Abstract
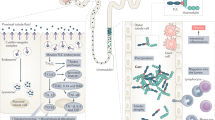
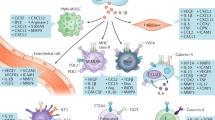
Since their introduction into clinical practice a decade ago, immune checkpoint inhibitors (ICIs) have had an overwhelming impact on cancer treatment. Use of these agents in oncology continues to grow; however, the increased use of these agents has been associated with a parallel increase in ICI-associated immune-related adverse events, which can affect virtually any organ, including the kidneys. ICI-associated acute kidney injury (ICI-AKI) occurs in 2–5% of patients treated with ICIs. Its occurrence can have important consequences, including the temporary or permanent discontinuation of ICIs or other concomitant anticancer therapies and the need for prolonged treatment with corticosteroids. Various mechanisms have been proposed to underlie the development of ICI-AKI, including loss of tolerance to self-antigens, reactivation of drug-specific effector T cells, and the production of kidney-specific autoantibodies. ICI-AKI most commonly manifests as acute tubulo-interstitial nephritis on kidney biopsy and generally shows a favourable response to early initiation of corticosteroids, with complete or partial remission achieved in most patients. The evaluation of patients with suspected ICI-AKI requires careful diagnostic work-up and kidney biopsy for patients with moderate-to-severe ICI-AKI to ensure accurate diagnosis and inform appropriate treatment.
Key points
-
Immune checkpoint inhibitors are frequently associated with the development of immune-related adverse events (irAEs).
-
Renal irAEs are rare, but potentially severe.
-
Acute tubulointerstitial nephritis is the most common irAE that affects the kidney, although other renal lesions have been described.
-
A kidney biopsy is central to making the correct diagnosis and guiding treatment in immune checkpoint inhibitor-associated acute kidney injury.
-
In general, the response of immune checkpoint inhibitor-associated acute kidney injury to corticosteroids is good and the prognosis is favourable.
-
The use of immune checkpoint inhibitors in kidney transplant recipients is associated with a significantly increased risk of allograft rejection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Matsushita, H. et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482, 400–404 (2012).
Robbins, P. F. et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 19, 747–752 (2013).
van Rooij, N. et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 31, e439–e442 (2013).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Kwon, E. D. et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc. Natl Acad. Sci. USA 96, 15074–15079 (1999).
Ahmadzadeh, M. et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544 (2009).
Xu, C. et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ 363, k4226 (2018).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Champiat, S. et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann. Oncol. 27, 559–574 (2016).
Khoja, L., Day, D., Wei-Wu, C. T., Siu, L. L. & Hansen, A. R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann. Oncol. 28, 2377–2385 (2017).
Wolchok, J. D. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377, 1345–1356 (2017).
Kanjanapan, Y. et al. Delayed immune-related adverse events in assessment for dose-limiting toxicity in early phase immunotherapy trials. Eur. J. Cancer 107, 1–7 (2019).
Salahudeen, A. K. et al. Incidence rate, clinical correlates, and outcomes of AKI in patients admitted to a comprehensive cancer center. Clin. J. Am. Soc. Nephrol. 8, 347–354 (2013).
Manohar, S. et al. Acute interstitial nephritis and checkpoint inhibitor therapy. Kidney360 1, 16–24 (2020).
Seethapathy, H. et al. Incidence and clinical features of immune-related acute kidney injury in patients receiving programmed cell death ligand-1 inhibitors. Kidney Int. Rep. 5, 1700–1705 (2020).
Sorah, J. D., Rose, T. L., Radhakrishna, R., Derebail, V. K. & Milowsky, M. I. Incidence and prediction of immune checkpoint inhibitor-related nephrotoxicity. J. Immunother. 44, 127–131 (2021).
Seethapathy, H. et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin. J. Am. Soc. Nephrol. 14, 1692–1700 (2019).
Meraz-Munoz, A. et al. Acute kidney injury associated with immune checkpoint inhibitor therapy: incidence, risk factors and outcomes. J. Immunother. Cancer 8, e000467 (2020).
