Key Points
-
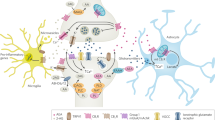
Endocannabinoids are lipid mediators derived from arachidonic acid and produced on demand to restore homeostasis. By activating cannabinoid CB1 and CB2 receptors, they produce neuromodulatory and immune modulatory actions.
-
Endocannabinoid levels and CB1 and CB2 expression vary during ageing and neuroinflammatory and neurodegenerative disorders. CB1 loss or overactivation may affect cognition negatively in advanced or young age, respectively.
-
CB2 receptors control inflammatory cytochine production in brain-invading T lymphocytes during neuroinflammatory conditions. CB1 and CB2 receptors also control the cellular features of 'inflammaging', whereas, with age, CB1 may participate in the neuroinflammation accompanying a disrupted hypothalamic–pituitary–adrenal (HPA) axis in obesity.
-
The endocannabinoid system controls the activity of molecular and subcellular master regulators of cell metabolism, such as mammalian target of rapamycin (mTOR) and mitochondria, lysosomes and autophagosomes, which are also involved in the correct functioning of the CNS during ageing and neurological conditions. These effects might also be mediated by intracellular populations of CB1 receptors.
-
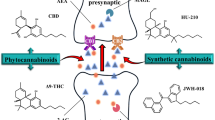
Endocannabinoids are accompanied in tissues by several metabolically related bioactive fatty acid amides and esters, which act at non-cannabinoid receptors. This renders the pharmacological manipulation of endocannabinoid levels, and hence the indirect modulation of cannabinoid receptor activity with inhibitors of endocannabinoid metabolic enzymes, more problematic.
-
Nevertheless, several preclinical studies indicate that CB1 and, particularly, CB2 agonists, as well as inhibitors of endocannabinoid inactivation, may be useful as disease-modifying agents for neurodegenerative and neuroinflammatory conditions such as multiple sclerosis. This, together with other mechanisms of action, may explain the recent clinical success of plant cannabinoids against spasticity and pain in multiple sclerosis.
Abstract
Ageing is characterized by the progressive impairment of physiological functions and increased risk of developing debilitating disorders, including chronic inflammation and neurodegenerative diseases. These disorders have common molecular mechanisms that can be targeted therapeutically. In the wake of the approval of the first cannabinoid-based drug for the symptomatic treatment of multiple sclerosis, we examine how endocannabinoid (eCB) signalling controls — and is affected by — normal ageing and neuroinflammatory and neurodegenerative disorders. We propose a conceptual framework linking eCB signalling to the control of the cellular and molecular hallmarks of these processes, and categorize the key components of endocannabinoid signalling that may serve as targets for novel therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mechoulam, R. (ed.) in Cannabis as Therapeutic Agent 1–19 (CRC Press Roca Ranton, 1986). [CE: Could not find reference to check]
Kmietowicz, Z. Cannabis based drug is licensed for spasticity in patients with MS. BMJ 340, c3363 (2010).
Pertwee, R. G. Cannabinoid pharmacology: the first 66 years. Br. J. Pharmacol. 147, S153–S171 (2006).
Bilkei-Gorzo, A. The endocannabinoid system in normal and pathological brain ageing. Phil. Trans. R. Soc. B 367, 3326–3341 (2012).
Velayudhan, L. et al. Therapeutic potential of cannabinoids in neurodegenerative disorders: a selective review. Curr. Pharm. Des. 20, 2218–2230 (2014).
Devane, W. A., Dysarz, F. A., Johnson, M. R., Melvin, L. S. & Howlett, A. C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 34, 605–613 (1988).
Matsuda, L. A., Lolait, S. J., Brownstein, M. J., Young, A. C. & Bonner, T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564 (1990).
Munro, S., Thomas, K. L. & Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65 (1993).
Devane, W. A. et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 (1992).
Mechoulam, R. et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50, 83–90 (1995).
Sugiura, T. et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Commun. 215, 89–97 (1995).
Di Marzo, V. Targeting the endocannabinoid system: to enhance or reduce? Nature Rev. Drug Discov. 7, 438–455 (2008).
