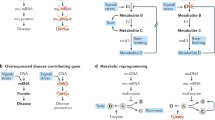
Abstract
RNA interference (RNAi) is a robust gene silencing mechanism that degrades mRNAs complementary to the antisense strands of double-stranded, short interfering RNAs (siRNAs). As a therapeutic strategy, RNAi has an advantage over small-molecule drugs, as virtually all genes are susceptible to targeting by siRNA molecules. This advantage is, however, counterbalanced by the daunting challenge of achieving safe, effective delivery of oligonucleotides to specific tissues in vivo. Lipid-based carriers of siRNA therapeutics can now target the liver in metabolic diseases and are being assessed in clinical trials for the treatment of hypercholesterolemia. For this indication, a chemically modified oligonucleotide that targets endogenous small RNA modulators of gene expression (microRNAs) is also under investigation in clinical trials. Emerging 'self-delivery' siRNAs that are covalently linked to lipophilic moieties show promise for the future development of therapies. Besides the liver, inflammation of the adipose tissue in patients with obesity and type 2 diabetes mellitus may be an attractive target for siRNA therapeutics. Administration of siRNAs encapsulated within glucan microspheres can silence genes in inflammatory phagocytic cells, as can certain lipid-based carriers of siRNA. New technologies that combine siRNA molecules with antibodies or other targeting molecules also appear encouraging. Although still at an early stage, the emergence of RNAi-based therapeutics has the potential to markedly influence our clinical future.
Key Points
-
RNA interference (RNAi) represents a therapeutic approach for targeting any expressed gene with a high degree of specificity
-
Chemical modifications of siRNAs enhance their potency, stability and efficacy, while reducing immunostimulatory effects
-
Targeting hepatic lipid synthesis with RNAi can alleviate hyperlipidemia and hepatic steatosis in patients with the metabolic syndrome
-
Clinical trials have shown that liposomal RNAi delivery systems are efficacious at lowering serum lipid profiles
-
The metabolic syndrome in patients with obesity is associated with adipose tissue inflammation
-
Targeting inflammation with RNAi may offer an exciting but challenging goal for the treatment of metabolic disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jornayvaz, F. R., Samuel, V. T. & Shulman, G. I. The role of muscle insulin resistance in the pathogenesis of atherogenic dyslipidemia and nonalcoholic fatty liver disease associated with the metabolic syndrome. Annu. Rev. Nutr. 30, 273–290 (2010).
Reaven, G. M. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu. Rev. Nutr. 25, 391–406 (2005).
Guilherme, A., Virbasius, J. V., Puri, V. & Czech, M. P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 367–377 (2008).
Ergens, R. & Yukhimenko, S. S. Gyrodactylus somnaensis sp. n (Monogenea: Gyrodactylidae), a new fish parasite from the basin of the River Amur. Folia Parasitol. (Praha) 37, 313–314 (1990).
Zheng, C. J. et al. Therapeutic targets: progress of their exploration and investigation of their characteristics. Pharmacol. Rev. 58, 259–279 (2006).
De, A. & DiMarchi, R. D. Synthesis and characterization of ester-based prodrugs of glucagon-like peptide 1. Biopolymers 94, 448–456 (2010).
Mello, C. C. & Conte, D. Jr. Revealing the world of RNA interference. Nature 431, 338–342 (2004).
Ghildiyal, M. & Zamore, P. D. Small silencing RNAs: an expanding universe. Nat. Rev. Genet. 10, 94–108 (2009).
Ambros, V. The functions of animal microRNAs. Nature 431, 350–355 (2004).
Guo, H., Ingolia, N. T., Weissman, J. S. & Bartel, D. P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840 (2010).
Bumcrot, D., Manoharan, M., Koteliansky, V. & Sah, D. W. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat. Chem. Biol. 2, 711–719 (2006).
Shabtai, M., Waltzer, W. C., Anaise, D., Miller, F. & Rapaport, F. T. Implication of IgA and complement in the alterations in renal blood flow associated with allograft rejection. Transplant Proc. 21, 352–353 (1989).
Wolfrum, C. et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 25, 1149–1157 (2007).
Sioud, M. Recent advances in small interfering RNA sensing by the immune system. N. Biotechnol. 27, 236–242 (2010).
Kleinman, M. E. et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 452, 591–597 (2008).
