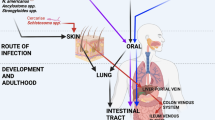
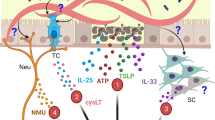
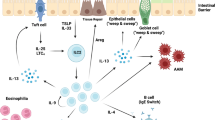
Abstract
Helminth-induced type 2 immune responses, which are characterized by the T helper 2 cell-associated cytokines interleukin-4 (IL-4) and IL-13, mediate host protection through enhanced tissue repair, the control of inflammation and worm expulsion. In this Opinion article, we consider type 2 immunity in the context of helminth-mediated tissue damage. We examine the relationship between the control of helminth infection and the mechanisms of wound repair, and we provide a new understanding of the adaptive type 2 immune response and its contribution to both host tolerance and resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Allen, J. E. & Wynn, T. A. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 7, e1002003 (2011).
Artis, D., Maizels, R. M. & Finkelman, F. D. Forum: Immunology: Allergy challenged. Nature 484, 458–459 (2012).
Palm, N. W., Rosenstein, R. K. & Medzhitov, R. Allergic host defences. Nature 484, 465–472 (2012).
Deter, J., Cosson, J. F., Chaval, Y., Charbonnel, N. & Morand, S. The intestinal nematode Trichuris arvicolae affects the fecundity of its host, the common vole Microtus arvalis. Parasitol. Res. 101, 1161–1164 (2007).
Coop, R. L. & Kyriazakis, I. Influence of host nutrition on the development and consequences of nematode parasitism in ruminants. Trends Parasitol. 17, 325–330 (2001).
Gulland, F. M. The role of nematode parasites in Soay sheep (Ovis aries L.) mortality during a population crash. Parasitology 105, 493–503 (1992).
King, C. H. Health metrics for helminthic infections. Adv. Parasitol. 73, 51–69 (2010).
Schneider, D. S. & Ayres, J. S. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nature Rev. Immunol. 8, 889–895 (2008).
Chen, F. et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nature Med. 18, 260–266 (2012).
Monticelli, L. A. et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature Immunol. 12, 1045–1054 (2011).
Herbert, D. R. et al. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J. Immunol. 184, 6438–6446 (2010).
Pesce, J. T. et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 5, e1000371 (2009).
Read, A. F., Graham, A. L. & Raberg, L. Animal defenses against infectious agents: is damage control more important than pathogen control. PLoS Biol. 6, e4 (2008).
Jackson, J. A., Friberg, I. M., Little, S. & Bradley, J. E. Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology 126, 18–27 (2009).
Eming, S. A., Krieg, T. & Davidson, J. M. Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 127, 514–525 (2007).
Nguyen, K. D. et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480, 104–108 (2011).
Hernandez, J. L., Leung, G. & McKay, D. M. Cestode regulation of inflammation and inflammatory diseases. Int. J. Parasitol. 43, 233–243 (2012).
Elliott, D. E. & Weinstock, J. V. Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Ann. NY Acad. Sci. 1247, 83–96 (2012).
Diegelmann, R. F. & Evans, M. C. Wound healing: an overview of acute, fibrotic and delayed healing. Front. Biosci. 9, 283–289 (2004).
Mills, K. H. TLR-dependent T cell activation in autoimmunity. Nature Rev. Immunol. 11, 807–822 (2011).
Everts, B., Smits, H. H., Hokke, C. H. & Yazdanbakhsh, M. Helminths and dendritic cells: sensing and regulating via pattern recognition receptors, Th2 and Treg responses. Eur. J. Immunol. 40, 1525–1537 (2010).
Saenz, S. A. et al. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature 464, 1362–1366 (2010).
Ramalingam, T. R. et al. Regulation of helminth-induced Th2 responses by thymic stromal lymphopoietin. J. Immunol. 182, 6452–6459 (2009).
Smithgall, M. D. et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 20, 1019–1030 (2008).
Zaph, C. et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 446, 552–556 (2007).
Siracusa, M. C. et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 477, 229–233 (2011).
Massacand, J. C. et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc. Natl Acad. Sci. USA 106, 13968–13973 (2009).
Townsend, M. J., Fallon, P. G., Matthews, D. J., Jolin, H. E. & McKenzie, A. N. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 191, 1069–1076 (2000).
Humphreys, N. E., Xu, D., Hepworth, M. R., Liew, F. Y. & Grencis, R. K. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J. Immunol. 180, 2443–2449 (2008).
Wang, Y. H. et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J. Exp. Med. 204, 1837–1847 (2007).
Fallon, P. G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116 (2006).
Kuroda, E. et al. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity 34, 514–526 (2011).