Onaivi, E. S., Ishiguro, H., Gu, S. & Liu, Q. R. CNS effects of CB2 cannabinoid receptors: beyond neuro-immuno-cannabinoid activity. J. Psychopharmacol. 26, 92–103 (2012).
Mackie, K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp. Pharmacol. 168, 299–325 (2005).
Dalton, G. D., Bass, C. E., Van Horn, C. G. & Howlett, A. C. Signal transduction via cannabinoid receptors. CNS Neurol. Disord. Drug Targets 8, 422–431 (2009).
Gonsiorek, W. et al. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol. Pharmacol. 57, 1045–1050 (2000).
Di Marzo, V. et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372, 686–691 (1994).
Okamoto, Y., Morishita, J., Tsuboi, K., Tonai, T. & Ueda, N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem. 279, 5298–5305 (2004).
Simon, G. M. & Cravatt, B. F. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J. Biol. Chem. 283, 9341–9349 (2008).
Liu, J. et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology 54, 1–7 (2008).
Bisogno, T. et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 163, 463–468 (2003).
Tanimura, A. et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase α mediates retrograde suppression of synaptic transmission. Neuron 65, 320–327 (2010).
Lehmann, D. M., Yuan, C. & Smrcka, A. V. Analysis and pharmacological targeting of phospholipase C β interactions with G proteins. Methods Enzymol. 434, 29–48 (2007).
Cadas, H., di Tomaso, E. & Piomelli, D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J. Neurosci. 17, 1226–1242 (1997).
Fowler, C. J. Transport of endocannabinoids across the plasma membrane and within the cell. FEBS J. 280, 1895–1904 (2013).
Cravatt, B. F. et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384, 83–87 (1996).
Dinh, T. P. et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl Acad. Sci. USA 99, 10819–10824 (2002).
Marrs, W. R. et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nature Neurosci. 13, 951–957 (2010).
Blankman, J. L., Simon, G. M. & Cravatt, B. F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 14, 1347–1356 (2007).
Kozak, K. R. et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J. Biol. Chem. 277, 44877–44885 (2002).
Ohno-Shosaku, T. & Kano, M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr. Opin. Neurobiol. 29, 1–8 (2014).
Di Marzo, V., Bifulco, M. & De Petrocellis, L. The endocannabinoid system and its therapeutic exploitation. Nature Rev. Drug Discov. 3, 771–784 (2004).
Zygmunt, P. M. et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457 (1999).
Bouaboula, M. et al. Anandamide induced PPARγ transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur. J. Pharmacol. 517, 174–181 (2005).
Rodriguez de Fonseca, F., Ramos, J. A., Bonnin, A. & Fernández-Ruiz, J. J. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport 4, 135–138 (1993).
Berrendero, F. et al. Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochim. Biophys. Acta 1407, 205–214 (1998).
McLaughlin, C. R., Martin, B. R., Compton, D. R. & Abood, M. E. Cannabinoid receptors in developing rats: detection of mRNA and receptor binding. Drug Alcohol Depend. 36, 27–31 (1994).
Wang, L., Liu, J., Harvey-White, J., Zimmer, A. & Kunos, G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc. Natl Acad. Sci. USA 100, 1393–1398 (2003).
Morishita, J. et al. Regional distribution and age-dependent expression of N-acylphosphatidylethanolamine-hydrolyzing phospholipase D in rat brain. J. Neurochem. 94, 753–762 (2005).
Berrendero, F., Sepe, N., Ramos, J. A., Di Marzo, V. & Fernández-Ruiz, J. J. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse 33, 181–191 (1999).
Wenger, T. et al. The hypothalamic levels of the endocannabinoid, anandamide, peak immediately before the onset of puberty in female rats. Life Sci. 70, 1407–1414 (2002).
Glass, M., Dragunow, M. & Faull, R. L. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77, 299–318 (1997). The first study showing impaired endocannabinoid signalling in Huntington's disease.
Choi, K. et al. Expression pattern of the cannabinoid receptor genes in the frontal cortex of mood disorder patients and mice selectively bred for high and low fear. J. Psych Res. 46, 882–889 (2012).
