Key Points
-
Childhood maltreatment (specifically, physical, sexual and emotional abuse, and physical and emotional neglect) exerts a prepotent influence on trajectories of child brain development and constitutes a major risk factor for adult psychopathology.
-
Brain alterations resulting from maltreatment are highly specific, depend on the type and timing of exposure, and probably were once phenotypic adaptations that enhanced species survival and reproductive success but are now associated with substantial medical and psychiatric disadvantages.
-
Maltreatment reduces the volume of the hippocampus (particularly in adults), as well as the volume of anterior cingulate and ventromedial and dorsomedial cortices; affects the development of key fibre tracts (including the corpus callosum, superior longitudinal fasciculus, uncinate fasciculus and cingulum bundle); and appears to alter the development of sensory systems that process and convey stressful experiences.
-
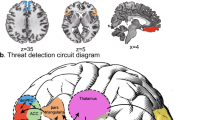
This Review reveals consistent reports of augmented amygdala response to threatening stimuli, diminished ventral striatal response to anticipation or receipt of reward, diminished connectivity between prefrontal regions and the amygdala, and increased volume and network centrality of the precuneus in maltreated individuals.
-
Maltreated and non-maltreated individuals with the same primary psychiatric diagnoses differ clinically, neurobiologically and genetically, such that maltreated individuals seem to represent distinct ecophenotypes of established psychiatric disorders. Thus, maltreatment may be an unrecognized confound in psychiatric neuroimaging studies.
-
Maltreatment-associated brain changes are frequently reported in resilient individuals who show no past or current symptoms of psychopathology. Other neurobiological or molecular alterations are probably present that enable these individuals to effectively compensate for stress-related neurobiological alterations.
Abstract
Maltreatment-related childhood adversity is the leading preventable risk factor for mental illness and substance abuse. Although the association between maltreatment and psychopathology is compelling, there is a pressing need to understand how maltreatment increases the risk of psychiatric disorders. Emerging evidence suggests that maltreatment alters trajectories of brain development to affect sensory systems, network architecture and circuits involved in threat detection, emotional regulation and reward anticipation. This Review explores whether these alterations reflect toxic effects of early-life stress or potentially adaptive modifications, the relationship between psychopathology and brain changes, and the distinction between resilience, susceptibility and compensation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Takesian, A. E. & Hensch, T. K. Balancing plasticity/stability across brain development. Prog. Brain Res. 207, 3–34 (2013).
Teicher, M. H. & Samson, J. A. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry 170, 1114–1133 (2013). This article argues that psychiatric disorders need to be subtyped based on maltreatment history.
Dube, S. R., Felitti, V. J., Dong, M., Giles, W. H. & Anda, R. F. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev. Med. 37, 268–277 (2003).
Dube, S. R. et al. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics 111, 564–572 (2003).
Anda, R. F. et al. Adverse childhood experiences and prescribed psychotropic medications in adults. Am. J. Prev. Med. 32, 389–394 (2007).
Brown, D. W. et al. Adverse childhood experiences and the risk of premature mortality. Am. J. Prev. Med. 37, 389–396 (2009).
Price, L. H., Kao, H. T., Burgers, D. E., Carpenter, L. L. & Tyrka, A. R. Telomeres and early-life stress: an overview. Biol. Psychiatry 73, 15–23 (2013).
Ito, Y. et al. Increased prevalence of electrophysiological abnormalities in children with psychological, physical, and sexual abuse. J. Neuropsychiatry Clin. Neurosci. 5, 401–408 (1993).
Schiffer, F., Teicher, M. H. & Papanicolaou, A. C. Evoked potential evidence for right brain activity during the recall of traumatic memories. J. Neuropsychiatry Clin. Neurosci. 7, 169–175 (1995).
Teicher, M. H. Wounds that time won't heal: the neurobiology of child abuse. Cerebrum 4, 50–67 (2000).
Teicher, M. H. & Samson, J. A. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry 57, 241–266 (2016).