Castanotto, D. & Rossi, J. J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 457, 426–433 (2009).
Tiemann, K. & Rossi, J. J. RNAi-based therapeutics-current status, challenges and prospects. EMBO Mol. Med. 1, 142–151 (2009).
Jackson, A. L. & Linsley, P. S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 9, 57–67 (2010).
Vaishnaw, A. K. et al. A status report on RNAi therapeutics. Silence 1, 14 (2010).
Zimmermann, T. S. et al. RNAi-mediated gene silencing in non-human primates. Nature 441, 111–114 (2006).
Semple, S. C. et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 28, 172–176 (2010).
Schroeder, A., Levins, C. G., Cortez, C., Langer, R. & Anderson, D. G. Lipid-based nanotherapeutics for siRNA delivery. J. Intern. Med. 267, 9–21 (2010).
Rozema, D. B. et al. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc. Natl Acad. Sci. USA 104, 12982–12987 (2007).
Sato, A., Takagi, M., Shimamoto, A., Kawakami, S. & Hashida, M. Small interfering RNA delivery to the liver by intravenous administration of galactosylated cationic liposomes in mice. Biomaterials 28, 1434–1442 (2007).
Morrissey, D. V. et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 23, 1002–1007 (2005).
Nishina, K. et al. Efficient in vivo delivery of siRNA to the liver by conjugation of alpha-tocopherol. Mol. Ther. 16, 734–740 (2008).
Love, K. T. et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl Acad. Sci. USA 107, 1864–1869 (2010).
McCaffrey, A. P. et al. RNA interference in adult mice. Nature 418, 38–39 (2002).
Lewis, D. L., Hagstrom, J. E., Loomis, A. G., Wolff, J. A. & Herweijer, H. Efficient delivery of siRNA for inhibition of gene expression in postnatal mice. Nat. Genet. 32, 107–108 (2002).
Gomez-Valades, A. G. et al. Pck1 gene silencing in the liver improves glycemia control, insulin sensitivity, and dyslipidemia in db/db mice. Diabetes 57, 2199–2210 (2008).
Judge, A. D., Bola, G., Lee, A. C. & MacLachlan, I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol. Ther. 13, 494–505 (2006).
Kim, S. I. et al. Systemic and specific delivery of small interfering RNAs to the liver mediated by apolipoprotein A-I. Mol. Ther. 15, 1145–1152 (2007).
Giladi, H. et al. Small interfering RNA inhibits hepatitis B virus replication in mice. Mol. Ther. 8, 769–776 (2003).
Frank-Kamenetsky, M. et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl Acad. Sci. USA 105, 11915–11920 (2008).
Akinc, A. et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 26, 561–569 (2008).
Akinc, A. et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 18, 1357–1364 (2010).
Akinc, A. et al. Development of lipidoid-siRNA formulations for systemic delivery to the liver. Mol. Ther. 17, 872–879 (2009).
Watts, J. K., Deleavey, G. F. & Damha, M. J. Chemically modified siRNA: tools and applications. Drug Discov. Today 13, 842–855 (2008).
Behlke, M. A. Chemical modification of siRNAs for in vivo use. Oligonucleotides 18, 305–319 (2008).
Chernolovskaya, E. L. & Zenkova, M. A. Chemical modification of siRNA. Curr. Opin. Mol. Ther. 12, 158–167 (2010).
Chiu, Y. L. & Rana, T. M. siRNA function in RNAi: a chemical modification analysis. RNA 9, 1034–1048 (2003).
Allerson, C. R. et al. Fully 2′-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J. Med. Chem. 48, 901–904 (2005).
Judge, A. & MacLachlan, I. Overcoming the innate immune response to small interfering RNA. Hum. Gene Ther. 19, 111–124 (2008).
Veedu, R. N. & Wengel, J. Locked nucleic acids: promising nucleic acid analogs for therapeutic applications. Chem. Biodivers. 7, 536–542 (2010).
Gaglione, M. & Messere, A. Recent progress in chemically modified siRNAs. Mini Rev. Med. Chem. 10, 578–595 (2010).
Bennett, C. F. & Swayze, E. E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 50, 259–293 (2010).
Elmen, J. et al. LNA-mediated microRNA silencing in non-human primates. Nature 452, 896–899 (2008).
Krutzfeldt, J. et al. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 35, 2885–2892 (2007).