Long, L. E., Lind, J., Webster, M. & Weickert, C. S. Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neurosci. 13, 87 (2012).
Westlake, T. M., Howlett, A. C., Bonner, T. I., Matsuda, L. A. & Herkenham, M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer's brains. Neuroscience 63, 637–652 (1994).
Bilkei-Gorzo, A. et al. Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc. Natl Acad. Sci. USA 102, 15670–15675 (2005). This paper shows that brain ageing is accelerated in mice lacking cannabinoid CB1 receptors.
Albayram, O. et al. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc. Natl Acad. Sci. USA 108, 11256–11261 (2011).
Albayram, O., Bilkei-Gorzó, A. & Zimmer, A. Loss of CB1 receptors leads to differential age-related changes in reward-driven learning and memory. Front. Aging Neurosci. 4, 34 (2012).
Han, J. et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148, 1039–1050 (2012).
Solowij, N. et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology 216, 131–144 (2011).
Bolla, K. I., Brown, K., Eldreth, D., Tate, K. & Cadet, J. L. Dose-related neurocognitive effects of marijuana use. Neurology 59, 1337–1343 (2002).
Mata, I. et al. Gyrification brain abnormalities associated with adolescence and early-adulthood cannabis use. Brain Res. 1317, 297–304 (2010).
Solowij, N. et al. Cerebellar white-matter changes in cannabis users with and without schizophrenia. Psychol. Med. 41, 2349–2359 (2011).
Lundqvist, T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol. Biochem. Behav. 81, 319–330 (2005).
Solowij, N. & Battisti, R. The chronic effects of cannabis on memory in humans: a review. Curr. Drug Abuse Rev. 1, 81–98 (2008).
Bilkei-Gorzó, A. et al. Early onset of aging-like changes is restricted to cognitive abilities and skin structure in Cnr1−/− mice. Neurobiol. Aging 33, 200.e11–22 (2012).
Ofek, O. et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl Acad. Sci. USA 103, 696–701 (2006). This paper demonstrates that mice lacking cannabinoid CB2 receptors have an age-related osteoporosis phenotype.
Bab, I. & Zimmer, A. Cannabinoid receptors and the regulation of bone mass. Br. J. Pharmacol. 153, 182–188 (2008).
López- Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013). This review provides a comprehensive overview of prominent physiological changes that are associated with the ageing process.
Hamilton, L. K., Joppé, S. E., Cochard, M. & Fernandes, K. J. L. Aging and neurogenesis in the adult forebrain: what we have learned and where we should go from here. Eur. J. Neurosci. 37, 1978–1986 (2013).
Molofsky, A. V. et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443, 448–452 (2006).
Jin, K. et al. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell 2, 175–183 (2003).
Jiang, W. et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Invest. 115, 3104–3116 (2005).
Palazuelos, J. et al. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 20, 2405–2407 (2006).
Goncalves, M. B. et al. A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol. Cell Neurosci. 38, 526–536 (2008).
Díaz-Alonso, J., Guzmán, M. & Galve-Roperh, I. Endocannabinoids via CB□ receptors act as neurogenic niche cues during cortical development. Phil. Trans. R. Soc. B 367, 3229–3241 (2012).
Gao, Y. et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J. Neurosci. 30, 2017–2024 (2010).
Oudin, M. J., Hobbs, C. & Doherty, P. DAGL-dependent endocannabinoid signalling: roles in axonal pathfinding, synaptic plasticity and adult neurogenesis. Eur. J. Neurosci. 34, 1634–1646 (2011).
Jin, K. et al. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol. Pharmacol. 66, 204–208 (2004).
Aguado, T. et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 19, 1704–1706 (2005).
Tortoriello, G. et al. Miswiring the brain: Δ9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 33, 668–685 (2014). An important study showing for the first time the molecular mechanisms through which overactivation of CB1 may disrupt cortical development.
Arévalo- Martín, A. et al. Cannabinoids modulate Olig2 and polysialylated neural cell adhesion molecule expression in the subventricular zone of post-natal rats through cannabinoid receptor 1 and cannabinoid receptor 2. Eur. J. Neurosci. 26, 1548–1559 (2007).