Tottenham, N. The importance of early experiences for neuro-affective development. Curr. Top. Behav. Neurosci. 16, 109–129 (2014).
McLaughlin, K. A., Sheridan, M. A. & Lambert, H. K. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev. 47, 578–591 (2014).
Brewin, C. R., Andrews, B. & Gotlib, I. H. Psychopathology and early experience: a reappraisal of retrospective reports. Psychol. Bull. 113, 82–98 (1993).
De Bellis, M. D. et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol. Psychiatry 52, 1066–1078 (2002).
Teicher, M. H., Tomoda, A. & Andersen, S. L. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann. NY Acad. Sci. 1071, 313–323 (2006).
National Scientific Council on the Developing Child. Excessive stress disrupts the architecture of the developing brain: working paper #3 (Center on the Developing Child Harvard Univ., 2005).
Lupien, S. J., McEwen, B. S., Gunnar, M. R. & Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445 (2009).
Teicher, M. H. Scars that won't heal: the neurobiology of child abuse. Sci. Am. 286, 68–75 (2002).
Teicher, M. H. et al. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 27, 33–44 (2003).
Gibb, B. E., Schofield, C. A. & Coles, M. E. Reported history of childhood abuse and young adults' information-processing biases for facial displays of emotion. Child Maltreat. 14, 148–156 (2009).
Pollak, S. D. Experience-dependent affective learning and risk for psychopathology in children. Ann. NY Acad. Sci. 1008, 102–111 (2003).
Rutter, M. Achievements and challenges in the biology of environmental effects. Proc. Natl Acad. Sci. USA 109, 17149–17153 (2012).
Belsky, J. & Pluess, M. Beyond risk, resilience, and dysregulation: phenotypic plasticity and human development. Dev. Psychopathol. 25, 1243–1261 (2013).
Tomoda, A. et al. Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage 54, S280–S286 (2011).
Choi, J., Jeong, B., Rohan, M. L., Polcari, A. M. & Teicher, M. H. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol. Psychiatry 65, 227–234 (2009).
Tomoda, A., Polcari, A., Anderson, C. M. & Teicher, M. H. Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PLoS ONE 7, e52528 (2012).
Choi, J., Jeong, B., Polcari, A., Rohan, M. L. & Teicher, M. H. Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage 59, 1071–1079 (2012).
Tomoda, A., Navalta, C. P., Polcari, A., Sadato, N. & Teicher, M. H. Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biol. Psychiatry 66, 642–648 (2009).
Heim, C. M., Mayberg, H. S., Mletzko, T., Nemeroff, C. B. & Pruessner, J. C. Decreased cortical representation of genital somatosensory field after childhood sexual abuse. Am. J. Psychiatry 170, 616–623 (2013). This study provides evidence for sensory-specific damage after exposure to childhood sexual abuse.
Tottenham, N. et al. Elevated amygdala response to faces following early deprivation. Dev. Sci. 14, 190–204 (2011).
McCrory, E. J. et al. Heightened neural reactivity to threat in child victims of family violence. Curr. Biol. 21, R947–R948 (2011).
Garrett, A. S. et al. Brain activation to facial expressions in youth with PTSD symptoms. Depress. Anxiety 29, 449–459 (2012).
Grant, M. M., Cannistraci, C., Hollon, S. D., Gore, J. & Shelton, R. Childhood trauma history differentiates amygdala response to sad faces within MDD. J. Psychiatr. Res. 45, 886–895 (2011).
Bogdan, R., Williamson, D. E. & Hariri, A. R. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am. J. Psychiatry 169, 515–522 (2012).
van Harmelen, A. L. et al. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc. Cogn. Affect. Neurosci. 8, 362–369 (2013).
Dannlowski, U. et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry 71, 286–293 (2012). This paper shows evidence of morphometric abnormalities and amygdala hyperreactivity in maltreated subjects without psychopathology.
LeDoux, J. Emotional networks and motor control: a fearful view. Prog. Brain Res. 107, 437–446 (1996).
