Key Points
-
The most common complications of surgical treatments for gastrointestinal disorders include stenosis or leakage; tissue engineering and regenerative medicine could reduce these complications
-
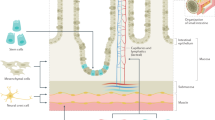
The gastrointestinal tract is complex in structure and function, which makes its regeneration challenging; however, a wide selection of cell sources and scaffolding materials are available
-
Alignment and innervation of the smooth muscle have important roles in mediating coordinated peristalsis and propulsion of luminal content; regeneration of the neuromuscular apparatus is of critical importance
-
The epithelial lining of the gastrointestinal tract has multiple roles in providing nutrition, innate immunity and protection; regeneration of a functional epithelium is essential for translational purposes
-
New stem cell strategies for in vitro modelling and in vivo therapies are emerging
-
Less is known about regeneration of the lymphatic system in the gastrointestinal tract and future studies must incorporate the lymphatics as part of the bioengineering process
Abstract
Functions of the gastrointestinal tract include motility, digestion and absorption of nutrients. These functions are mediated by several specialized cell types including smooth muscle cells, neurons, interstitial cells and epithelial cells. In gastrointestinal diseases, some of the cells become degenerated or fail to accomplish their normal functions. Surgical resection of the diseased segments of the gastrointestinal tract is considered the gold-standard treatment in many cases, but patients might have surgical complications and quality of life can remain low. Tissue engineering and regenerative medicine aim to restore, repair, or regenerate the function of the tissues. Gastrointestinal tissue engineering is a challenging process given the specific phenotype and alignment of each cell type that colonizes the tract — these properties are critical for proper functionality. In this Review, we summarize advances in the field of gastrointestinal tissue engineering and regenerative medicine. Although the findings are promising, additional studies and optimizations are needed for translational purposes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Said, H. M. Physiology of the Gastrointestinal Tract, Two Volume Set (Academic Press, 2012).
Sanders, K. M., Koh, S. D., Ro, S. & Ward, S. M. Regulation of gastrointestinal motility — insights from smooth muscle biology. Nat. Rev. Gastroenterol. Hepatol. 9, 633–645 (2012).
Kiela, P. R. & Ghishan, F. K. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 30, 145–159 (2016).
Alexander, J., Ganta, V. C., Jordan, P. & Witte, M. H. Gastrointestinal lymphatics in health and disease. Pathophysiology 17, 315–335 (2010).
Kulig, K. M. & Vacanti, J. P. Hepatic tissue engineering. Transpl. Immunol. 12, 303–310 (2004).
Takahashi, Y., Takebe, T. & Taniguchi, H. Engineering pancreatic tissues from stem cells towards therapy. Regenerative Ther. 3, 15–23 (2016).
Moharamzadeh, K., Brook, I., Van Noort, R., Scutt, A. & Thornhill, M. Tissue-engineered oral mucosa: a review of the scientific literature. J. Dental Res. 86, 115–124 (2007).
Szymanski, P. T., Chacko, T. K., Rovner, A. S. & Goyal, R. K. Differences in contractile protein content and isoforms in phasic and tonic smooth muscles. Am. J. Physiol. 275, C684–C692 (1998).
Patel, C. A. & Rattan, S. Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G830–G837 (2006).
Gerthoffer, W., Murphey, K., Mangini, J., Boman, S. & Lattanzio, F. Myosin phosphorylation and calcium in tonic and phasic contractions of colonic smooth muscle. Am. J. Physiol. 260, G958–G964 (1991).
Smith, G. T. et al. Anatomic localization of cholecystokinin receptors to the pyloric sphincter. Am. J. Physiol. 246, R127–R130 (1984).
Hori, Y. et al. Experimental study on tissue engineering of the small intestine by mesenchymal stem cell seeding. J. Surg. Res. 102, 156–160 (2002).
Somara, S., Gilmont, R. R., Dennis, R. G. & Bitar, K. N. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology 137, 53–61 (2009).
Raghavan, S. et al. Bioengineered three-dimensional physiological model of colonic longitudinal smooth muscle in vitro. Tissue Eng. Part C Methods 16, 999–1009 (2010).