Stein, C. et al. Acute kidney injury in patients treated with anti-programmed death receptor-1 for advanced melanoma: a real-life study in a single-centre cohort. Nephrol. Dial. Transplant. 36, 1664–1674 (2021).
García-Carro, C. et al. Acute kidney injury as a risk factor for mortality in oncological patients receiving checkpoint inhibitors. Nephrol. Dial. Transplant. 37, 887–894 (2022).
Manohar, S. et al. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol. Dial. Transpl. 34, 108–117 (2019).
Cortazar, F. B. et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90, 638–647 (2016).
Sznol, M. et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J. Clin. Oncol. 35, 3815–3822 (2017).
Wanchoo, R. et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am. J. Nephrol. 45, 160–169 (2017).
Gupta, S. et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J. Immunother. Cancer https://doi.org/10.1136/jitc-2021-003467 (2021).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Wing, K. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 (2008).
Sharma, A. et al. Anti-CTLA-4 immunotherapy does not deplete FOXP3+ regulatory T cells (Tregs) in human cancers. Clin. Cancer Res 25, 1233–1238 (2019).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Larkin, J., Hodi, F. S. & Wolchok, J. D. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 1270–1271 (2015).
Menke, J. et al. Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: distinct roles. J. Immunol. 179, 7466–7477 (2007).
Nishimura, H., Nose, M., Hiai, H., Minato, N. & Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151 (1999).
Nishimura, H. et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319–322 (2001).
Okazaki, T. et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 9, 1477–1483 (2003).
Tivol, E. A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 (1995).
Waterhouse, P. et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988 (1995).
Barreto, M. et al. Evidence for CTLA4 as a susceptibility gene for systemic lupus erythematosus. Eur. J. Hum. Genet. 12, 620–626 (2004).
Vaidya, B. et al. An association between the CTLA4 exon 1 polymorphism and early rheumatoid arthritis with autoimmune endocrinopathies. Rheumatology 41, 180–183 (2002).
Ueda, H. et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423, 506–511 (2003).
Prokunina, L. et al. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat. Genet. 32, 666–669 (2002).
Huang, C.-H. et al. Effects of genetic polymorphisms of programmed cell death 1 and its ligands on the development of ankylosing spondylitis. Rheumatology 50, 1809–1813 (2011).
Boutros, C. et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 13, 473–486 (2016).
Pillai, R. N. et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: a systematic analysis of the literature. Cancer 124, 271–277 (2018).
Xu, H. et al. Antitumor activity and treatment-related toxicity associated with nivolumab plus ipilimumab in advanced malignancies: a systematic review and meta-analysis. Front. Pharmacol. 10, 1300 (2019).
Lozano, A. X. et al. T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat. Med. 28, 353–362 (2022).
Subudhi, S. K. et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc. Natl Acad. Sci. USA 113, 11919–11924 (2016).
Johnson, D. B. et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 375, 1749–1755 (2016).
Ding, H., Wu, X. & Gao, W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin. Immunol. 115, 184–191 (2005).
Schoop, R. et al. Suppressed T-cell activation by IFN-γ-induced expression of PD-L1 on renal tubular epithelial cells. Nephrol. Dial. Transplant. 19, 2713–2720 (2004).
Starke, A. et al. Renal tubular PD-L1 (CD274) suppresses alloreactive human T-cell responses. Kidney Int 78, 38–47 (2010).
Jaworska, K. et al. Both PD-1 ligands protect the kidney from ischemia reperfusion injury. J. Immunol. 194, 325–333 (2015).
Hakroush, S. et al. Variable expression of programmed cell death protein 1-ligand 1 in kidneys independent of immune checkpoint inhibition. Front. Immunol. 11, 624547 (2020).
Cortazar, F. B. et al. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. J. Am. Soc. Nephrol. 31, 435–446 (2020).
Koda, R. et al. Immune checkpoint inhibitor (nivolumab)-associated kidney injury and the importance of recognizing concomitant medications known to cause acute tubulointerstitial nephritis: a case report. BMC Nephrol. 19, 48 (2018).