LeDoux, J. Synaptic Self: How Our Brains Become Who We Are (Viking Penguin, 2002).
Herman, J. P. & Mueller, N. K. Role of the ventral subiculum in stress integration. Behav. Brain Res. 174, 215–224 (2006).
Maren, S., Phan, K. L. & Liberzon, I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 14, 417–428 (2013).
Linke, R., Braune, G. & Schwegler, H. Differential projection of the posterior paralaminar thalamic nuclei to the amygdaloid complex in the rat. Exp. Brain Res. 134, 520–532 (2000).
Shang, C. et al. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science 348, 1472–1477 (2015).
De Bellis, M. D., Keshavan, M. S., Spencer, S. & Hall, J. N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am. J. Psychiatry 157, 1175–1177 (2000).
Cohen, R. A. et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol. Psychiatry 59, 975–982 (2006).
Kelly, P. A. et al. Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: neural markers of vulnerability? Biol. Psychiatry 74, 845–852 (2013).
Jensen, S. K. et al. Effect of early adversity and childhood internalizing symptoms on brain structure in young men. JAMA Pediatr. 169, 938–946 (2015).
Chugani, H. T. et al. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage 14, 1290–1301 (2001).
Edmiston, E. E. et al. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch. Pediatr. Adolesc. Med. 165, 1069–1077 (2011).
Andersen, S. L. et al. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J. Neuropsychiatry Clin. Neurosci. 20, 292–301 (2008). This study provides the initial evidence for sensitive periods in the hippocampus, corpus callosum and PFC.
Opel, N. et al. Hippocampal atrophy in major depression: a function of childhood maltreatment rather than diagnosis? Neuropsychopharmacology 39, 2723–2731 (2014). This paper shows that hippocampal volume abnormalities are associated more directly with maltreatment than with major depression.
Teicher, M. H., Anderson, C. M. & Polcari, A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl Acad. Sci. USA 109, E563–E572 (2012).
Hanson, J. L. et al. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J. Neurosci. 30, 7466–7472 (2010).
Kumari, V. et al. Reduced thalamic volume in men with antisocial personality disorder or schizophrenia and a history of serious violence and childhood abuse. Eur. Psychiatry 28, 225–234 (2013).
Huang, H., Gundapuneedi, T. & Rao, U. White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology 37, 2693–2701 (2012).
Benedetti, F. et al. Adverse childhood experiences influence white matter microstructure in patients with bipolar disorder. Psychol. Med. 44, 3069–3082 (2014).
Eluvathingal, T. J. et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics 117, 2093–2100 (2006).
Birn, R. M., Patriat, R., Phillips, M. L., Germain, A. & Herringa, R. J. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress. Anxiety 31, 880–892 (2014).
Cisler, J. M. et al. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol. Med. 43, 507–518 (2013).
Herringa, R. J. et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl Acad. Sci. USA 110, 19119–19124 (2013).
Wang, L. et al. Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum. Brain Mapp. 35, 1154–1166 (2014). This study demonstrates functional connectivity abnormalities in depressed individuals with and without histories of maltreatment.
Morris, J. S., Ohman, A. & Dolan, R. J. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc. Natl Acad. Sci. USA 96, 1680–1685 (1999).
Dannlowski, U. et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum. Brain Mapp. 34, 2899–2909 (2013).
Mehta, M. A. et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees Study Pilot. J. Child Psychol. Psychiatry 50, 943–951 (2009).
Tottenham, N. et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 13, 46–61 (2010).
Lupien, S. J. et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc. Natl Acad. Sci. USA 108, 14324–14329 (2011).
Pechtel, P., Lyons-Ruth, K., Anderson, C. M. & Teicher, M. H. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage 97, 236–244 (2014).
Whittle, S. et al. Childhood maltreatment and psychopathology affect brain development during adolescence. J. Am. Acad. Child Adolesc. Psychiatry 52, 940–952.e1 (2013).