Nakase, Y. et al. Tissue engineering of small intestinal tissue using collagen sponge scaffolds seeded with smooth muscle cells. Tissue Eng. 12, 403–412 (2006).
Huber, A. & Badylak, S. F. Phenotypic changes in cultured smooth muscle cells: limitation or opportunity for tissue engineering of hollow organs? J. Tissue Eng. Regen. Med. 6, 505–511 (2012).
Arey, L. & Tremaine, M. The muscle content of the lower oesophagus of man. Anat. Rec. 56, 315–320 (1933).
Tan, J. et al. Esophageal tissue engineering: an in-depth review on scaffold design. Biotechnol. Bioeng. 109, 1–15 (2012).
Furness, J. & Costa, M. Types of nerves in the enteric nervous system. Neuroscience 5, 1–20 (1980).
Hao, M. M. & Young, H. M. Development of enteric neuron diversity. J. Cell. Mol. Med. 13, 1193–1210 (2009).
Furness, J. Types of neurons in the enteric nervous system. J. Autonom. Nerv. Syst. 81, 87–96 (2000).
Sanders, K. M., Koh, S. D. & Ward, S. M. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu. Rev. Physiol. 68, 307–343 (2006).
Dickens, E. J., Edwards, F. & Hirst, G. Selective knockout of intramuscular interstitial cells reveals their role in the generation of slow waves in mouse stomach. J. Physiol. 531, 827–833 (2001).
Zheng, H., Park, K. S., Koh, S. D. & Sanders, K. M. Expression and function of a T-type Ca2+ conductance in interstitial cells of Cajal of the murine small intestine. Am. J. Physiol. Cell Physiol. 306, C705–C713 (2014).
Komuro, T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J. Physiol. 576, 653–658 (2006).
Ward, S. & Sanders, K. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol. Motil. 16, 112–117 (2004).
Kraehenbuh, J. P., Pringault, E., & Neutra, M. R. Review article: intestinal epithelia and barrier functions. Aliment. Pharmacol. Ther. 11, 3–9 (1997).
Peterson, L. W. & Artis, D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14, 141–153 (2014).
Martin-Belmonte, F. & Perez-Moreno, M. Epithelial cell polarity, stem cells and cancer. Nat. Rev. Cancer 12, 23–38 (2012).
Karlsson, J. et al. Regional variations in Paneth cell antimicrobial peptide expression along the mouse intestinal tract. BMC Immunol. 9, 37 (2008).
van der Flier, L. G. & Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71, 241–260 (2009).
Goto, Y. & Kiyono, H. Epithelial barrier: an interface for the cross-communication between gut flora and immune system. Immunol. Rev. 245, 147–163 (2012).
Camilleri, M., Madsen, K., Spiller, R., Van Meerveld, B. & Verne, G. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 24, 503–512 (2012).
Marchiando, A. M. et al. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology 140, 1208–1218.e2 (2011).
Ulluwishewa, D. et al. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 141, 769–776 (2011).
Swartz, M. A. The physiology of the lymphatic system. Adv. Drug Deliv. Rev. 50, 3–20 (2001).
Sommer, F. & Bäckhed, F. The gut microbiota — masters of host development and physiology. Nat. Rev. Microbiol. 11, 227–238 (2013).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Dommett, R., Zilbauer, M., George, J. T. & Bajaj-Elliott, M. Innate immune defence in the human gastrointestinal tract. Mol. Immunol. 42, 903–912 (2005).
Atala, A., Bauer, S. B., Soker, S., Yoo, J. J. & Retik, A. B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367, 1241–1246 (2006).
Macchiarini, P. et al. Clinical transplantation of a tissue-engineered airway. Lancet 372, 2023–2030 (2008).
Fisher, M. B. & Mauck, R. L. Tissue engineering and regenerative medicine: recent innovations and the transition to translation. Tissue Eng. Part B Rev. 19, 1–13 (2013).
Zhang, Y. Epidemiology of esophageal cancer. World J. Gastroenterol. 19, 5598–5606 (2013).
Crew, K. D. & Neugut, A. I. Epidemiology of gastric cancer. World J. Gastroenterol. 12, 354–362 (2006).
