Key Points
-
Potent androgen receptor (AR)-targeted therapies have increased survival rates for metastatic castration-resistant prostate cancer (mCRPC), but correlate with the emergence of 'treatment-induced lineage crisis' characterized by visceral and bulky metastases and low PSA secretion
-
In prostate cancer, lineage crisis can occur either in the form of treatment-induced neuroendocrine differentiation, which results in a neuroendocrine phenotype, or in the form of treatment-induced epithelial-to-mesenchymal transition
-
Regardless of the mechanism responsible for lineage crisis, a proposed common checkpoint that precedes such crisis is the loss of expression and/or activity of AR pathway (AR-lo prostate cancer)
-
Drug-cycling designs used to prevent multidrug resistance (or 'superbugs') in infectious diseases might delay treatment-induced lineage crisis in prostate cancer, owing to the partial similarities between both phenomena
-
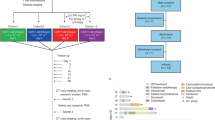
The PRINT protocol is a phase II trial designed to alternate administration of FDA-approved drugs in rapid cycles of 3 months to prevent treatment-induced lineage crisis for mCRPC, which might provide a rationale for testing drug cycling in the setting of first-line treatment for mCRPC
-
Collateral sensitivity might result in increased cytotoxic effects compared with standard approaches for mCRPC (a heterogeneous disease); this treatment strategy uses synergistic drug pairs because drug resistance results in competitive fitness
Abstract
The increasing potency of therapies that target the androgen receptor (AR) signalling axis has correlated with a rise in the proportion of patients with prostate cancer harbouring an adaptive phenotype, termed treatment-induced lineage crisis. This phenotype is characterized by features that include soft-tissue metastasis and/or resistance to standard anticancer therapies. Potent anticancer treatments might force cancer cells to evolve and develop alternative cell lineages that are resistant to primary therapies, a mechanism similar to the generation of multidrug- resistant microorganisms after continued antibiotic use. Herein, we assess the hypothesis that treatment-adapted phenotypes harbour reduced AR expression and/or activity, and acquire compensatory strategies for cell survival. We highlight the striking similarities between castration-resistant prostate cancer and triple-negative breast cancer, another poorly differentiated endocrine malignancy. Alternative treatment paradigms are needed to avoid therapy-induced resistance. Herein, we present a new clinical trial strategy designed to evaluate the potential of rapid drug cycling as an approach to delay the onset of resistance and treatment-induced lineage crisis in patients with metastatic castration-resistant prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Beltran, H. et al. Challenges in recognizing treatment-related neuroendocrine prostate cancer. J. Clin. Oncol. 30, e386–e389 (2012).
Sun, Y. et al. Androgen deprivation causes epithelial–mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res. 72, 527–536 (2012).
Martin, S. K. et al. Multinucleation and mesenchymal-to-epithelial transition alleviate resistance to combined cabazitaxel and antiandrogen therapy in advanced prostate cancer. Cancer Res. 76, 912–926 (2016).
Aparicio, A. M. et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin. Cancer Res. 19, 3621–3630 (2013).
Aparicio, A. & Tzelepi, V. Neuroendocrine (small-cell) carcinomas: why they teach us essential lessons about prostate cancer. Oncology (Williston Park) 28, 831–838 (2014).
Beltran, H. et al. Aggressive variants of castration-resistant prostate cancer. Clin. Cancer Res. 20, 2846–2850 (2014).
Wang, H. T. et al. Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis — a systematic review and pooled analysis. J. Clin. Oncol. 32, 3383–3390 (2014).
Tsao, C. K., Galsky, M. D. & Oh, W. K. Is metastatic prostate cancer changing, and how will we know it? It's time for standard nomenclature for nonosseous metastases in clinical trials of patients with metastatic castration resistant prostate cancer. Eur. Urol. 66, 184–185 (2014).
Berglund, R. K. et al. Comparison of observed biochemical recurrence-free survival in patients with low PSA values undergoing radical prostatectomy and predictions of preoperative nomogram. Urology 73, 1098–1103 (2009).
McGuire, B. B. et al. Outcomes in patients with Gleason score 8–10 prostate cancer: relation to preoperative PSA level. BJU Int. 109, 1764–1769 (2012).
Beltran, H. et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 22, 298–305 (2016).
Mahal, B. A., Aizer, A. A., Efstathiou, J. A. & Nguyen, P. L. Association of very low prostate-specific antigen levels with increased cancer-specific death in men with high-grade prostate cancer. Cancer 122, 78–83 (2016).
Pezaro, C. J. et al. Visceral disease in castration-resistant prostate cancer. Eur. Urol. 65, 270–273 (2014).
Small, E. J. et al. Characterization of neuroendocrine prostate cancer (NEPC) in patients with metastatic castration resistant prostate cancer (mCRPC) resistant to abiraterone (Abi) or enzalutamide (Enz): Preliminary results from the SU2C/PCF/AACR West Coast Prostate Cancer Dream Team (WCDT) [abstract]. J. Clin. Oncol. 33 (Suppl.), 5003 (2015).
Bianchini, G., Balko, J. M., Mayer, I. A., Sanders, M. E. & Gianni, L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 13, 674–690 (2016).
Terry, S. & Beltran, H. The many faces of neuroendocrine differentiation in prostate cancer progression. Front. Oncol. 4, 60 (2014).
Dolgin, E. 'Game changer' antibiotic and others in works for superbug. Nat. Med. 17, 10 (2011).
Lowy, F. D. Secrets of a superbug. Nat. Med. 13, 1418–1420 (2007).