Kuo, J. R., Kaloupek, D. G. & Woodward, S. H. Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Arch. Gen. Psychiatry 69, 1080–1086 (2012).
Hanson, J. L. et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol. Psychiatry 77, 314–323 (2015).
Lyons-Ruth, K., Pechtel, P., Yoon, S. A., Anderson, C. M. & Teicher, M. H. Disorganized attachment in infancy predicts greater amygdala volume in adulthood. Behav. Brain Res. 308, 83–93 (2016).
Fetterman, A. K., Ode, S. & Robinson, M. D. For which side the bell tolls: the laterality of approach-avoidance associative networks. Motiv. Emot. 37, 33–38 (2013).
Kolb, B. & Gibb, R. Searching for the principles of brain plasticity and behavior. Cortex 58, 251–260 (2014).
Caldji, C., Diorio, J. & Meaney, M. J. Variations in maternal care alter GABAA receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology 28, 1950–1959 (2003).
Baker, L. M. et al. Impact of early versus late childhood early life stress on brain morphometrics. Brain Imag. Behav. 7, 196–203 (2013).
Hodel, A. S. et al. Duration of early adversity and structural brain development in post-institutionalized adolescents. Neuroimage 105, 112–119 (2015).
Riem, M. M., Alink, L. R., Out, D., Van Ijzendoorn, M. H. & Bakermans-Kranenburg, M. J. Beating the brain about abuse: empirical and meta-analytic studies of the association between maltreatment and hippocampal volume across childhood and adolescence. Dev. Psychopathol. 27, 507–520 (2015).
Masten, C. L. et al. Recognition of facial emotions among maltreated children with high rates of post-traumatic stress disorder. Child Abuse Negl. 32, 139–153 (2008).
Gorka, A. X., Hanson, J. L., Radtke, S. R. & Hariri, A. R. Reduced hippocampal and medial prefrontal gray matter mediate the association between reported childhood maltreatment and trait anxiety in adulthood and predict sensitivity to future life stress. Biol. Mood Anxiety Disord. 4, 12 (2014).
Whittle, S. et al. Hippocampal volume and sensitivity to maternal aggressive behavior: a prospective study of adolescent depressive symptoms. Dev. Psychopathol. 23, 115–129 (2011).
Morey, R. A., Haswell, C. C., Hooper, S. R. & De Bellis, M. D. Amygdala, hippocampus, and ventral medial prefrontal cortex volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacology 41, 791–801 (2016).
Takiguchi, S. et al. Ventral striatum dysfunction in children and adolescents with reactive attachment disorder: a functional MRI Study. BJPsych Open 1, 121–128 (2015).
Hanson, J. L., Hariri, A. R. & Williamson, D. E. Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol. Psychiatry 78, 598–605 (2015). This study shows an association between childhood emotional neglect, reduced ventral striatal reward activation and depression.
Mehta, M. A. et al. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J. Cogn. Neurosci. 22, 2316–2325 (2010).
Boecker, R. et al. Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PLoS ONE 9, e104185 (2014).
Hanson, J. L. et al. Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Soc. Cogn. Affect. Neurosci. 11, 405–412 (2015).
Dillon, D. G. et al. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol. Psychiatry 66, 206–213 (2009).
Haber, S. N. & Knutson, B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35, 4–26 (2010).
Thomaes, K. et al. Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. J. Clin. Psychiatry 71, 1636–1644 (2010).
Teicher, M. H., Anderson, C. M., Ohashi, K. & Polcari, A. Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biol. Psychiatry 76, 297–305 (2014). This study shows maltreatment-associated cortical network abnormalities in the cingulate, precuneus and insula.
van der Werff, S. J. et al. Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychol. Med. 43, 1825–1836 (2013).
Gerritsen, L. et al. BDNF Val66Met genotype modulates the effect of childhood adversity on subgenual anterior cingulate cortex volume in healthy subjects. Mol. Psychiatry 17, 597–603 (2012).