Siegel, R., DeSantis, C. & Jemal, A. Colorectal cancer statistics, 2014. CA Cancer J. Clin. 64, 104–117 (2014).
Fujita, H. et al. Optimum treatment strategy for superficial esophageal cancer: endoscopic mucosal resection versus radical esophagectomy. World J. Surg. 25, 424–431 (2001).
Gordon, A. C., Kojima, K., Inokuchi, M., Kato, K. & Sugihara, K. Long-term comparison of laparoscopy-assisted distal gastrectomy and open distal gastrectomy in advanced gastric cancer. Surg. Endosc. 27, 462–470 (2013).
Lujan, J. et al. Laparoscopic versus open surgery for rectal cancer: results of a prospective multicentre analysis of 4,970 patients. Surg. Endosc. 27, 295–302 (2013).
Isomoto, H., Yamaguchi, N., Minami, H. & Nakao, K. Management of complications associated with endoscopic submucosal dissection/endoscopic mucosal resection for esophageal cancer. Dig. Endosc. 25, 29–38 (2013).
Fukuda, Y. et al. Prevalence of malnutrition among gastric cancer patients undergoing gastrectomy and optimal preoperative nutritional support for preventing surgical site infections. Ann. Surg. Oncol. 22, 778–785 (2015).
Nour, S., Beck, J. & Stringer, M. Colostomy complications in infants and children. Ann. R. Coll. Surg. Engl. 78, 526 (1996).
Dabirian, A., Yaghmaei, F., Rassouli, M. & Tafreshi, M. Z. Quality of life in ostomy patients: a qualitative study. Patient Prefer. Adherence 5, 1–5 (2010).
Schalamon, J., Mayr, J. & Höllwarth, M. Mortality and economics in short bowel syndrome. Best Pract. Res. Clin. Gastroenterol. 17, 931–942 (2003).
Winkler, M. F. & Smith, C. E. Clinical, social, and economic impacts of home parenteral nutrition dependence in short bowel syndrome. JPEN J. Parenter. Enteral Nutr. 38 (1 Suppl.), 32S–37S (2014).
Buchman, A. L., Scolapio, J. & Fryer, J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 124, 1111–1134 (2003).
Fishbein, T. M. Intestinal transplantation. N. Engl. J. Med. 361, 998–1008 (2009).
Sudan, D. Cost and quality of life after intestinal transplantation. Gastroenterology 130, S158–S162 (2006).
Grant, D. et al. Intestinal transplant registry report: global activity and trends. Am. J. Transplant. 15, 210–219 (2015).
Freeman, R. et al. Liver and intestine transplantation in the United States, 1997–2006. Am. J. Transplant. 8, 958–976 (2008).
Brown, B. N. & Badylak, S. F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 163, 268–285 (2014).
Crapo, P. M., Gilbert, T. W. & Badylak, S. F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233–3243 (2011).
Badylak, S. F., Taylor, D. & Uygun, K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 13, 27–53 (2011).
Gilbert, T. W., Sellaro, T. L. & Badylak, S. F. Decellularization of tissues and organs. Biomaterials 27, 3675–3683 (2006).
Syed, O., Walters, N. J., Day, R. M., Kim, H.-W. & Knowles, J. C. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomaterialia 10, 5043–5054 (2014).
Badylak, S., Meurling, S., Chen, M., Spievack, A. & Simmons-Byrd, A. Resorbable bioscaffold for esophageal repair in a dog model. J. Pediatr. Surg. 35, 1097–1103 (2000).
Doede, T., Bondartschuk, M., Joerck, C., Schulze, E. & Goernig, M. Unsuccessful alloplastic esophageal replacement with porcine small intestinal submucosa. Artif. Organs 33, 328–333 (2009).
Wang, Z. Q., Watanabe, Y. & Toki, A. Experimental assessment of small intestinal submucosa as a small bowel graft in a rat model. J. Pediatr. Surg. 38, 1596–1601 (2003).
Nakao, M. et al. Proposal of intestinal tissue engineering combined with Bianchi's procedure. J. Pediatr. Surg. 50, 573–580 (2014).
