Abstract
The development of immunosuppressants with minimal adverse and nephrotoxic effects is important to improve outcomes, such as acute and chronic antibody-mediated rejection, after organ transplantation. In addition, the application of expanded criteria for donors and transplantation in immunized patients necessitates the development of new therapies. Drug development over the past 10 years has generally been disappointing, but several new promising compounds have been or are being developed to prevent acute and chronic transplant rejection. In this Review, we report on several compounds that have been developed to remove allogenic T cells and/or to inhibit T-cell activation. We also discuss compounds that interfere with antibody-mediated rejection.
Key Points
-
Allogenic organ transplantation is limited by drug-associated toxicity and the occurrence of antibody-mediated or chronic rejection
-
Improved understanding of the molecular mechanisms of rejection has led to the development of agents that regulate T-cell function, complement activation and/or the survival of plasma and B cells in immunized or naive patients
-
These new agents may control acute, antibody-mediated rejection and chronic rejection to increase long-term renal transplantation outcomes
-
The safety of these new agents remains to be evaluated to ensure that they do not increase the risk of infections or tumor formation in transplanted patients
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hibberd, A. D. et al. Cancer risk associated with ATG/OKT3 in renal transplantation. Transplant. Proc. 31, 1271–1272 (1999).
Danielian, S., Fagard, R., Alcover, A., Acuto, O. & Fischer, S. The tyrosine kinase activity of p56lck is increased in human T cells activated via CD2. Eur. J. Immunol. 21, 1967–1970 (1991).
June, C. H., Fletcher, M. C., Ledbetter, J. A. & Samelson, L. E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J. Immunol. 144, 1591–1599 (1990).
Déas, O. et al. Caspase-independent cell death induced by anti-CD2 or staurosporine in activated human peripheral T lymphocytes. J. Immunol. 161, 3375–3383 (1998).
Dumont, C. et al. Targeting additional costimulatory pathways: a subtle role for CD2. Transplant. Proc. 33, 199–200 (2001).
Mollereau, B., Deas, O., Dumont, C., Charpentier, B. & Senik, A. Effects of anti-CD2 monoclonal antibody: CD2- and CD95-mediated apoptosis of human peripheral T cells. Transplant. Proc. 31, 1245 (1999).
Mollereau, B. et al. CD2-induced apoptosis in activated human peripheral T cells: a Fas-independent pathway that requires early protein tyrosine phosphorylation. J. Immunol. 156, 3184–3190 (1996).
Rouleau, M. et al. Mitogenic CD2 monoclonal antibody pairs predispose peripheral T cells to undergo apoptosis on interaction with a third CD2 monoclonal antibody. J. Immunol. 152, 4861–4872 (1994).
Snanoudj, R. et al. A role for CD2 antibodies (BTI-322 and its humanized form) in the in vivo elimination of human T lymphocytes infiltrating an allogeneic human skin graft in SCID mice: an Fcgamma receptor-related mechanism involving co-injected human NK cells. Transplantation 78, 50–58 (2004).
Kaplon, R. J. et al. Short course single agent therapy with an LFA-3-IgG1 fusion protein prolongs primate cardiac allograft survival. Transplantation 61, 356–363 (1996).
Majeau, G. R., Meier, W., Jimmo, B., Kioussis, D. & Hochman, P. S. Mechanism of lymphocyte function-associated molecule 3-Ig fusion proteins inhibition of T cell responses. Structure/function analysis in vitro and in human CD2 transgenic mice. J. Immunol. 152, 2753–2767 (1994).
Miller, G. T. et al. Specific interaction of lymphocyte function-associated antigen 3 with CD2 can inhibit T cell responses. J. Exp. Med. 178, 211–222 (1993).
Krueger, G. G. et al. A randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. J. Am. Acad. Dermatol. 47, 821–833 (2002).
Lebwohl, M. et al. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch. Dermatol. 139, 719–727 (2003).
Weaver, T. A. et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat. Med. 15, 746–749 (2009).
Shapira, M. Y. et al. Alefacept treatment for refractory chronic extensive GVHD. Bone Marrow Transplant. 43, 339–343 (2009).