Balodis, I. M. & Potenza, M. N. Anticipatory reward processing in addicted populations: a focus on the monetary incentive delay task. Biol. Psychiatry 77, 434–444 (2015).
De Bellis, M. D. et al. Developmental traumatology part II: brain development. Biol. Psychiatry 45, 1271–1284 (1999). A classic study on childhood trauma, PTSD and altered brain morphology in children.
Teicher, M. H. et al. Childhood neglect is associated with reduced corpus callosum area. Biol. Psychiatry 56, 80–85 (2004).
Teicher, M. H., Samson, J. A., Sheu, Y. S., Polcari, A. & McGreenery, C. E. Hurtful words: association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. Am. J. Psychiatry 167, 1464–1471 (2010).
Bucker, J. et al. Childhood maltreatment and corpus callosum volume in recently diagnosed patients with bipolar I disorder: data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM). J. Psychiatr. Res. 48, 65–72 (2014).
Paul, R. et al. The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatr. Dis. Treat. 4, 193–201 (2008).
Luders, E., Thompson, P. M. & Toga, A. W. The development of the corpus callosum in the healthy human brain. J. Neurosci. 30, 10985–10990 (2010).
Luders, E. et al. Positive correlations between corpus callosum thickness and intelligence. Neuroimage 37, 1457–1464 (2007).
Teicher, M. H. et al. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Ann. NY Acad. Sci. 821, 160–175 (1997).
De Bellis, M. D. & Keshavan, M. S. Sex differences in brain maturation in maltreatment-related pediatric posttraumatic stress disorder. Neurosci. Biobehav. Rev. 27, 103–117 (2003).
Juraska, J. M. & Kopcik, J. R. Sex and environmental influences on the size and ultrastructure of the rat corpus callosum. Brain Res. 450, 1–8 (1988).
Galinowski, A. et al. Resilience and corpus callosum microstructure in adolescence. Psychol. Med. 45, 2285–2294 (2015).
Sheridan, M. A., Fox, N. A., Zeanah, C. H., McLaughlin, K. A. & Nelson, C. A. III. Variation in neural development as a result of exposure to institutionalization early in childhood. Proc. Natl Acad. Sci. USA 109, 12927–12932 (2012).
Rauch, S. L. et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch. Gen. Psychiatry 53, 380–387 (1996).
Schutter, D. J. & Harmon-Jones, E. The corpus callosum: a commissural road to anger and aggression. Neurosci. Biobehav. Rev. 37, 2481–2488 (2013).
van den Heuvel, M. P. & Hulshoff Pol, H. E. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 20, 519–534 (2010).
He, Y. & Evans, A. Graph theoretical modeling of brain connectivity. Curr. Opin. Neurol. 23, 341–350 (2010).
Stevens, F. L., Hurley, R. A. & Taber, K. H. Anterior cingulate cortex: unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci. 23, 121–125 (2011).
Ross, L. A. & Olson, I. R. Social cognition and the anterior temporal lobes. Neuroimage 49, 3452–3462 (2010).
Amodio, D. M. & Frith, C. D. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277 (2006).
Cavanna, A. E. & Trimble, M. R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583 (2006).
Li, B. et al. A treatment-resistant default mode subnetwork in major depression. Biol. Psychiatry 74, 48–54 (2013).
Craig, A. D. How do you feel — now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70 (2009).
Philip, N. S. et al. Early life stress is associated with greater default network deactivation during working memory in healthy controls: a preliminary report. Brain Imag. Behav. 7, 204–212 (2013).
Sripada, R. K., Swain, J. E., Evans, G. W., Welsh, R. C. & Liberzon, I. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology 39, 2244–2251 (2014).
Bluhm, R. L. et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J. Psychiatry Neurosci. 34, 187–194 (2009).
Krause-Utz, A. et al. Amygdala and anterior cingulate resting-state functional connectivity in borderline personality disorder patients with a history of interpersonal trauma. Psychol. Med. 44, 2889–2901 (2014).
Marusak, H. A., Etkin, A. & Thomason, M. E. Disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth. Neuroimage Clin. 8, 516–525 (2015).