Briel, J. W. et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J. Am. Coll. Surg. 198, 536–541 (2004).
Badylak, S. F. et al. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng. Part A 17, 1643–1650 (2011).
Nieponice, A. et al. Patch esophagoplasty: esophageal reconstruction using biologic scaffolds. Ann. Thorac. Surg. 97, 283–288 (2014).
Badylak, S. F., Valentin, J. E., Ravindra, A. K., McCabe, G. P. & Stewart-Akers, A. M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng. Part A 14, 1835–1842 (2008).
Sjöqvist, S. et al. Experimental orthotopic transplantation of a tissue-engineered oesophagus in rats. Nat. Commun. 5, 3562 (2014).
Nieponice, A., Gilbert, T. W., Johnson, S. A., Turner, N. J. & Badylak, S. F. Bone marrow-derived cells participate in the long-term remodeling in a mouse model of esophageal reconstruction. J. Surg. Res. 182, e1–e7 (2013).
Diemer, P., Markoew, S., Le, D. & Qvist, N. Poly-ε-caprolactone mesh as a scaffold for in vivo tissue engineering in rabbit esophagus. Dis. Esophagus 28, 240–245 (2014).
Freud, E., Efrati, I., Kidron, D. & Mares, A. Comparative experimental study of esophageal wall regeneration after prosthetic replacement. J. Biomed. Mater. Res. 45, 84–91 (1999).
Miyazawa, M. et al. Extensive regeneration of the stomach using bioabsorbable polymer sheets. Surgery 158, 1283–1290 (2015).
Hori, Y. et al. Functional analysis of the tissue-engineered stomach wall. Artif. Organs 26, 868–872 (2002).
Araki, M. et al. Development of a new tissue-engineered sheet for reconstruction of the stomach. Artif. Organs 33, 818–826 (2009).
Denost, Q. et al. Colorectal tissue engineering: a comparative study between porcine small intestinal submucosa (SIS) and chitosan hydrogel patches. Surgery 158, 1714–1723 (2015).
Nakatsu, H. et al. Influence of mesenchymal stem cells on stomach tissue engineering using small intestinal submucosa. J. Tissue Eng. Regen. Med. 9, 296–604 (2013).
Qin, H. H. & Dunn, J. C. Small intestinal submucosa seeded with intestinal smooth muscle cells in a rodent jejunal interposition model. J. Surg. Res. 171, e21–e26 (2011).
Walthers, C. M., Nazemi, A. K., Patel, S. L., Wu, B. M. & Dunn, J. C. The effect of scaffold macroporosity on angiogenesis and cell survival in tissue-engineered smooth muscle. Biomaterials 35, 5129–5137 (2014).
Walthers, C. M., Lee, M., Wu, B. M. & Dunn, J. C. Smooth muscle strips for intestinal tissue engineering. PLoS ONE 9, e114850 (2014).
Raghavan, S. & Bitar, K. N. The influence of extracellular matrix composition on the differentiation of neuronal subtypes in tissue engineered innervated intestinal smooth muscle sheets. Biomaterials 35, 7429–7440 (2014).
Zakhem, E., Elbahrawy, M., Orlando, G. & Bitar, K. N. Successful implantation of an engineered tubular neuromuscular tissue composed of human cells and chitosan scaffold. Surgery 158, 1598–1608 (2015).
Franck, D. et al. In vitro evaluation of bi-layer silk fibroin scaffolds for gastrointestinal tissue engineering. J. Tissue Eng. 5, 2041731414556849 (2014).
Metzger, M. et al. Expansion and differentiation of neural progenitors derived from the human adult enteric nervous system. Gastroenterology 137, 2063–2073.e4 (2009).
Anitha, M. et al. Characterization of fetal and postnatal enteric neuronal cell lines with improvement in intestinal neural function. Gastroenterology 134, 1424–1435 (2008).
Hotta, R. et al. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J. Clin. Invest. 123, 1182–1191 (2013).
Pan, W. K., Zheng, B. J., Gao, Y., Qin, H. & Liu, Y. Transplantation of neonatal gut neural crest progenitors reconstructs ganglionic function in benzalkonium chloride-treated homogenic rat colon. J. Surg. Res. 167, e221–e230 (2011).