Stotler, C. J., Eghtesad, B., Hsi, E. & Silver, B. Rapid resolution of GVHD after orthotopic liver transplantation in a patient treated with alefacept. Blood 113, 5365–5366 (2009).
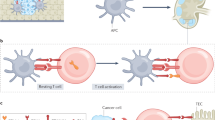
Bromley, S. K. et al. The immunological synapse. Annu. Rev. Immunol. 19, 375–396 (2001).
Greenwald, R. J., Freeman, G. J. & Sharpe, A. H. The B7 family revisited. Annu. Rev. Immunol. 23, 515–548 (2005).
Vincenti, F. et al. Costimulation blockade with belatacept in renal transplantation. N. Engl. J. Med. 353, 770–781 (2005).
Linsley, P. S. et al. Human B7–1 (CD80) and B7–2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1, 793–801 (1994).
Finger, E. B. & Bluestone, J. A. When ligand becomes receptor--tolerance via B7 signaling on DCs. Nat. Immunol. 3, 1056–1057 (2002).
Grohmann, U. et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol. 3, 1097–1101 (2002).
Chavez, H. et al. Absence of CD4CD25 regulatory T cell expansion in renal transplanted patients treated in vivo with belatacept mediated CD28-CD80/86 blockade. Transpl. Immunol. 17, 243–248 (2007).
Akalin, E. et al. CD28-B7 T cell costimulatory blockade by CTLA4Ig in the rat renal allograft model: inhibition of cell-mediated and humoral immune responses in vivo. Transplantation 62, 1942–1945 (1996).
Larsen, C. P. et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 381, 434–438 (1996).
Lenschow, D. J. et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science 257, 789–792 (1992).
Pearson, T. C. et al. Transplantation tolerance induced by CTLA4-Ig. Transplantation 57, 1701–1706 (1994).
Turka, L. A. et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc. Natl Acad. Sci. USA 89, 11102–11105 (1992).
Kirk, A. D. et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc. Natl Acad. Sci. USA 94, 8789–8794 (1997).
Kirk, A. D. et al. Induction therapy with monoclonal antibodies specific for CD80 and CD86 delays the onset of acute renal allograft rejection in non-human primates. Transplantation 72, 377–384 (2001).
Durrbach, A. et al. A Phase III Study of Belatacept vs Cyclosporine in Kidney Transplants from Extended Criteria Donors (BENEFIT-EXT Study). Am. J. Transplant. (in press).
Vincenti, F. et al. A Phase III Study of Belatacept-based Immunosuppression Regimens vs Cyclosporine in Renal Transplant Recipients (BENEFIT Study). Am. J. Transplant. (in press).
Evenou, J. P. et al. The potent protein kinase C selective inhibitor AEB071 (sotrastaurin) represents a new class of immunosuppressive agents affecting early T-cell activation. J. Pharmacol. Exp. Ther. 330, 792–801 (2009).
Takai, Y., Kishimoto, A., Inoue, M. & Nishizuka, Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J. Biol. Chem. 252, 7603–7609 (1977).
Baier, G. The PKC gene module: molecular biosystematics to resolve its T cell functions. Immunol. Rev. 192, 64–79 (2003).
Spitaler, M. & Cantrell, D. A. Protein kinase C and beyond. Nat. Immunol. 5, 785–790 (2004).
Tan, S. L. & Parker, P. J. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem. J. 376, 545–552 (2003).
Long, A., Kelleher, D., Lynch, S. & Volkov, Y. Cutting edge: protein kinase C beta expression is critical for export of Il-2 from T cells. J. Immunol. 167, 636–640 (2001).
Pfeifhofer, C. et al. Defective IgG2a/2b class switching in PKC alpha-/- mice. J. Immunol. 176, 6004–6011 (2006).
Pfeifhofer, C. et al. Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J. Exp. Med. 197, 1525–1535 (2003).
Sun, Z. et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 404, 402–407 (2000).
Volkov, Y., Long, A., McGrath, S., Ni Eidhin, D. & Kelleher, D. Crucial importance of PKC-beta(I) in LFA-1-mediated locomotion of activated T cells. Nat. Immunol. 2, 508–514 (2001).