Mishra, P. K. et al. Micrometer-sized titanium particles can induce potent Th2-type responses through TLR4-independent pathways. J. Immunol. 187, 6491–6498 (2011).
Kool, M. et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity 34, 527–540 (2011).
Kouzaki, H., Iijima, K., Kobayashi, T., O'Grady, S. M. & Kita, H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J. Immunol. 186, 4375–4387 (2011).
Strid, J., Sobolev, O., Zafirova, B., Polic, B. & Hayday, A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science 334, 1293–1297 (2011).
Carvalho, L. et al. Review series on helminths, immune modulation and the hygiene hypothesis: mechanisms underlying helminth modulation of dendritic cell function. Immunology 126, 28–34 (2009).
Puneet, P. et al. The helminth product ES-62 protects against septic shock via Toll-like receptor 4-dependent autophagosomal degradation of the adaptor MyD88. Nature Immunol. 12, 344–351 (2011).
Everts, B. et al. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J. Exp. Med. 209, 1753–1767 (2012).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Price, A. E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl Acad. Sci. USA 107, 11489–11494 (2010).
Allen, J. E. & Maizels, R. M. Diversity and dialogue in immunity to helminths. Nature Rev. Immunol. 11, 375–388 (2011).
Wynn, T. A. IL-13 effector functions. Annu. Rev. Immunol. 21, 425–456 (2003).
Pulendran, B. & Artis, D. New paradigms in type 2 immunity. Science 337, 431–435 (2012).
Rosenberg, H. F., Dyer, K. D. & Foster, P. S. Eosinophils: changing perspectives in health and disease. Nature Rev. Immunol. 13, 9–22 (2013).
Wynn, T. A. & Ramalingam, T. R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature Med. 18, 1028–1040 (2012).
Loke, P. et al. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J. Immunol. 179, 3926–3936 (2007).
Sandler, N. G., Mentink-Kane, M. M., Cheever, A. W. & Wynn, T. A. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J. Immunol. 171, 3655–3667 (2003).
Harris, N. & Gause, W. C. To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol. 32, 80–88 (2011).
Anthony, R. M. et al. Protective immune mechanisms in helminth infection. Nature Rev. Immunol. 7, 975–987 (2007).
Anthony, R. M. et al. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nature Med. 12, 955–960 (2006).
Harvie, M. et al. The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect. Immun. 78, 3753–3762 (2010).
Gurish, M. F. et al. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J. Immunol. 172, 1139–1145 (2004).
Ushio, H., Watanabe, N., Kiso, Y., Higuchi, S. & Matsuda, H. Protective immunity and mast cell and eosinophil responses in mice infested with larval Haemaphysalis longicornis ticks. Parasite Immunol. 15, 209–214 (1993).
Matsuda, H. et al. Necessity of IgE antibodies and mast cells for manifestation of resistance against larval Haemaphysalis longicornis ticks in mice. J. Immunol. 144, 259–262 (1990).
Okumura, S., Sagara, H., Fukuda, T., Saito, H. & Okayama, Y. FcɛRI-mediated amphiregulin production by human mast cells increases mucin gene expression in epithelial cells. J. Allergy Clin. Immunol. 115, 272–279 (2005).
Hoffmann, K. F., Cheever, A. W. & Wynn, T. A. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164, 6406–6416 (2000).
Pearce, E. J. et al. Schistosoma mansoni in IL-4-deficient mice. Int. Immunol. 8, 435–444 (1996).
Reece, J. J., Siracusa, M. C. & Scott, A. L. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect. Immun. 74, 4970–4981 (2006).
McNeil, K. S., Knox, D. P. & Proudfoot, L. Anti-inflammatory responses and oxidative stress in Nippostrongylus brasiliensis-induced pulmonary inflammation. Parasite Immunol. 24, 15–22 (2002).
Thomas, G. D. et al. The biology of nematode- and IL4Rα-dependent murine macrophage polarization in vivo as defined by RNA-Seq and targeted lipidomics. Blood 120, e93–e104 (2012).
Wynes, M. W., Frankel, S. K. & Riches, D. W. IL-4-induced macrophage-derived IGF-I protects myofibroblasts from apoptosis following growth factor withdrawal. J. Leukoc. Biol. 76, 1019–1027 (2004).
Gillery, P., Leperre, A., Maquart, F. X. & Borel, J. P. Insulin-like growth factor-I (IGF-I) stimulates protein synthesis and collagen gene expression in monolayer and lattice cultures of fibroblasts. J. Cell. Physiol. 152, 389–396 (1992).
Toulon, A. et al. A role for human skin-resident T cells in wound healing. J. Exp. Med. 206, 743–750 (2009).