Almond, S., Lindley, R. M., Kenny, S. E., Connell, M. G. & Edgar, D. H. Characterisation and transplantation of enteric nervous system progenitor cells. Gut 56, 489–496 (2007).
Raghavan, S., Gilmont, R. R. & Bitar, K. N. Neuroglial differentiation of adult enteric neuronal progenitor cells as a function of extracellular matrix composition. Biomaterials 34, 6649–6658 (2013).
Macheiner, T., Ackbar, R. & Saxena, A. K. Isolation, identification and culture of myenteric plexus cells from ovine esophagus. Esophagus 10, 144–148 (2013).
Hanani, M. Multiple myenteric networks in the human appendix. Auton. Neurosci. 110, 49–54 (2004).
Hagl, C. I., Heumüller-Klug, S., Wink, E., Wessel, L. & Schäfer, K.-H. The human gastrointestinal tract, a potential autologous neural stem cell source. PLoS ONE 8, e72948 (2013).
Bitar, K. N. & Zakhem, E. Is bioengineering a possibility in gastrointestinal disorders? Expert Rev. Gastroenterol. Hepatol. 9, 1463–1465 (2015).
Guilak, F. et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17–26 (2009).
Rego, S. L., Raghavan, S., Zakhem, E. & Bitar, K. N. Enteric neural differentiation in innervated, physiologically functional, smooth muscle constructs is modulated by bone morphogenic protein 2 secreted by sphincteric smooth muscle cells. J. Tissue Eng. Regen. Med. http://dx.doi.org/10.1002/term.2027 (2015).
Anitha, M. et al. BMP2 promotes differentiation of nitrergic and catecholaminergic enteric neurons through a Smad1-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G375–G383 (2010).
Zakhem, E. & Bitar, K. N. Development of chitosan scaffolds with enhanced mechanical properties for intestinal tissue engineering applications. J. Funct. Biomater. 6, 999–1011 (2015).
Zakhem, E., Raghavan, S., Gilmont, R. R. & Bitar, K. N. Chitosan-based scaffolds for the support of smooth muscle constructs in intestinal tissue engineering. Biomaterials 33, 4810–4817 (2012).
Zakhem, E., Raghavan, S. & Bitar, K. N. Neo-innervation of a bioengineered intestinal smooth muscle construct around chitosan scaffold. Biomaterials 35, 1882–1889 (2014).
Vrana, N. E. et al. Engineering functional epithelium for regenerative medicine and in vitro organ models: a review. Tissue Eng. Part B Rev. 19, 529–543 (2013).
Paz, A. C. et al. Challenges and opportunities for tissue-engineering polarized epithelium. Tissue Eng. Part B Rev. 20, 56–72 (2013).
Macheiner, T., Kuess, A., Dye, J. & Saxena, A. K. A novel method for isolation of epithelial cells from ovine esophagus for tissue engineering. Biomed. Mater. Eng. 24, 1457–1468 (2014).
Ohki, T. et al. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut 55, 1704–1710 (2006).
Ohki, T. et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 143, 582–588.e2 (2012).
Poghosyan, T. et al. In vitro development and characterization of a tissue-engineered conduit resembling esophageal wall using human and pig skeletal myoblast, oral epithelial cells, and biologic scaffolds. Tissue Eng. Part A 19, 2242–2252 (2013).
Shabafrooz, V. et al. The effect of hyaluronic acid on biofunctionality of gelatin–collagen intestine tissue engineering scaffolds. J. Biomed. Mater. Res. Part A 102, 3130–3139 (2014).
Costello, C. M. et al. Synthetic small intestinal scaffolds for improved studies of intestinal differentiation. Biotechnol. Bioeng. 111, 1222–1232 (2014).
Yu, J., Peng, S., Luo, D. & March, J. C. In vitro 3D human small intestinal villous model for drug permeability determination. Biotechnol. Bioeng. 109, 2173–2178 (2012).
Nelson, R. L. Epidemiology of fecal incontinence. Gastroenterology 126, S3–S7 (2004).
Bharucha, A. E. Fecal incontinence 1, 2. Gastroenterology 124, 1672–1685 (2003).