Stella, N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58, 1017–1030 (2010).
Hegde, V. L., Nagarkatti, M. & Nagarkatti, P. S. Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur. J. Immunol. 40, 3358–3371 (2010).
Maresz, K., Carrier, E. J., Ponomarev, E. D., Hillard, C. J. & Dittel, B. N. Modulation of the cannabinoid CB receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 95, 437–445 (2005).
Stella, N. Endocannabinoid signaling in microglial cells. Neuropharmacology 56, 244–253 (2009).
Maresz, K. et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nature Med. 13, 492–497 (2007).
Jean-Gilles, L. et al. Plasma endocannabinoid levels in multiple sclerosis. J. Neurol. Sci. 287, 212–215 (2009).
Centonze, D. Finazzi-Agro, A. Bernardi, G. & Maccarrone, M. The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends Pharmacol. Sci. 28, 180–187 (2007).
Russell, S. J. & Kahn, C. R. Endocrine regulation of ageing. Nature Rev. Mol. Cell. Biol. 8, 681–691 (2007).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. NY Acad. Sci. 908, 244–254 (2000).
Patel, S. et al. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology 145, 5431–5438 (2004).
Zoppi, S. et al. Regulatory role of cannabinoid receptor 1 in stress-induced excitotoxicity and neuroinflammation. Neuropsychopharmacology 36, 805–818 (2011).
Cutando, L. et al. Microglial activation underlies cerebellar deficits produced by repeated cannabis exposure. J. Clin. Invest. 123, 2816–2831 (2013). This paper identifies microglial cell activation as the non-cell-autonomous mechanism mediating cerebellar deficits induced by semi-chronic exposure to THC, emphasizing the remarkable specificity in cell damage occurring under such regimen.
Zhang, G. et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497, 211–216 (2013).
Di Marzo, V. et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410, 822–825 (2001).
Conde, J. et al. At the crossroad between immunity and metabolism: focus on leptin. Expert Rev. Clin. Immunol. 6, 801–808 (2010).
Cristino, L. et al. Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proc. Natl Acad. Sci. USA 110, E2229–E2238 (2013). The first paper describing endocannabinoid-mediated mechanisms through which the orexinergic control of basic functions can be disrupted in obesity.
Matarese, G. et al. Leptin as a metabolic link to multiple sclerosis. Nature Rev. Neurol. 6, 455–461 (2010).
Martínez de Morentin, P. B. et al. Hypothalamic mTOR: the rookie energy sensor. Curr. Mol. Med. 14, 3–21 (2014).
Puighermanal, E. et al. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nature Neurosci. 12, 1152–1158 (2009).
Puighermanal, E. et al. Dissociation of the Pharmacological Effects of THC by mTOR Blockade. Neuropsychopharmacology 38, 1334–1343 (2013).
Busquets-Garcia, A. et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nature Med. 19, 603–607 (2013). The first study indicating how blockade of CB1 receptors might be useful in some rare forms of autism.
Rubinsztein, D. C., Mariño, G. & Kroemer, G. Autophagy and aging. Cell 146, 682–695 (2011).
Kapahi, P. et al. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell. Metab. 11, 453–465 (2010).
Salazar, M. et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Invest. 119, 1359–1372 (2009).
Salazar, M. et al.TRB3 links ER stress to autophagy in cannabinoid anti-tumoral action. Autophagy 57, 1048–1049 (2009).
Koay, L. C., Rigby, R. J. & Wright, K. L. Cannabinoid-induced autophagy regulates suppressor of cytokine signaling-3 in intestinal epithelium. Am. J. Physiol. Gastrointest Liver Physiol. 307, G140–G148 (2014).
Hiebel, C., Kromm, T., Stark, M. & Behl, C. Cannabinoid receptor 1 modulates the autophagic flux independent of mTOR- and BECLIN1-complex. J Neurochem. 131, 484–497 (2014).