Esau, C. C. Inhibition of microRNA with antisense oligonucleotides. Methods 44, 55–60 (2008).
Visser, M. E., Kastelein, J. J. & Stroes, E. S. Apolipoprotein B synthesis inhibition: results from clinical trials. Curr. Opin. Lipidol. 21, 319–323 (2010).
Unger, R. H. & Orci, L. Paracrinology of islets and the paracrinopathy of diabetes. Proc. Natl Acad. Sci. USA 107, 16009–16012 (2010).
Cheung, N., Mitchell, P. & Wong, T. Y. Diabetic retinopathy. Lancet 376, 124–136 (2010).
Boulton, A. J. What you can't feel can hurt you. J. Am. Podiatr. Med. Assoc. 100, 349–352 (2010).
Wu, S. C., Marston, W. & Armstrong, D. G. Wound care: the role of advanced wound-healing technologies. J. Am. Podiatr. Med. Assoc. 100, 385–394 (2010).
van Dieren, S., Beulens, J. W., van der Schouw, Y. T., Grobbee, D. E. & Neal, B. The global burden of diabetes and its complications: an emerging pandemic. Eur. J. Cardiovasc. Prev. Rehabil. 17 (Suppl. 1), S3–S8 (2010).
Postic, C. & Girard, J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 34, 643–648 (2008).
Brown, M. S. & Goldstein, J. L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 (2008).
Abdelmalek, M. F. & Diehl, A. M. Nonalcoholic fatty liver disease as a complication of insulin resistance. Med. Clin. North Am. 91, 1125–1149, ix (2007).
Brown, M. S. & Goldstein, J. L. A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34–47 (1986).
Baigude, H., McCarroll, J., Yang, C. S., Swain, P. M. & Rana, T. M. Design and creation of new nanomaterials for therapeutic RNAi. ACS Chem. Biol. 2, 237–241 (2007).
Seidah, N. G. et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc. Natl Acad. Sci. USA 100, 928–933 (2003).
Bassi, D. E., Fu, J., Lopez de Cicco, R. & Klein-Szanto, A. J. Proprotein convertases: “master switches” in the regulation of tumor growth and progression. Mol. Carcinog. 44, 151–161 (2005).
Horton, J. D., Cohen, J. C. & Hobbs, H. H. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem. Sci. 32, 71–77 (2007).
Allard, D. et al. Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Hum. Mutat. 26, 497 (2005).
Abifadel, M. et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34, 154–156 (2003).
Rashid, S. et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl Acad. Sci. USA 102, 5374–5379 (2005).
Graham, M. J. et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J. Lipid Res. 48, 763–767 (2007).
Capeau, J. Insulin resistance and steatosis in humans. Diabetes Metab. 34, 649–657 (2008).
Meshkani, R. & Adeli, K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin. Biochem. 42, 1331–1346 (2009).
Fabbrini, E., Sullivan, S. & Klein, S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51, 679–689 (2010).
Liang, G. et al. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J. Biol. Chem. 277, 9520–9528 (2002).
Iizuka, K., Bruick, R. K., Liang, G., Horton, J. D. & Uyeda, K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl Acad. Sci. USA 101, 7281–7286 (2004).
Moore, K. J., Rayner, K. J., Suárez, Y. & Fernández-Hernando, C. microRNAs and cholesterol metabolism. Trends Endocrinol. Metab. 21, 699–706 (2010).
Lagos-Quintana, M. et al. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 12, 735–739 (2002).
Xu, H. et al. Liver-enriched transcription factors regulate microRNA-122 that targets CUTL1 during liver development. Hepatology 52, 1431–1442 (2010).
Esau, C. et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 3, 87–98 (2006).
Elmén, J. et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 36, 1153–1162 (2008).
Krützfeldt, J. et al. Silencing of microRNAs in vivo with 'antagomirs'. Nature 438, 685–689 (2005).
Gómez-Valadés, A. G. et al. Overcoming diabetes-induced hyperglycemia through inhibition of hepatic phosphoenolpyruvate carboxykinase (GTP) with RNAi. Mol. Ther. 13, 401–410 (2006).
Bosi, E. Metformin—the gold standard in type 2 diabetes: what does the evidence tell us? Diabetes Obes. Metab. 11 (Suppl. 2), 3–8 (2009).