Dimitriou, F. et al. Cytokine release syndrome during sequential treatment with immune checkpoint inhibitors and kinase inhibitors for metastatic melanoma. J. Immunother. 42, 29–32 (2019).
Bridge, J. A., Lee, J. C., Daud, A., Wells, J. W. & Bluestone, J. A. Cytokines, chemokines, and other biomarkers of response for checkpoint inhibitor therapy in skin cancer. Front. Med. 5, 351 (2018).
Wang, H. et al. Interleukin-10 is a promising marker for immune-related adverse events in patients with non-small cell lung cancer receiving immunotherapy. Front. Immunol. 13, 840313 (2022).
Hailemichael, Y. et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 40, 509–523.e506 (2022).
Fadel, F., El Karoui, K. & Knebelmann, B. Anti-CTLA4 antibody-induced lupus nephritis. N. Engl. J. Med. 361, 211–212 (2009).
Leaf, D. E. Excessive diagnostic testing in acute kidney injury. BMC Nephrol. 17, 9 (2016).
Muriithi, A. K., Nasr, S. H. & Leung, N. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin. J. Am. Soc. Nephrol. 8, 1857–1862 (2013).
Kitchlu, A. et al. A systematic review of checkpoint inhibitor-associated glomerular disease. KI Rep. 6, 66–77 (2020).
Schneider, B. J. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J. Clin. Oncol. 39, 4073–4126 (2021).
Thompson, J. A. et al. Management of immunotherapy-related toxicities, version 1.2019. J. Natl Compr. Canc. Netw. 17, 255–289 (2019).
Brahmer, J. R. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 9, e002435 (2021).
Thompson, J. et al. NCCN Guidelines Version 1.2022: Management of Immunotherapy-Related Toxicities. https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1486 (2022).
Haanen, J. et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 28, iv119–iv142 (2017).
Oleas, D. et al. Acute interstitial nephritis associated with immune checkpoint inhibitors: a single-centre experience. Clin. Kidney J. 14, 1364–1370 (2021).
Andrulli, S. et al. The risks associated with percutaneous native kidney biopsies: a prospective study. Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/gfac177 (2022).
Qualls, D. et al. Positron emission tomography as an adjuvant diagnostic test in the evaluation of checkpoint inhibitor-associated acute interstitial nephritis. J. Immunother. Cancer 7, 356 (2019).
Lee, M. D. et al. Rapid corticosteroid taper versus standard of care for immune checkpoint inhibitor induced nephritis: a single-center retrospective cohort study. J. Immunother. Cancer 9, e002292 (2021).
Waljee, A. K. et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 357, j1415 (2017).
Zhang, H. et al. Impact of corticosteroid use on outcomes of non-small-cell lung cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. J. Clin. Pharm. Ther. 46, 927–935 (2021).
Baker, M. L. et al. Mortality after acute kidney injury and acute interstitial nephritis in patients prescribed immune checkpoint inhibitor therapy. J. Immunother. Cancer https://doi.org/10.1136/jitc-2021-004421 (2022).
Lin, J. S. et al. Infliximab for the treatment of patients with checkpoint inhibitor-associated acute tubular interstitial nephritis. Oncoimmunology 10, 1877415 (2021).
Dimitriou, F., Hogan, S., Menzies, A. M., Dummer, R. & Long, G. V. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur. J. Cancer 157, 214–224 (2021).
Allouchery, M. et al. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J. Immunother. Cancer 8, e001622 (2020).
Perazella, M. A. & Sprangers, B. AKI in patients receiving immune checkpoint inhibitors. Clin. J. Am. Soc. Nephrol. 14, 1077–1079 (2019).
Mamlouk, O. et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J. Immunother. Cancer 7, 2 (2019).
Abdelahim, M. et al. Incidence, predictors, and survival impact of acute kidney injury in patients with melanoma treated with immune checkpoint inhibitors: a 10-year single-institution analysis. Oncoimmunology 10, 1927313 (2021).