Philip, N. S. et al. Decreased default network connectivity is associated with early life stress in medication-free healthy adults. Eur. Neuropsychopharmacol. 23, 24–32 (2013).
Graham, A. M., Pfeifer, J. H., Fisher, P. A., Carpenter, S. & Fair, D. A. Early life stress is associated with default system integrity and emotionality during infancy. J. Child Psychol. Psychiatry 56, 1212–1222 (2015).
Tursich, M. et al. Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatr. Scand. 132, 29–38 (2015).
Cole, J., Costafreda, S. G., McGuffin, P. & Fu, C. H. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J. Affect. Disord. 134, 483–487 (2011).
Vythilingam, M. et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am. J. Psychiatry 159, 2072–2080 (2002).
Chaney, A. et al. Effect of childhood maltreatment on brain structure in adult patients with major depressive disorder and healthy participants. J. Psychiatry Neurosci. 39, 50–59 (2014).
Gerritsen, L. et al. Childhood maltreatment modifies the relationship of depression with hippocampal volume. Psychol. Med. 45, 3517–3526 (2015).
Samplin, E., Ikuta, T., Malhotra, A. K., Szeszko, P. R. & Derosse, P. Sex differences in resilience to childhood maltreatment: effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. J. Psychiatr. Res. 47, 1174–1179 (2013).
Geuze, E., Vermetten, E. & Bremner, J. D. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol. Psychiatry 10, 160–184 (2005).
Malykhin, N. V., Carter, R., Hegadoren, K. M., Seres, P. & Coupland, N. J. Fronto-limbic volumetric changes in major depressive disorder. J. Affect. Disord. 136, 1104–1113 (2012).
Kumari, V. et al. Lower anterior cingulate volume in seriously violent men with antisocial personality disorder or schizophrenia and a history of childhood abuse. Aust. N. Z. J. Psychiatry 48, 153–161 (2014).
Sheffield, J. M., Williams, L. E., Woodward, N. D. & Heckers, S. Reduced gray matter volume in psychotic disorder patients with a history of childhood sexual abuse. Schizophr. Res. 143, 185–191 (2013).
Bremner, J. D. et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am. J. Psychiatry 160, 924–932 (2003).
Shin, L. M. et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am. J. Psychiatry 156, 575–584 (1999).
De Bellis, M. D. et al. Posterior structural brain volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Dev. Psychopathol. 27, 1555–1576 (2015).
van Harmelen, A. L. et al. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol. Psychiatry 68, 832–838 (2010).
Van Dam, N. T., Rando, K., Potenza, M. N., Tuit, K. & Sinha, R. Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiatry 71, 917–925 (2014).
van Harmelen, A. L. et al. Hypoactive medial prefrontal cortex functioning in adults reporting childhood emotional maltreatment. Soc. Cogn. Affect. Neurosci. 9, 2026–2033 (2014).
Ugwu, I. D., Amico, F., Carballedo, A., Fagan, A. J. & Frodl, T. Childhood adversity, depression, age and gender effects on white matter microstructure: a DTI study. Brain Struct. Funct. 220, 1997–2009 (2015).
Seckfort, D. L. et al. Early life stress on brain structure and function across the lifespan: a preliminary study. Brain Imag. Behav. 2, 49–58 (2008).
Carballedo, A. et al. Early life adversity is associated with brain changes in subjects at family risk for depression. World J. Biol. Psychiatry 13, 569–578 (2012).
Everaerd, D. et al. Sex modulates the interactive effect of the serotonin transporter gene polymorphism and childhood adversity on hippocampal volume. Neuropsychopharmacology 37, 1848–1855 (2012).
Frodl, T. et al. Effects of early-life adversity on white matter diffusivity changes in patients at risk for major depression. J. Psychiatry Neurosci. 37, 37–45 (2012).