Elbashir, S. M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
McCaffrey, A. P. et al. Inhibition of hepatitis B virus in mice by RNA interference. Nat.Biotechnol. 21, 639–644 (2003).
Song, E. et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat. Med. 9, 347–351 (2003).
Soutschek, J. et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432, 173–178 (2004).
Gao, S. et al. The effect of chemical modification and nanoparticle formulation on stability and biodistribution of siRNA in mice. Mol. Ther. 17, 1225–1233 (2009).
Weisman, S., Hirsch-Lerner, D., Barenholz, Y. & Talmon, Y. Nanostructure of cationic lipid-oligonucleotide complexes. Biophys. J. 87, 609–614 (2004).
Torchilin, V. P. et al. Poly(ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevity. Biochim. Biophys. Acta 1195, 11–20 (1994).
Hafez, I. M., Maurer, N. & Cullis, P. R. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 8, 1188–1196 (2001).
Xu, Y. & Szoka, F. C. Jr. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry 35, 5616–5623 (1996).
Zelphati, O. & Szoka, F. C. Jr. Mechanism of oligonucleotide release from cationic liposomes. Proc. Natl Acad. Sci. USA 93, 11493–11498 (1996).
Torchilin, V. P. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu. Rev. Biomed. Eng. 8, 343–375 (2006).
Malek, A. et al. In vivo pharmacokinetics, tissue distribution and underlying mechanisms of various PEI(-PEG)/siRNA complexes. Toxicol. Appl. Pharmacol. 236, 97–108 (2009).
Lanford, R. E. et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327, 198–201 (2010).
Hardy, O. T. et al. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg. Obes. Relat. Dis. 7, 60–67 (2011).
Winer, S. et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 15, 921–929 (2009).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009).
Liu, J. et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 15, 940–945 (2009).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Wentworth, J. M. et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes 59, 1648–1656 (2010).
Heilbronn, L. K. & Campbell, L. V. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr. Pharm. Des 14, 1225–1230 (2008).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Halberg, N., Wernstedt-Asterholm, I. & Scherer, P. E. The adipocyte as an endocrine cell. Endocrinol. Metab. Clin. North Am. 37, 753–768 (2008).
Scherer, P. E. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 55, 1537–1545 (2006).
Wang, P., Mariman, E., Renes, J. & Keijer, J. The secretory function of adipocytes in the physiology of white adipose tissue. J. Cell Physiol. 216, 3–13 (2008).
Kamei, N. et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 281, 26602–26614 (2006).
Kanda, H. et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 (2006).
Kirk, E. A., Sagawa, Z. K., McDonald, T. O., O'Brien, K. D. & Heinecke, J. W. Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue [corrected]. Diabetes 57, 1254–1261 (2008).
Inouye, K. E. et al. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes 56, 2242–2250 (2007).
Yuan, M. et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 293, 1673–1677 (2001).
Goldfine, A. B. et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin. Transl. Sci. 1, 36–43 (2008).
Hundal, R. S. et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J. Clin. Invest. 109, 1321–1326 (2002).
Larsen, C. M. et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 (2007).
Dinarello, C. A. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N. Engl. J. Med. 343, 732–734 (2000).
Barbuio, R., Milanski, M., Bertolo, M. B., Saad, M. J. & Velloso, L. A. Infliximab reverses steatosis and improves insulin signal transduction in liver of rats fed a high-fat diet. J. Endocrinol. 194, 539–550 (2007).
Hotamisligil, G. S., Arner, P., Caro, J. F., Atkinson, R. L. & Spiegelman, B. M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 95, 2409–2415 (1995).
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 (1993).
Ofei, F., Hurel, S., Newkirk, J., Sopwith, M. & Taylor, R. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 45, 881–885 (1996).
Bernstein, L. E., Berry, J., Kim, S., Canavan, B. & Grinspoon, S. K. Effects of etanercept in patients with the metabolic syndrome. Arch. Intern. Med. 166, 902–908 (2006).
Dominguez, H. et al. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J. Vasc. Res. 42, 517–525 (2005).
Stanley, T. L. et al. TNF-α antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J. Clin. Endocrinol. Metab. 96, E146–E150 (2011).
Choi, B. et al. Tumor necrosis factor α small interfering RNA decreases herpes simplex virus-induced inflammation in a mouse model. J. Dermatol. Sci. 52, 87–97 (2008).