Perazella, M. A. & Shirali, A. C. Nephrotoxicity of cancer immunotherapies: past, present and future. J. Am. Soc. Nephrol. 29, 2039–2052 (2018).
Murakami, N. et al. A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int. 100, 196–205 (2021).
Carroll, R. P. et al. Immune checkpoint inhibitors in kidney transplant recipients: a multicentre, single-arm, phase 1 study. Lancet Oncol. https://doi.org/10.1016/s1470-2045(22)00368-0 (2022).
Kitchlu, A., Jhaveri, K. D., Sprangers, B., Yanagita, M. & Wanchoo, R. Immune checkpoint inhibitor use in patients with end-stage kidney disease: an analysis of reported cases and literature review. Clin. Kidney J. 14, 2012–2022 (2021).
Chute, D. F. et al. Incidence and predictors of CKD and estimated GFR decline in patients receiving immune checkpoint inhibitors. Am. J. Kidney Dis. 79, 134–137 (2022).
Isik, B. et al. Biomarkers, clinical features, and rechallenge for immune checkpoint inhibitor renal immune-related adverse events. Kidney Int. Rep. 6, 1022–1031 (2021).
Moledina, D. G. et al. Urine TNF-α and IL-9 for clinical diagnosis of acute interstitial nephritis. JCI Insight 4, e127456 (2019).
Heybeli, C., Nathan, M. A. & Herrmann, S. Renal injury in the setting of immune checkpoint inhibitor: report of a case of hypothyroidism and the role of positron emission tomography. J. Onconephrol. 4, 112–116 (2020).
Herrmann, S. M., Alexander, M. P., Romero, M. F. & Zand, L. Renal tubular acidosis and immune checkpoint inhibitor therapy: an immune-related adverse event of PD-1 inhibitor-a report of 3 cases. Kidney Med. 2, 657–662 (2020).
Okawa, S. et al. Rapidly progressive acute kidney injury associated with nivolumab treatment. Case Rep. Oncol. 13, 85–90 (2020).
Acknowledgements
B.S. is a senior clinical investigator of The Research Foundation Flanders (F.W.O. 1842919 N) and receives funding from the Foundation against Cancer (Stichting tegen Kanker; C/2020/1380). D.E.L. is funded by National Institutes of Health grants R01HL144566, R01DK125786 and R01DK126685. M.J.S. is funded by Fondo de Investigación Sanitaria-FEDER, ISCIII, PI17/00257, REDINREN, RD16/0009/0030 and EIN2020-112338. We would like to thank Albert Herelixka, University Hospitals Leuven, Belgium, for drawing the figures included in the original submission of this manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Immune checkpoints
-
Regulators of the immune system that can be activating (promoting immune cell activation) or negative (inhibiting immune cell activation).
- T cell exhaustion
-
A state of T cell dysfunction arising during chronic infections and cancer, characterized by poor T cell effector function, continued expression of inhibitor receptors and a specific transcriptional signature.
- Tumour-draining lymph nodes
-
Lymph nodes located immediately downstream of tumours and where the anti-tumour immune response will be activated.
- Thymic negative selection
-
Process of intra-thymic deletion of self-reactive thymocytes (developing T cells).
- Haptens
-
Small molecules that elicit an immune response only when bound to a large carrier such as a protein.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sprangers, B., Leaf, D.E., Porta, C. et al. Diagnosis and management of immune checkpoint inhibitor-associated acute kidney injury. Nat Rev Nephrol 18, 794–805 (2022). https://doi.org/10.1038/s41581-022-00630-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00630-8
This article is cited by
-
Akute und chronische Nierenerkrankungen in der Differenzialdiagnose einer akuten Nierenfunktionsstörung
Die Nephrologie (2024)
-
Effect of cardiac function in patients with gastrointestinal cancer with or without acute kidney injury assessed using a non-invasive impedance cardiography: a case-control study
BMC Cardiovascular Disorders (2023)
-
Immunotherapy in hematologic malignancies: achievements, challenges and future prospects
Signal Transduction and Targeted Therapy (2023)