Marsland, B. J. & Kopf, M. T-cell fate and function: PKC-theta and beyond. Trends Immunol. 29, 179–185 (2008).
Monks, C. R., Kupfer, H., Tamir, I., Barlow, A. & Kupfer, A. Selective modulation of protein kinase C-theta during T-cell activation. Nature 385, 83–86 (1997).
Anderson, K. et al. Mice deficient in PKC theta demonstrate impaired in vivo T cell activation and protection from T cell-mediated inflammatory diseases. Autoimmunity 39, 469–478 (2006).
Kovarik, J. M., Huang, H. L., Slade, A., Sfikas, N. & Chandler, P. A. The effect on sotrastaurin pharmacokinetics of strong CYP3A inhibition by ketoconazole. Br. J. Clin. Pharmacol. 68, 381–385 (2009).
Vincenti, F. & Kirk, A. D. What's next in the pipeline. Am. J. Transplant. 8, 1972–1981 (2008).
Budde, K. et al. AEB071, a novel protein kinase C-inhibitor: first clinical results of an AEB071 (sotrastaurin) plus tacrolimus regimen in renal transplant recipients [abstract]. AST 391 (2009).
Friman, S. et al. AEB071 (sotrastaurin) a novel protein kinase C-inhibitor: evaluation of an AEB071 plus mycophenolate regiman in renal transplant recipients [abstract]. AST 458 (2009).
Abraham, R. T. Mammalian target of rapamycin: immunosuppressive drugs uncover a novel pathway of cytokine receptor signaling. Curr. Opin. Immunol. 10, 330–336 (1998).
Denton, M. D., Magee, C. C. & Sayegh, M. H. Immunosuppressive strategies in transplantation. Lancet 353, 1083–1091 (1999).
Schorle, H., Holtschke, T., Hünig, T., Schimpl, A. & Horak, I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature 352, 621–624 (1991).
Steiger, J., Nickerson, P. W., Steurer, W., Moscovitch-Lopatin, M. & Strom, T. B. IL-2 knockout recipient mice reject islet cell allografts. J. Immunol. 155, 489–498 (1995).
Thèze, J., Alzari, P. M. & Bertoglio, J. Interleukin 2 and its receptors: recent advances and new immunological functions. Immunol. Today 17, 481–486 (1996).
Giri, J. G. et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 13, 2822–2830 (1994).
Willerford, D. M. et al. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3, 521–530 (1995).
Liu, K. D., Gaffen, S. L. & Goldsmith, M. A. JAK/STAT signaling by cytokine receptors. Curr. Opin. Immunol. 10, 271–278 (1998).
Nelson, B. H. & Willerford, D. M. Biology of the interleukin-2 receptor. Adv. Immunol. 70, 1–81 (1998).
Thomis, D. C. & Berg, L. J. The role of Jak3 in lymphoid development, activation, and signaling. Curr. Opin. Immunol. 9, 541–547 (1997).
Hofmann, S. R. et al. Cytokines and their role in lymphoid development, differentiation and homeostasis. Curr. Opin. Allergy Clin. Immunol. 2, 495–506 (2002).
Leonard, W. J. & O'Shea, J. J. Jaks and STATs: biological implications. Annu. Rev. Immunol. 16, 293–322 (1998).
O'Shea, J. J., Pesu, M., Borie, D. C. & Changelian, P. S. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat. Rev. Drug Discov. 3, 555–564 (2004).
Nosaka, T. et al. Defective lymphoid development in mice lacking Jak3. Science 270, 800–802 (1995).
Park, S. Y. et al. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity 3, 771–782 (1995).
Thomis, D. C., Gurniak, C. B., Tivol, E., Sharpe, A. H. & Berg, L. J. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science 270, 794–797 (1995).
Macchi, P. et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 377, 65–68 (1995).
Russell, S. M. et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270, 797–800 (1995).
Gazit, A. et al. Tyrphostins. 2. Heterocyclic and alpha-substituted benzylidenemalononitrile tyrphostins as potent inhibitors of EGF receptor and ErbB2/neu tyrosine kinases. J. Med. Chem. 34, 1896–1907 (1991).