Hecker, L., Baar, K., Dennis, R. G. & Bitar, K. N. Development of a three-dimensional physiological model of the internal anal sphincter bioengineered in vitro from isolated smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G188–G196 (2005).
Raghavan, S. et al. Successful implantation of bioengineered, intrinsically innervated, human internal anal sphincter. Gastroenterology 141, 310–319 (2011).
Gilmont, R. R., Raghavan, S., Somara, S. & Bitar, K. N. Bioengineering of physiologically functional intrinsically innervated human internal anal sphincter constructs. Tissue Eng. Part A 20, 1603–1611 (2014).
Raghavan, S. et al. Perianal implantation of bioengineered human internal anal sphincter constructs intrinsically innervated with human neural progenitor cells. Surgery 155, 668–674 (2014).
Bitar, K. N. et al. 600 in situ implantation of autologous Biosphincter™ re-instates continence in a large animal model of passive fecal incontinence. Gastroenterology 148, S-117 (2015).
Rego, S. L., Zakhem, E., Orlando, G. & Bitar, K. N. Bioengineering functional human sphincteric and non-sphincteric gastrointestinal smooth muscle constructs. Methods 99, 128–134 (2015).
Rego, S. L., Zakhem, E., Orlando, G. & Khalil, B. Bioengineered human pyloric sphincters using autologous smooth muscle and neural progenitor cells. Tissue Eng. Part A 22, 151–160 (2016).
Kellersman, R., Zhong, R., Kiyochi, H., Garcia, B. & Grant, D. R. Reconstruction of the intestinal lymphatic drainage after small bowel transplantation. Transplantation 69, 10–16 (2000).
Duxbury, M. S. et al. Lymphangiogenesis in tissue-engineered small intestine. Transplantation 77, 1162–1166 (2004).
Perez, A. et al. Tissue-engineered small intestine: ontogeny of the Immune System12. Transplantation 74, 619–623 (2002).
Dixon, J. B., Raghunathan, S. & Swartz, M. A. A tissue-engineered model of the intestinal lacteal for evaluating lipid transport by lymphatics. Biotechnol. Bioeng. 103, 1224–1235 (2009).
Gardner-Thorpe, J. et al. Angiogenesis in tissue-engineered small intestine. Tissue Eng. 9, 1255–1261 (2003).
Choi, R. & Vacanti, J. Preliminary studies of tissue-engineered intestine using isolated epithelial organoid units on tubular synthetic biodegradable scaffolds. Transplant. Proc. 29, 848–851 (1997).
van Rijn, J. M., Schneeberger, K., Wiegerinck, C. L., Nieuwenhuis, E. E. & Middendorp, S. Novel approaches: tissue engineering and stem cells — in vitro modelling of the gut. Best Pract. Res. Clin. Gastroenterol. 30, 281–293 (2016).
Grikscheit, T. C. et al. Tissue-engineered colon exhibits function in vivo. Surgery 132, 200–204 (2002).
Grikscheit, T. C. et al. Tissue-engineered large intestine resembles native colon with appropriate in vitro physiology and architecture. Ann. Surg. 238, 35 (2003).
Grikscheit, T., Ochoa, E. R., Srinivasan, A., Gaissert, H. & Vacanti, J. P. Tissue-engineered esophagus: experimental substitution by onlay patch or interposition. J. Thorac. Cardiovasc. Surg. 126, 537–544 (2003).
Grikscheit, T., Srinivasan, A. & Vacanti, J. P. Tissue-engineered stomach: a preliminary report of a versatile in vivo model with therapeutic potential. J. Pediatr. Surg. 38, 1305–1309 (2003).
Grikscheit, T. C. et al. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann. Surg. 240, 748–754 (2004).
Sala, F. G., Kunisaki, S. M., Ochoa, E. R., Vacanti, J. & Grikscheit, T. C. Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J. Surg. Res. 156, 205–212 (2009).
Speer, A. L., Sala, F. G., Matthews, J. A. & Grikscheit, T. C. Murine tissue-engineered stomach demonstrates epithelial differentiation. J. Surg. Res. 171, 6–14 (2011).