Teicher, M. H., Ohashi, K., Lowen, S. B., Polcari, A. & Fitzmaurice, G. M. Mood dysregulation and affective instability in emerging adults with childhood maltreatment: an ecological momentary assessment study. J. Psychiatr. Res. 70, 1–8 (2015).
van der Werff, S. J. et al. Resilience to childhood maltreatment is associated with increased resting-state functional connectivity of the salience network with the lingual gyrus. Child Abuse Negl. 37, 1021–1029 (2013).
Pagliaccio, D. et al. Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology 39, 1245–1253 (2014).
Walsh, N. D. et al. General and specific effects of early-life psychosocial adversities on adolescent grey matter volume. Neuroimage Clin. 4, 308–318 (2014).
White, M. G. et al. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes Brain Behav. 11, 869–878 (2012).
Hyman, S. E. How adversity gets under the skin. Nat. Neurosci. 12, 241–243 (2009).
Perroud, N. et al. Methylation of serotonin receptor 3A in ADHD, borderline personality, and bipolar disorders: link with severity of the disorders and childhood maltreatment. Depress. Anxiety 33, 45–55 (2016).
Plotsky, P. M. & Meaney, M. J. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res. Mol. Brain Res. 18, 195–200 (1993).
Barr, C. S. et al. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcohol Clin. Exp. Res. 27, 812–817 (2003).
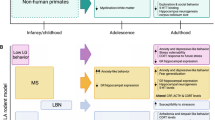
Jackowski, A. et al. Early-life stress, corpus callosum development, hippocampal volumetrics, and anxious behavior in male nonhuman primates. Psychiatry Res. 192, 37–44 (2011).
Weaver, I. C. et al. Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 (2004).
Maestripieri, D., Lindell, S. G., Ayala, A., Gold, P. W. & Higley, J. D. Neurobiological characteristics of rhesus macaque abusive mothers and their relation to social and maternal behavior. Neurosci. Biobehav. Rev. 29, 51–57 (2005).
Meaney, M. J., Brake, W. & Gratton, A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology 27, 127–138 (2002).
Suderman, M. et al. Conserved epigenetic sensitivity to early life experience in the rat and human hippocampus. Proc. Natl Acad. Sci. USA 109, 17266–17272 (2012).
Berrebi, A. S. et al. Corpus callosum: region-specific effects of sex, early experience and age. Brain Res. 438, 216–224 (1988).
Sapolsky, R. M. Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress 1, 1–19 (1996).
Andersen, S. L. & Teicher, M. H. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology 29, 1988–1993 (2004).
Andersen, S. L. & Teicher, M. H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 31, 183–191 (2008).
Bock, J., Gruss, M., Becker, S. & Braun, K. Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: correlation with developmental time windows. Cereb. Cortex 15, 802–808 (2005).
McEwen, B. S. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol. Aging 23, 921–939 (2002).
Champagne, D. L. et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 28, 6037–6045 (2008).
Sanchez, M. M., Hearn, E. F., Do, D., Rilling, J. K. & Herndon, J. G. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 812, 38–49 (1998).
Howell, B. R. et al. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: relation to amygdala volume. Dev. Psychobiol. 56, 1735–1746 (2014).
Jackowski, A. P. et al. Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Res. 162, 256–261 (2008).
Carrion, V. G. et al. Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: an MRI study. Psychiatry Res. 172, 226–234 (2009).
Moutsiana, C. et al. Insecure attachment during infancy predicts greater amygdala volumes in early adulthood. J. Child Psychol. Psychiatry 56, 540–548 (2015).
Carrion, V. G., Weems, C. F. & Reiss, A. L. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics 119, 509–516 (2007).
Thomason, M. E. et al. Altered amygdala connectivity in urban youth exposed to trauma. Soc. Cogn. Affect. Neurosci. 10, 1460–1468 (2015).
Sheu, Y. S., Polcari, A., Anderson, C. M. & Teicher, M. H. Harsh corporal punishment is associated with increased T2 relaxation time in dopamine-rich regions. Neuroimage 53, 412–419 (2010).