Nau, G. J. et al. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc. Natl Acad. Sci. USA 94, 6414–6419 (1997).
Giachelli, C. M., Lombardi, D., Johnson, R. J., Murry, C. E. & Almeida, M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am. J. Pathol. 152, 353–358 (1998).
Ashkar, S. et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287, 860–864 (2000).
Bruemmer, D. et al. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J. Clin. Invest. 112, 1318–1331 (2003).
Nomiyama, T. et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J. Clin. Invest. 117, 2877–2888 (2007).
Kaneider, N. C., Leger, A. J. & Kuliopulos, A. Therapeutic targeting of molecules involved in leukocyte-endothelial cell interactions. FEBS J. 273, 4416–4424 (2006).
Feral, C. C. et al. Blocking the α4 integrin-paxillin interaction selectively impairs mononuclear leukocyte recruitment to an inflammatory site. J. Clin. Invest. 116, 715–723 (2006).
Ghosh, S. et al. Natalizumab for active Crohn's disease. N. Engl. J. Med. 348, 24–32 (2003).
Miller, D. H. et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 348, 15–23 (2003).
Sheridan, C. Third Tysabri adverse case hits drug class. Nat. Rev. Drug Discov. 24, 357–358 (2005).
Sheridan, C. Tysabri raises alarm bells on drug class. Nat. Biotechnol. 23, 397–398 (2005).
Herre, J., Gordon, S. & Brown, G. D. Dectin-1 and its role in the recognition of beta-glucans by macrophages. Mol. Immunol. 40, 869–876 (2004).
Aouadi, M. et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 458, 1180–1184 (2009).
Tesz, G. J. et al. Glucan particles for selective delivery of siRNA to phagocytic cells in mice. Biochem. J. doi: 10.1042/BJ20110352.
Khoury, M. et al. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor alpha in experimental arthritis. Arthritis Rheum. 54, 1867–1877 (2006).
Zheng, X., Vladau, C., Shunner, A. & Min, W. P. siRNA specific delivery system for targeting dendritic cells. Methods Mol. Biol. 623, 173–188 (2010).
Zheng, X. et al. A novel in vivo siRNA delivery system specifically targeting dendritic cells and silencing CD40 genes for immunomodulation. Blood 113, 2646–2654 (2009).
Lee, S., Yang, S. C., Kao, C. Y., Pierce, R. H. & Murthy, N. Solid polymeric microparticles enhance the delivery of siRNA to macrophages in vivo. Nucleic Acids Res. 37, e145 (2009).
Alshamsan, A. et al. STAT3 silencing in dendritic cells by siRNA polyplexes encapsulated in PLGA nanoparticles for the modulation of anticancer immune response. Mol. Pharm. doi: 10.1021/mp100067u.
Shukla, A. K., Verma, M. & Singh, K. N. Superoxide induced deprotection of 1,3-dithiolanes: a convenient method of dedithioacetalization. Indian J. Chem. 43B, 1748–1752 (2004).
Lih-Brody, L. et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig. Dis. Sci. 41, 2078–2086 (1996).
Brunner, T., Cohen, S. & Monsonego, A. Silencing of proinflammatory genes targeted to peritoneal-residing macrophages using siRNA encapsulated in biodegradable microspheres. Biomaterials 31, 2627–2636 (2010).
Kumar, P. et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell 134, 577–586 (2008).
Song, E. et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat. Biotechnol. 23, 709–717 (2005).
Peer, D., Zhu, P., Carman, C. V., Lieberman, J. & Shimaoka, M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proc. Natl Acad. Sci. USA 104, 4095–4100 (2007).
Kim, S. S. et al. Targeted delivery of siRNA to macrophages for anti-inflammatory treatment. Mol. Ther. 18, 993–1001 (2010).
Subramanya, S. et al. Targeted delivery of small interfering RNA to human dendritic cells to suppress dengue virus infection and associated proinflammatory cytokine production. J. Virol. 84, 2490–2501 (2010).
Nishimura, S. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 (2009).
Wellen, K. E. & Hotamisligil, G. S. Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119 (2005).
Asano, M., Toda, M., Sakaguchi, N. & Sakaguchi, S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184, 387–396 (1996).
Ilan, Y. et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc. Natl Acad. Sci. USA 107, 9765–9770 (2010).