Spurrier, R. G., Speer, A. L., Hou, X., El-Nachef, W. N. & Grikscheit, T. C. Murine and human tissue-engineered esophagus form from sufficient stem/progenitor cells and do not require microdesigned biomaterials. Tissue Eng. Part A 21, 906–915 (2015).
Levin, D. E. et al. Human tissue-engineered small intestine forms from postnatal progenitor cells. J. Pediatr. Surg. 48, 129–137 (2013).
Grant, C. N. et al. Human and mouse tissue-engineered small intestine both demonstrate digestive and absorptive function. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G664–G677 (2015).
Yen, T.-H. & Wright, N. A. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2, 203–212 (2006).
DeWard, A. D., Cramer, J. & Lagasse, E. Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 9, 701–711 (2014).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Sato, T. et al. Single Lgr5 stem cells build crypt villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Yui, S. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 18, 618–623 (2012).
Watson, C. L. et al. An in vivo model of human small intestine using pluripotent stem cells. Nat. Med. 20, 1310–1314 (2014).
Takebe, T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013).
Takebe, T. et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 16, 556–565 (2015).
Taka-aki, K. N. et al. Generation of stomach tissue from mouse embryonic stem cells. Nat. Cell Biol. 17, 984–993 (2015).
Lee, M., Wu, B. M., Stelzner, M., Reichardt, H. M. & Dunn, J. C. Intestinal smooth muscle cell maintenance by basic fibroblast growth factor. Tissue Eng. Part A 14, 1395–1402 (2008).
Heise, R. L., Ivanova, J., Parekh, A. & Sacks, M. S. Generating elastin-rich small intestinal submucosa-based smooth muscle constructs utilizing exogenous growth factors and cyclic mechanical stimulation. Tissue Eng. Part A 15, 3951–3960 (2009).
Yazdani, S. K. et al. Smooth muscle cell seeding of decellularized scaffolds: the importance of bioreactor preconditioning to development of a more native architecture for tissue-engineered blood vessels. Tissue Eng. Part A 15, 827–840 (2009).
Rühl, A. Glial cells in the gut. Neurogastroenterol. Motil. 17, 777–790 (2005).
Bassotti, G., Villanacci, V., Antonelli, E., Morelli, A. & Salerni, B. Enteric glial cells: new players in gastrointestinal motility? Lab. Invest. 87, 628–632 (2007).
Sanders, K. M. & Ward, S. M. Interstitial cells of Cajal: a new perspective on smooth muscle function. J. Physiol. 576, 721–726 (2006).
Watters, D., Smith, A., Eastwood, M., Anderson, K. & Elton, R. Mechanical properties of the rat colon: the effect of age, sex and different conditions of storage. Q. J. Exp. Physiol. 70, 151–162 (1985).
Watters, D. et al. Mechanical properties of the colon: comparison of the features of the African and European colon in vitro. Gut 26, 384–392 (1985).
Sarkar, S., Salacinski, H., Hamilton, G. & Seifalian, A. The mechanical properties of infrainguinal vascular bypass grafts: their role in influencing patency. Eur. J. Vasc. Endovasc. Surg. 31, 627–636 (2006).
Lee, S. J. et al. Development of a composite vascular scaffolding system that withstands physiological vascular conditions. Biomaterials 29, 2891–2898 (2008).
Lovett, M., Lee, K., Edwards, A. & Kaplan, D. L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 15, 353–370 (2009).
Rouwkema, J., Rivron, N. C. & van Blitterswijk, C. A. Vascularization in tissue engineering. Trends Biotechnol. 26, 434–441 (2008).
Lee, K. Y., Peters, M. C., Anderson, K. W. & Mooney, D. J. Controlled growth factor release from synthetic extracellular matrices. Nature 408, 998–1000 (2000).
Ovsianikov, A. et al. Laser fabrication of three-dimensional CAD scaffolds from photosensitive gelatin for applications in tissue engineering. Biomacromolecules 12, 851–858 (2011).
Gauvin, R. et al. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 33, 3824–3834 (2012).
Pennathur, A., Gibson, M. K., Jobe, B. A. & Luketich, J. D. Oesophageal carcinoma. Lancet 381, 400–412 (2013).