Kirken, R. A. et al. Tyrphostin AG-490 inhibits cytokine-mediated JAK3/STAT5a/b signal transduction and cellular proliferation of antigen-activated human T cells. J. Leukoc. Biol. 65, 891–899 (1999).
Meydan, N. et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature 379, 645–648 (1996).
Säemann, M. D. et al. Suppression of primary T-cell responses and induction of alloantigen-specific hyporesponsiveness in vitro by the Janus kinase inhibitor tyrphostin AG490. Transplantation 70, 1215–1225 (2000).
Wang, L., Chen, J. J., Gelman, B. B., Konig, R. & Cloyd, M. W. A novel mechanism of CD4 lymphocyte depletion involves effects of HIV on resting lymphocytes: induction of lymph node homing and apoptosis upon secondary signaling through homing receptors. J. Immunol. 162, 268–276 (1999).
Behbod, F. et al. Concomitant inhibition of Janus kinase 3 and calcineurin-dependent signaling pathways synergistically prolongs the survival of rat heart allografts. J. Immunol. 166, 3724–3732 (2001).
Paniagua, R. et al. Effects of JAK3 inhibition with CP-690,550 on immune cell populations and their functions in nonhuman primate recipients of kidney allografts. Transplantation 80, 1283–1292 (2005).
Conklyn, M., Andresen, C., Changelian, P. & Kudlacz, E. The JAK3 inhibitor CP-690550 selectively reduces NK and CD8+ cell numbers in cynomolgus monkey blood following chronic oral dosing. J. Leukoc. Biol. 76, 1248–1255 (2004).
Borie, D. C. et al. Immunosuppression by the JAK3 inhibitor CP-690,550 delays rejection and significantly prolongs kidney allograft survival in nonhuman primates. Transplantation 79, 791–801 (2005).
Borie, D. C. et al. Combined use of the JAK3 inhibitor CP-690,550 with mycophenolate mofetil to prevent kidney allograft rejection in nonhuman primates. Transplantation 80, 1756–1764 (2005).
Rousvoal, G. et al. Janus kinase 3 inhibition with CP-690,550 prevents allograft vasculopathy. Transpl. Int. 19, 1014–1021 (2006).
Velotta, J. B. et al. A novel JAK3 inhibitor, R348, attenuates chronic airway allograft rejection. Transplantation 87, 653–659 (2009).
Crespo, M. et al. Acute humoral rejection in renal allograft recipients: I. Incidence, serology and clinical characteristics. Transplantation 71, 652–658 (2001).
Rocha, P. N. et al. Beneficial effect of plasmapheresis and intravenous immunoglobulin on renal allograft survival of patients with acute humoral rejection. Transplantation 75, 1490–1495 (2003).
Shah, A. et al. Treatment of C4d-positive acute humoral rejection with plasmapheresis and rabbit polyclonal antithymocyte globulin. Transplantation 77, 1399–1405 (2004).
Gloor, J. M. et al. Overcoming a positive crossmatch in living-donor kidney transplantation. Am. J. Transplant. 3, 1017–1023 (2003).
Jordan, S. C. et al. Intravenous immune globulin treatment inhibits crossmatch positivity and allows for successful transplantation of incompatible organs in living-donor and cadaver recipients. Transplantation 76, 631–636 (2003).
Montgomery, R. A. et al. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation 70, 887–895 (2000).
Park, W. D. et al. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am. J. Transplant. 3, 952–960 (2003).
Tanabe, K. et al. Long-term results of ABO-incompatible living kidney transplantation: a single-center experience. Transplantation 65, 224–228 (1998).
Racusen, L. C. & Haas, M. Antibody-mediated rejection in renal allografts: lessons from pathology. Clin. J. Am. Soc. Nephrol. 1, 415–420 (2006).
Terasaki, P. I. Humoral theory of transplantation. Am. J. Transplant. 3, 665–673 (2003).
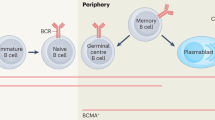
McHeyzer-Williams, M. G. & Ahmed, R. B cell memory and the long-lived plasma cell. Curr. Opin. Immunol. 11, 172–179 (1999).
