. Functional polymer gel–exosomes hybrids for drug delivery system and tissue engineering. [abstract P4C-192], Presented at the 2014 ISEV Annual Meeting (2014).
Malda, J. et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv. Mater. 25, 5011–5028 (2013).
Visser, J. et al. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 5, 035007 (2013).
Dai, S. et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 16, 782–790 (2008).
Wiklander, O. P. et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 4, 26316 (2015).
Ohno, S. et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 21, 185–191 (2013).
Hurtig, M. B. et al. Preclinical studies for cartilage repair: recommendations from the International Cartilage Repair Society. Cartilage 2, 137–152 (2011).
Bergknut, N. et al. The dog as an animal model for intervertebral disc degeneration? Spine (Phila. Pa 1976) 37, 351–358 (2012).
Malda, J. et al. Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthr. Cartil. 20, 1147–1151 (2012).
Lotvall, J. et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 3, 26913 (2014).
Gyorgy, B. et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood 117, e39–e48 (2011).
Acknowledgements
The authors' research work is supported by the Dutch Arthritis Foundation (grant numbers LLP-12 and LLP-22; J.M., P.R.W.), the EU Seventh Framework Programme (FP7/2007–2013, grant agreement 309962 [HydroZONES]) (J.M.), the European Research Council (grant agreement 647426 [3D-JOINT]) (J.M., P.R.W.), and a grant from the Dutch government to the Netherlands Institute for Regenerative Medicine (NIRM, grant number FES0908) (J.B.).
Author information
Authors and Affiliations
Contributions
J.M. and J.B. contributed equally to researching data for the article and writing the manuscript. All authors made substantial contributions to discussion of content and to review and edit the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Malda, J., Boere, J., van de Lest, C. et al. Extracellular vesicles — new tool for joint repair and regeneration. Nat Rev Rheumatol 12, 243–249 (2016). https://doi.org/10.1038/nrrheum.2015.170
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.170
This article is cited by
-
Extracellular vesicles: a rising star for therapeutics and drug delivery
Journal of Nanobiotechnology (2023)
-
Small extracellular vesicles have distinct CD81 and CD9 tetraspanin expression profiles in plasma from rheumatoid arthritis patients
Clinical and Experimental Medicine (2023)
-
Synovial fibroblast-miR-214-3p-derived exosomes inhibit inflammation and degeneration of cartilage tissues of osteoarthritis rats
Molecular and Cellular Biochemistry (2023)
-
Exosome application in treatment and diagnosis of B-cell disorders: leukemias, multiple sclerosis, and arthritis rheumatoid
Cellular & Molecular Biology Letters (2022)
-
Engineering stem cells to produce exosomes with enhanced bone regeneration effects: an alternative strategy for gene therapy
Journal of Nanobiotechnology (2022)