Whittle, S. et al. Observed measures of negative parenting predict brain development during adolescence. PLoS ONE 11, e0147774 (2016).
Acknowledgements
Studies in the authors' laboratory have been supported by RO1 awards (MH-091391, DA-017846 and HD-079484 to M.H.T.) from the US National Institutes of Health (National Institute of Mental Health (NIMH), National Institute on Drug Abuse (NIDA) and National Institute of Child Health and Human Development (NICHD)), the Harvard Clinical and Translational Science Center (UL1 TR001102) and donor support from S. Miller. The authors thank former and present staff members including S. L. Andersen, E. Bolger, J. Choi, C. E. McGreenery, A. Khan, A. Tomoda and G. Vitaliano for their myriad contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary Box and tables (PDF 6898 kb)
Glossary
- Resilient
-
Able to withstand stress and trauma so as to maintain or rapidly regain physical and mental well-being.
- Ecophenotype
-
Observable outward characteristics or traits that result from adaptation to environmental agents and that may closely mimic more-intrinsic, genetically determined phenotypes.
- Voxel-based morphometry
-
(VBM). An unbiased technique to identify brain anatomical differences in regional density of grey or white matter between groups.
- Tract-based spatial statistics
-
(TBSS). An unbiased global analysis for assessing group differences in fractional anisotropy and other diffusion measures in white-matter pathways.
- Fractional anisotropy
-
(FA). The degree to which the diffusion of molecules is directionally dependent. Its measures reflect the integrity (involving fibre density, axon diameter and myelination) of white matter.
- Sensitive-period analysis
-
A statistical procedure used to identify time periods when exposure to a particular experience most strongly influences a future outcome.
- Negative valence system
-
A functional construct domain involved in responses to acute threat, potential harm, sustained threat, frustrative non-reward and loss.
- Monetary incentive delay task
-
A task in which individuals respond to target stimuli that are presented after incentive cues to win or avoid losing indicated rewards.
- Approach–avoidance situation
-
A situation in which the same goal has elements that both attract and repel. Behavioural responses depend on the disparity between the drive to approach versus the drive to avoid.
- Bucharest Early Intervention Project
-
A randomized, longitudinal, controlled trial of high-level foster care as an intervention for children placed in one of six institutions in Bucharest, Romania, at birth.
- Probe auditory evoked potentials
-
Electroencephalogram responses to irrelevant auditory probes such as clicks. The degree to which these responses are attenuated reflects the level of brain involvement in a competing cognitive task.
- Graph theory
-
The study and use of graphs — collections of vertices (points or nodes) connected by edges (lines) — to represent, for example, brain regions and their interconnectivity.
- Centrality
-
A graph-theory measure that indicates the importance of nodes in a graph or network.
- Theory of mind
-
The ability to attribute mental states such as beliefs, intentions and desires to ourselves and others, and to recognize that the mental state of others is different from our own.
- Default-mode network
-
(DMN). A network of brain regions that are activated when the brain is resting and not engaged in cognitive or goal-directed tasks.
Rights and permissions
About this article
Cite this article
Teicher, M., Samson, J., Anderson, C. et al. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 17, 652–666 (2016). https://doi.org/10.1038/nrn.2016.111
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2016.111
This article is cited by
-
Early life stress, literacy and dyslexia: an evolutionary perspective
Brain Structure and Function (2024)
-
Cognitive and affective perspective taking amongst adolescent offenders with variants of callous–unemotional traits
European Child & Adolescent Psychiatry (2024)
-
Broadening the Scope of Resilience in Chronic Pain: Methods, Social Context, and Development
Current Rheumatology Reports (2024)
-
An Interpretive Phenomenological Analysis of Teachers’ Lived Experiences of Working with Traumatised Children in the Classroom
Journal of Child & Adolescent Trauma (2024)
-
Unmet needs of drugs for irritable bowel syndrome and inflammatory bowel diseases: interest of vagus nerve stimulation and hypnosis
Inflammopharmacology (2024)