Zeng, M. Y. et al. An efferocytosis-induced, IL-4-dependent macrophage-iNKT cell circuit suppresses sterile inflammation and is defective in murine CGD. Blood 121, 3473–3483 (2013).
Varin, A., Mukhopadhyay, S., Herbein, G. & Gordon, S. Alternative activation of macrophages by IL-4 impairs phagocytosis of pathogens but potentiates microbial-induced signalling and cytokine secretion. Blood 115, 353–362 (2010).
Schopf, L. R., Hoffmann, K. F., Cheever, A. W., Urban, J. F. Jr & Wynn, T. A. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J. Immunol. 168, 2383–2392 (2002).
Hesse, M. et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 167, 6533–6544 (2001).
Hesse, M. et al. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J. Immunol. 172, 3157–3166 (2004).
Shirey, K. A. et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4Rα-, TLR4-, and IFN-α-dependent. Mucosal Immunol. 3, 291–300 (2010).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nature Rev. Immunol. 8, 958–969 (2008).
Cooke, A. Review series on helminths, immune modulation and the hygiene hypothesis: how might infection modulate the onset of type 1 diabetes? Immunology 126, 12–17 (2009).
Zaccone, P. & Cooke, A. Infectious triggers protect from autoimmunity. Semin. Immunol. 23, 122–129 (2011).
Mishra, P. K., Patel, N., Wu, W., Bleich, D. & Gause, W. C. Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response. Mucosal Immunol. 6, 297–308 (2012).
Hubner, M. P. et al. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-β. J. Immunol. 188, 559–568 (2012).
Mentink-Kane, M. M. et al. Accelerated and progressive and lethal liver fibrosis in mice that lack interleukin (IL)-10, IL-12p40, and IL-13Rα2. Gastroenterology 141, 2200–2209 (2011).
Wilson, M. S. et al. Immunopathology of schistosomiasis. Immunol. Cell Biol. 85, 148–154 (2007).
Chiaramonte, M. G., Donaldson, D. D., Cheever, A. W. & Wynn, T. A. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Invest. 104, 777–785 (1999).
Chiaramonte, M. G., Cheever, A. W., Malley, J. D., Donaldson, D. D. & Wynn, T. A. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology 34, 273–282 (2001).
Wilson, M. S. et al. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 202, 1199–1212 (2005).
Egawa, M. et al. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity 38, 570–580 (2013).
Nair, M. G. et al. Alternatively activated macrophage-derived RELM-α is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 206, 937–952 (2009).
Pesce, J. T. et al. Retnlα (relmα/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 5, e1000393 (2009).
Wilson, M. S. et al. IL-13Rα2 and IL-10 coordinately suppress airway inflammation, airway-hyperreactivity, and fibrosis in mice. J. Clin. Invest. 117, 2941–2951 (2007).
Seno, H. et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc. Natl Acad. Sci. USA 106, 256–261 (2009).
Wu, D. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011).
Wills-Karp, M. & Finkelman, F. D. Innate lymphoid cells wield a double-edged sword. Nature Immunol. 12, 1025–1027 (2011).
Kool, M., Fierens, K. & Lambrecht, B. N. Alum adjuvant: some of the tricks of the oldest adjuvant. J. Med. Microbiol. 61, 927–934 (2012).
Grainger, J. R. et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med. 207, 2331–2341 (2010).
Piggott, D. A. et al. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. J. Clin. Invest. 115, 459–467 (2005).
Jenkins, S. J. et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288 (2011).
Acknowledgements
The authors wish to thank F. Finkelman at the Division of Allergy and Immunology, Cincinnati Children's Hospital Medical Center, USA, for providing excellent critical comments that strengthened this manuscript. This work was partly supported by the US National Institutes of Health (NIH) grants R01AI031678 and R01AI066188 awarded to W.C.G., and T.A.W. is supported by the Intramural Research Program from the NIH National Institute of Allergy and Infectious Diseases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
T.A.W. holds patents on the treatment of fibrosis by blocking interleukin-13 (IL-13) and IL-21.
Rights and permissions
About this article
Cite this article
Gause, W., Wynn, T. & Allen, J. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol 13, 607–614 (2013). https://doi.org/10.1038/nri3476
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3476
This article is cited by
-
How chemokines organize the tumour microenvironment
Nature Reviews Cancer (2024)
-
Type 2 immunity in the brain and brain borders
Cellular & Molecular Immunology (2023)
-
Unique adipose tissue invariant natural killer T cell subpopulations control adipocyte turnover in mice
Nature Communications (2023)
-
Type 2 cytokines in the thymus activate Sirpα+ dendritic cells to promote clonal deletion
Nature Immunology (2022)
-
Tissue-based IL-10 signalling in helminth infection limits IFNγ expression and promotes the intestinal Th2 response
Mucosal Immunology (2022)