Piyanova, A. et al. Loss of CB1 receptors leads to decreased cathepsin D levels and accelerated lipofuscin accumulation in the hippocampus. Mech. Ageing Dev. 134, 391–399 (2013).
Höhn, A. & Grune, T. Lipofuscin: formation, effects and role of macroautophagy. Redox Biol. 1, 140–144 (2013).
Andrew, S. E. et al. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nature Gen. 4, 398–403 (1993).
Byers, R. K. Gilles, F. H. & Fung, C. Huntington's disease in children. Neuropathologic study of four cases. Neurology 23, 561–569 (1973).
Blazquez, C. et al. Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington's disease. Brain 134, 119–136 (2011).
Naydenov, A. V. et al. Genetic rescue of CB1 receptors on medium spiny neurons prevents loss of excitatory striatal synapses but not motor impairment in HD mice. Neurobiol. Dis. 71, 140–150 (2014).
Chiarlone, A. et al. A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proc. Natl Acad. Sci. USA 111, 8257–8262 (2014).
Noonan, J. et al. Endocannabinoids prevent β-amyloid-mediated lysosomal destabilization in cultured neurons. J. Biol. Chem. 285, 38543–38554 (2010).
Sarnataro, D. et al. Plasma membrane and lysosomal localization of CB1 cannabinoid receptor are dependent on lipid rafts and regulated by anandamide in human breast cancer cells. FEBS Lett. 579, 6343–6349 (2005).
Rozenfeld, R. Type I cannabinoid receptor trafficking: all roads lead to lysosome. Traffic 12, 12–18 (2011).
Brailoiu, G. C. et al. Agonist-selective effects of opioid receptor ligands on cytosolic calcium concentration in rat striatal neurons. Drug Alcohol Depend. 123, 277–281 (2012).
Bénard, G. et al. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nature Neurosci. 15, 558–564 (2012). This paper suggests that CB1 receptors are present on mitochondria and that mitochondrial CB1 signalling modulates neuronal functions.
Morozov, Y. M. et al. Antibodies to cannabinoid type 1 receptor co-react with stomatin-like protein 2 in mouse brain mitochondria. Eur. J. Neurosci. 38, 2341–2348 (2013).
Witte, M. E., Mahad, D. J., Lassmann, H. & van Horssen, J. Mitochondrial dysfunction contributes to neurodegeneration in multiple sclerosis. Trends Mol. Med. 20, 179–187 (2014).
Cravatt, B. F. et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl Acad. Sci. USA 98, 9371–9376 (2001).
Taschler, U. et al. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J. Biol. Chem. 286, 17467–17477 (2011).
Morgese, M. G., Cassano, T., Cuomo, V. & Giuffrida, A. Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson's disease: role of CB(1) and TRPV1 receptors. Exp. Neurol. 208, 110–119 (2007).
Lastres-Becker, I. et al. Compounds acting at the endocannabinoid and/or endovanilloid systems reduce hyperkinesia in a rat model of Huntington's disease. J. Neurochem. 84, 1097–1109 (2003).
Huang, S. M. et al. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J. Biol. Chem. 276, 42639–42644 (2001).
Verhoeckx, K. C. et al. Presence, formation and putative biological activities of N-acyl serotonins, a novel class of fatty-acid derived mediators, in the intestinal tract. Biochim. Biophys. Acta 1811, 578–586 (2011).
Hu, S. S. et al. The biosynthesis of N-arachidonoyl dopamine (NADA), a putative endocannabinoid and endovanilloid, via conjugation of arachidonic acid with dopamine. Prostaglandins Leukot. Essent. Fatty Acids 81, 291–301 (2009).
Schmidt-Hieber, C., Jonas, P. & Bischofberger, J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429, 184–187 (2004).
Chemin, J., Cazade, M. & Lory, P. Modulation of T-type calcium channels by bioactive lipids. Pflugers Arch. 466, 689–700 (2014).
Alhouayek, M. & Muccioli, G. G. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol. Sci. 35, 284–292 (2014).
Woodward, D. F. et al. The pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 products. Pharmacol. Ther. 120, 71–80 (2008).