Rustgi, A. K. & El-Serag, H. B. Esophageal carcinoma. N. Engl. J. Med. 371, 2499–2509 (2014).
Bickenbach, K. & Strong, V. E. Comparisons of gastric cancer treatments: East versus West. J. Gastric Cancer 12, 55–62 (2012).
Huscher, C. G. et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann. Surg. 241, 232 (2005).
Jones, K. L. Gastroparesis: prevalence, clinical significance and treatment. Can. J. Gastroenterol. 14, 805 (2001).
Peyret, B. et al. Prevalence of liver complications in children receiving long-term parenteral nutrition. Eur. J. Clin. Nutr. 65, 743–749 (2011).
Yamamoto, T. & Keighley, M. R. Proctocolectomy is associated with a higher complication rate but carries a lower recurrence rate than total colectomy and ileorectal anastomosis in Crohn colitis. Scand. J. Gastroenterol. 34, 1212–1215 (1999).
Sohn, G. et al. Surgical outcomes after total colectomy with ileorectal anastomosis in patients with medically intractable slow transit constipation. J. Kor. Soc. Coloproctol. 27, 180–187 (2011).
Baumgart, D. C. & Carding, S. R. Inflammatory bowel disease: cause and immunobiology. Lancet 369, 1627–1640 (2007).
O'Dwyer, R. H. et al. Clinical features and colonic motor disturbances in chronic megacolon in adults. Dig. Dis. Sci. 60, 2398–2407 (2015).
Topor, L., Ulici, A., Malureanu, D., Stoica, I. & Moga, A. Difficulties in the diagnostics and treatment of near-total congenital megacolon. Chirurgia (Bucur.) 109, 701–704 (2014).
Kouranloo, J., Sadeghian, N. & Monfared, M. K. Treatment and postoperative complication of 420 patients with congenital megacolon. Saudi Med. J. 24, S25–S28 (2003).
Bharucha, A. E. & Rao, S. S. An update on anorectal disorders for gastroenterologists. Gastroenterology 146, 37–45.e2 (2014).
Whitehead, W. E. et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology 137, 512–517.e2 (2009).
Van Koughnett, J. A. & Wexner, S. D. Current management of fecal incontinence: choosing amongst treatment options to optimize outcomes. World J. Gastroenterol. 19, 9216–9230 (2013).
Micci, M. A. et al. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology 129, 1817–1824 (2005).
Hotta, R. et al. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J. Clin. Invest. 0, 0–0 (2013).
Liu, W., Wu, R. D., Dong, Y. L. & Gao, Y. M. Neuroepithelial stem cells differentiate into neuronal phenotypes and improve intestinal motility recovery after transplantation in the aganglionic colon of the rat. Neurogastroenterol. Motil. 19, 1001–1009 (2007).
Metzger, M., Caldwell, C., Barlow, A. J., Burns, A. J. & Thapar, N. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology 136, 2214–2225.e3 (2009).
Acknowledgements
The work of the authors is supported by Army, Navy, NIH, Air Force, VA and Health Affairs to support the AFIRM II effort, under Award No. W81XWH-13-2-0052; GU 7 and NIH/NIDDK R01DK071614 and R42DK105593.
Author information
Authors and Affiliations
Contributions
K.N.B. and E.Z. researched data for article, contributed to discussion of content, wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bitar, K., Zakhem, E. Bioengineering the gut: future prospects of regenerative medicine. Nat Rev Gastroenterol Hepatol 13, 543–556 (2016). https://doi.org/10.1038/nrgastro.2016.124
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.124
This article is cited by
-
Regenerative medicine for anal incontinence: a review of regenerative therapies beyond cells
International Urogynecology Journal (2021)
-
Innervation: the missing link for biofabricated tissues and organs
npj Regenerative Medicine (2020)
-
3D Synthetic Peptide-based Architectures for the Engineering of the Enteric Nervous System
Scientific Reports (2019)
-
Regenerative medicine for the esophagus
Surgery Today (2018)
-
The extracellular matrix of the gastrointestinal tract: a regenerative medicine platform
Nature Reviews Gastroenterology & Hepatology (2017)