Peipp, M. et al. A recombinant CD7-specific single-chain immunotoxin is a potent inducer of apoptosis in acute leukemic T cells. Cancer Res. 62, 2848–2855 (2002).
Bremer, E. et al. Target cell-restricted apoptosis induction of acute leukemic T cells by a recombinant tumor necrosis factor-related apoptosis-inducing ligand fusion protein with specificity for human CD7. Cancer Res. 65, 3380–3388 (2005).
Frankel, A. E. et al. Therapy of patients with T-cell lymphomas and leukemias using an anti-CD7 monoclonal antibody-ricin A chain immunotoxin. Leuk. Lymphoma 26, 287–298 (1997).
Lazarovits, A. I. et al. Human mouse chimeric CD7 monoclonal antibody (SDZCHH380) for the prophylaxis of kidney transplant rejection. Transplant Proc. 25, 820–822 (1993).
Peer, D., Park, E. J., Morishita, Y., Carman, C. V. & Shimaoka, M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science 319, 627–630 (2008).
Kim, S. S. et al. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol. Ther. 18, 370–376 (2010).
Kortylewski, M. et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat. Biotechnol. 27, 925–932 (2009).
Herrmann, A. et al. Targeting Stat3 in the myeloid compartment drastically improves the in vivo antitumor functions of adoptively transferred T cells. Cancer Res. 70, 7455–7464 (2010).
Kortylewski, M. et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 11, 1314–1321 (2005).
Davis, M. E. et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464, 1067–1070 (2010).
Brahmamdam, P. et al. Targeted delivery of siRNA to cell death proteins in sepsis. Shock 32, 131–139 (2009).
Newgard, C. B., Brady, M. J., O'Doherty, R. M. & Saltiel, A. R. Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes 49, 1967–1977 (2000).
Gross, D. N., van den Heuvel, A. P. & Birnbaum, M. J. The role of FoxO in the regulation of metabolism. Oncogene 27, 2320–2336 (2008).
Yamashita, H. et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl Acad. Sci. USA 98, 9116–9121 (2001).
Uyeda, K. & Repa, J. J. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 4, 107–110 (2006).
Cheloufi, S., Dos Santos, C. O., Chong, M. M. & Hannon, G. J. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 465, 584–589 (2010).
Cifuentes, D. et al. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science 328, 1694–1698 (2010).
Nowotny, M. & Yang, W. Structural and functional modules in RNA interference. Curr. Opin. Struct. Biol. 19, 286–293 (2009).
Naqvi, A. R., Islam, M. N., Choudhury, N. R. & Haq, Q. M. The fascinating world of RNA interference. Int. J. Biol. Sci. 5, 97–117 (2009).
Acknowledgements
The authors thank the members of their laboratory group for excellent discussions on the issues addressed in this Review. The studies from the authors' laboratory covered in this Review were supported by grants to M. P. Czech from the NIH (DK30898 and DK085753), a Juvenile Diabetes Research Foundation Award (17-2009-546) and by Core Facilities in the University of Massachusetts Diabetes and Endocrinology Research Center also funded by the NIH (DK325220).
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, provided a substantial contribution to discussions of the content, contributed equally to writing the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M. P. Czech declares an association with the following company: RXi Pharmaceuticals (stockholder/director, patent holder). M. Aouadi declares an association with the following company: RXi Pharmaceuticals (patent holder), G. J. Tesz declares no competing interests.
Rights and permissions
About this article
Cite this article
Czech, M., Aouadi, M. & Tesz, G. RNAi-based therapeutic strategies for metabolic disease. Nat Rev Endocrinol 7, 473–484 (2011). https://doi.org/10.1038/nrendo.2011.57
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2011.57
This article is cited by
-
A novel Anti-ROS osteoblast-specific delivery system for ankylosing spondylitis treatment via suppression of both inflammation and pathological new bone formation
Journal of Nanobiotechnology (2023)
-
Development of elastin-like polypeptide for targeted specific gene delivery in vivo
Journal of Nanobiotechnology (2020)
-
GALNT2 as a novel modulator of adipogenesis and adipocyte insulin signaling
International Journal of Obesity (2019)
-
MicroRNA regulatory networks in human adipose tissue and obesity
Nature Reviews Endocrinology (2015)
-
Managing diabetes with nanomedicine: challenges and opportunities
Nature Reviews Drug Discovery (2015)