Valdeolivas, S. et al. The inhibition of 2-arachidonoyl-glycerol (2-AG) biosynthesis, rather than enhancing striatal damage, protects striatal neurons from malonate-induced death: a potential role of cyclooxygenase-2-dependent metabolism of 2-AG. Cell Death Dis. 4, e862 (2013).
Nomura, D. K. et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334, 809–813 (2011). This study identifies COX2 as a major metabolism pathway of the endocannabinoid 2-AG that mediates neuroinflammation and neuronal loss in a mouse model of Parkinson's disease, suggesting that COX2 inhibitors might represent promising therapeutic approaches for this neurodegenerative disease.
Chen, X., Zhang, J. & Chen, C. Endocannabinoid 2-arachidonoylglycerol protects neurons against β-amyloid insults. Neuroscience 178, 159–168 (2011).
Chen, R. et al. Monoacylglycerol lipase is a therapeutic target for Alzheimer's disease. Cell Rep. 2, 1329–1339 (2012).
Ho, K. W., Ward, N. J. & Calkins, D. J. TRPV1: a stress response protein in the central nervous system. Am. J. Neurodegener. Dis. 1, 1–14 (2012).
Li, H. B. et al. Antistress effect of TRPV1 channel on synaptic plasticity and spatial memory. Biol. Psychiatry 64, 286–292 (2008).
González-Aparicio, R. & Moratalla, R. Oleoylethanolamide reduces l-DOPA-induced dyskinesia via TRPV1 receptor in a mouse model of Parkinson´s disease. Neurobiol. Dis. 62, 416–425 (2014).
Razavinasab, M. et al. Pharmacological blockade of TRPV1 receptors modulates the effects of 6-OHDA on motor and cognitive functions in a rat model of Parkinson's disease. Fundam. Clin. Pharmacol. 27, 632–640 (2013).
Sigel, E. et al. The major central endocannabinoid directly acts at GABAA receptors. Proc. Natl Acad. Sci. USA 108, 18150–18155 (2011).
Du, H., Chen, X., Zhang, J. & Chen, C. Inhibition of COX-2 expression by endocannabinoid 2-arachidonoylglycerol is mediated via PPAR-γ. Br. J. Pharmacol. 163, 1533–1549 (2011).
Naydenov, A. V. et al. ABHD6 blockade exerts antiepileptic activity in PTZ-induced seizures and in spontaneous seizures in R6/2 mice. Neuron 83, 361–371 (2014). This study identified ABHD6 as a therapeutically valid anti-epileptic target that is not associated with drug-treatment tolerance and psychotropic effects.
Pryce, G. et al. Control of experimental spasticity by targeting the degradation of endocannabinoids using selective fatty acid amide hydrolase inhibitors. Mult. Scler. 19, 1896–1904 (2013).
De Petrocellis, L. et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163, 1479–1494 (2011).
Leweke, F. M. et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2, e94 (2012).
Webb, M., Luo, L., Ma, J. Y. & Tham, C. S. Genetic deletion of fatty acid amide hydrolase results in improved long-term outcome in chronic autoimmune encephalitis. Neurosci. Lett. 439, 106–110 (2008).
Rossi, S. et al. Cannabinoid CB1 receptors regulate neuronal TNF-α effects in experimental autoimmune encephalomyelitis. Brain Behav. Immun. 25, 1242–1248 (2011).
Mazzola, C., Micale, V. & Drago, F. Amnesia induced by β-amyloid fragments is counteracted by cannabinoid CB1 receptor blockade. Eur. J. Pharmacol. 477, 219–225 (2003).
van der Stelt, M. et al. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson's disease. FASEB J. 19, 1140–1142 (2005).
Horne, E. A. et al. Downregulation of cannabinoid receptor 1 from neuropeptide Y interneurons in the basal ganglia of patients with Huntington's disease and mouse models. Eur. J. Neurosci. 37, 429–440 (2013).
Dowie, M. J. et al. Altered CB1 receptor and endocannabinoid levels precede motor symptom onset in a transgenic mouse model of Huntington's disease. Neuroscience 163, 456–465 (2009).
Bilsland, L. G. et al. Increasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 mice. FASEB J. 20, 1003–1005 (2006).