Slifka, M. K., Antia, R., Whitmire, J. K. & Ahmed, R. Humoral immunity due to long-lived plasma cells. Immunity 8, 363–372 (1998).
Perry, D. K. et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am. J. Transplant. 9, 201–209 (2009).
Caamaño, J. H. et al. Nuclear factor (NF)-kappa B2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J. Exp. Med. 187, 185–196 (1998).
Franzoso, G. et al. Mice deficient in nuclear factor (NF)-kappa B/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J. Exp. Med. 187, 147–159 (1998).
Ranger, A. M. et al. Delayed lymphoid repopulation with defects in IL-4-driven responses produced by inactivation of NF-ATc. Immunity 8, 125–134 (1998).
Barnes, P. J. & Karin, M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336, 1066–1071 (1997).
Baldwin, A. S. Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14, 649–683 (1996).
Thanos, D. & Maniatis, T. NF-kappa B: a lesson in family values. Cell 80, 529–532 (1995).
Sunwoo, J. B. et al. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin. Cancer Res. 7, 1419–1428 (2001).
Ghosh, S., May, M. J. & Kopp, E. B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16, 225–260 (1998).
McCarthy, P. L. Jr et al. Inhibition of interleukin-1 by an interleukin-1 receptor antagonist prevents graft-versus-host disease. Blood 78, 1915–1918 (1991).
Xun, C. Q., Thompson, J. S., Jennings, C. D., Brown, S. A. & Widmer, M. B. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood 83, 2360–2367 (1994).
Wang, X., Luo, H., Chen, H., Duguid, W. & Wu, J. Role of proteasomes in T cell activation and proliferation. J. Immunol. 160, 788–801 (1998).
Finn, P. W., Stone, J. R., Boothby, M. R. & Perkins, D. L. Inhibition of NF-kappaB-dependent T cell activation abrogates acute allograft rejection. J. Immunol. 167, 5994–6001 (2001).
Richardson, P. G. et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N. Engl. J. Med. 348, 2609–2617 (2003).
Everly, M. J. et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation 86, 1754–1761 (2008).
Trivedi, H. L. et al. Abrogation of anti-HLA antibodies via proteasome inhibition. Transplantation 87, 1555–1561 (2009).
Colvin, R. B. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J. Am. Soc. Nephrol. 18, 1046–1056 (2007).
Regele, H. et al. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: a contribution of humoral immunity to chronic allograft rejection. J. Am. Soc. Nephrol. 13, 2371–2380 (2002).
Williams, J. M. et al. Acute vascular rejection and accommodation: divergent outcomes of the humoral response to organ transplantation. Transplantation 78, 1471–1478 (2004).
Brodsky, R. A. How I treat paroxysmal nocturnal hemoglobinuria. Blood 113, 6522–6527 (2009).
Rother, R. P., Rollins, S. A., Mojcik, C. F., Brodsky, R. A. & Bell, L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat. Biotechnol. 25, 1256–1264 (2007).
Mache, C. J. et al. Complement inhibitor eculizumab in atypical hemolytic uremic syndrome. Clin. J. Am. Soc. Nephrol. 4, 1312–1316 (2009).
Locke, J. E. et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am. J. Transplant. 9, 231–235 (2009).
Acknowledgements
The authors thank Professor Lionel Rostaing (Head of the Department of Transplantation, Rangueil Teaching Hospital, Toulouse, France) for his constructive discussion and advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Durrbach, A., Francois, H., Beaudreuil, S. et al. Advances in immunosuppression for renal transplantation. Nat Rev Nephrol 6, 160–167 (2010). https://doi.org/10.1038/nrneph.2009.233
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2009.233
This article is cited by
-
Off-label use of the expensive orphan drug eculizumab in France 2009–2013 and the impact of literature: focus on the transplantation field
European Journal of Clinical Pharmacology (2016)
-
Production and characterization of LEA29Y, a variant of cytotoxic T-lymphocyte antigen 4-immunoglobulin, in Pichia pastoris
Applied Microbiology and Biotechnology (2011)