Steindel, F. et al. Neuron-type specific cannabinoid-mediated G protein signalling in mouse hippocampus. J. Neurochem. 124, 795–807 (2013). This study shows that CB1 receptor coupling efficacy in glutamatergic neurons is greater than in GABAergic neurons, providing evidence for an additional level of complexity in the differential signalling measured for this GPCR depending on the cell type.
van der Stelt, M. et al. Endocannabinoids and β-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell. Mol. Life Sci. 63, 1410–1424 (2006). The first study showing different time-dependent changes in anandamide and 2-AG levels, and a dual beneficial and exacerbating action on disease signs by endocannabinoids, in a model of amyloid-β-induced toxicity.
Mulder, J. et al. Molecular reorganization of endocannabinoid signalling in Alzheimer's disease. Brain. 134, 1041–1060 (2011). This study, along with Ref. 147 identifies increased 2-AG levels as a possible maladaptive mechanism contributing to some signs in amyloid-β-induced toxicity.
Bari, M. et al. In vitro and in vivo models of Huntington's disease show alterations in the endocannabinoid system. FEBS J. 280, 3376–3388 (2013).
Johnston, T. H. et al. Fatty acid amide hydrolase (FAAH) inhibition reduces L-3,4-dihydroxyphenylalanine-induced hyperactivity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned non-human primate model of Parkinson's disease. J. Pharmacol. Exp. Ther. 336, 423–430 (2011).
Bouchard, J. et al. Cannabinoid receptor 2 signaling in peripheral immune cells modulates disease onset and severity in mouse models of Huntington's disease. J. Neurosci. 32, 18259–18268 (2012).
Fernandez-Ruiz, J. et al. Prospects for cannabinoid therapies in basal ganglia disorders. Br. J. Pharmacol. 163, 1365–1378 (2011).
Kim, K., Moore, D. H., Makriyannis, A. & Abood, M. E. AM1241, a cannabinoid CB2 receptor selective compound, delays disease progression in a mouse model of amyotrophic lateral sclerosis. Eur. J. Pharmacol. 542, 100–105 (2006).
González, S. et al. Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of Parkinson's disease. Brain Res. 1073–1074, 209–219 (2006).
Acknowledgements
Research in the laboratory of A.Z. was funded by the Deutsche Forschungsgemeinschaft (FOR926, SFB645) and is a member of Excellence Cluster Immunosensation. N.S. is funded by the US National Institutes of Health (NIH; DA014486 and DA026430). V.D. acknowledges Progetto Operativo Nazionale (PON01_02512) and FIRB-MERIT grant number RBNE08HWLZ_006, for funding, and is a recipient of unrestricted grants from GW Pharmaceuticals, UK, and Allergan, USA. The authors wish to thank L. De Petrocellis and E. Drews for their help during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
VD acts as a consultant for GW Pharmaceuticals, UK, VD receives unrestricted grants from GW Pharmaceuticals, UK, and Allergan, USA
Supplementary information
Supplementary information S1 (table)
The “endocannabinoidome”. Endocannabinoids, endocannabinoid-related mediators and their metabolic enzymes and receptors. (PDF 469 kb)
Rights and permissions
About this article
Cite this article
Di Marzo, V., Stella, N. & Zimmer, A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci 16, 30–42 (2015). https://doi.org/10.1038/nrn3876
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3876
This article is cited by
-
The association of circulating endocannabinoids with neuroimaging and blood biomarkers of neuro-injury
Alzheimer's Research & Therapy (2023)
-
Pharmacologic antagonism of CB1 receptors improves electrophysiological alterations in Purkinje cells exposed to 3-AP
BMC Neuroscience (2023)
-
Postsynaptic synucleins mediate endocannabinoid signaling
Nature Neuroscience (2023)
-
Chronic low-dose Δ9-tetrahydrocannabinol (THC) treatment stabilizes dendritic spines in 18-month-old mice
Scientific Reports (2023)
-
Biological aspects of nitrogen heterocycles for amyotrophic lateral sclerosis
Applied Microbiology and Biotechnology (2023)