US National Library of Medicine. ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT02903160?term=NCT02903160&rank=1 (2016)
Nikaido, H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 78, 119–146 (2009).
Niederman, M. S. Appropriate use of antimicrobial agents: challenges and strategies for improvement. Crit. Care Med. 31, 608–616 (2003).
Gillessen, S. et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann. Oncol. 26, 1589–1604 (2015).
Tomlins, S. A. et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci. Transl Med. 3, 94ra72 (2011).
Beltran, H. et al. The initial detection and partial characterization of circulating tumor cells in neuroendocrine prostate cancer. Clin. Cancer Res. 22, 1510–1519 (2016).
Simanainen, U. et al. Disruption of prostate epithelial androgen receptor impedes prostate lobe-specific growth and function. Endocrinology 148, 2264–2272 (2007).
Vander Griend, D. J., Litvinov, I. V. & Isaacs, J. T. Conversion of androgen receptor signaling from a growth suppressor in normal prostate epithelial cells to an oncogene in prostate cancer cells involves a gain of function in c-Myc regulation. Int. J. Biol. Sci. 10, 627–642 (2014).
Wu, C. T. et al. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc. Natl Acad. Sci. USA 104, 12679–12684 (2007).
Xin, L. et al. Progression of prostate cancer by synergy of AKT with genotropic and nongenotropic actions of the androgen receptor. Proc. Natl Acad. Sci. USA 103, 7789–7794 (2006).
Cunha, G. R., Chung, L. W., Shannon, J. M., Taguchi, O. & Fujii, H. Hormone-induced morphogenesis and growth: role of mesenchymal–epithelial interactions. Recent Prog. Horm. Res. 39, 559–598 (1983).
Niu, Y. et al. Differential androgen receptor signals in different cells explain why androgen-deprivation therapy of prostate cancer fails. Oncogene 29, 3593–3604 (2010).
Scher, H. I. et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 26, 1148–1159 (2008).
Scher, H. I., Morris, M. J., Basch, E. & Heller, G. End points and outcomes in castration-resistant prostate cancer: from clinical trials to clinical practice. J. Clin. Oncol. 29, 3695–3704 (2011).
Huang, J. et al. Immunohistochemical characterization of neuroendocrine cells in prostate cancer. Prostate 66, 1399–1406 (2006).
Fleischmann, A. et al. Androgen receptors are differentially expressed in Gleason patterns of prostate cancer and down-regulated in matched lymph node metastases. Prostate 71, 453–460 (2011).
Pouessel, D. et al. Liver metastases in prostate carcinoma: clinical characteristics and outcome. BJU Int. 99, 807–811 (2007).
Falchook, A. D. et al. Stage at presentation and survival outcomes of patients with Gleason 8–10 prostate cancer and low prostate-specific antigen. Urol. Oncol. 34, 119.e19–119.e26 (2016).
Cinar, B. et al. Androgen receptor mediates the reduced tumor growth, enhanced androgen responsiveness, and selected target gene transactivation in a human prostate cancer cell line. Cancer Res. 61, 7310–7317 (2001).
Bonaccorsi, L. et al. Androgen receptor expression in prostate carcinoma cells suppresses α6β4 integrin-mediated invasive phenotype. Endocrinology 141, 3172–3182 (2000).
Chuu, C. P., Hiipakka, R. A., Fukuchi, J., Kokontis, J. M. & Liao, S. Androgen causes growth suppression and reversion of androgen-independent prostate cancer xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res. 65, 2082–2084 (2005).
Moehren, U. et al. Wild-type but not mutant androgen receptor inhibits expression of the hTERT telomerase subunit: a novel role of AR mutation for prostate cancer development. FASEB J. 22, 1258–1267 (2008).
Akashi, T., Koizumi, K., Nagakawa, O., Fuse, H. & Saiki, I. Androgen receptor negatively influences the expression of chemokine receptors (CXCR4, CCR1) and ligand-mediated migration in prostate cancer DU-145. Oncol. Rep. 16, 831–836 (2006).
Joly-Pharaboz, M. O. et al. Inhibition of growth and induction of apoptosis by androgens of a variant of LNCaP cell line. J. Steroid Biochem. Mol. Biol. 73, 237–249 (2000).
Cheng, H., Snoek, R., Ghaidi, F., Cox, M. E. & Rennie, P. S. Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res. 66, 10613–10620 (2006).
Qin, J. et al. The PSA−/lo prostate cancer cell population harbors self-renewing long-term tumor-propagating cells that resist castration. Cell Stem Cell 10, 556–569 (2012).
Mucci, N. R., Akdas, G., Manely, S. & Rubin, M. A. Neuroendocrine expression in metastatic prostate cancer: evaluation of high throughput tissue microarrays to detect heterogeneous protein expression. Hum. Pathol. 31, 406–414 (2000).
Shah, R. B. et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 64, 9209–9216 (2004).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015).
Mendiratta, P. et al. Genomic strategy for targeting therapy in castration-resistant prostate cancer. J. Clin. Oncol. 27, 2022–2029 (2009).
Sharma, N. L. et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell 23, 35–47 (2013).
Tomlins, S. A. et al. Integrative molecular concept modeling of prostate cancer progression. Nat. Genet. 39, 41–51 (2007).
Wang, Q. et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 138, 245–256 (2009).
van Soest, R. J. et al. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. Eur. J. Cancer 49, 3821–3830 (2013).
Zhu, M. L. et al. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 70, 7992–8002 (2010).
Mezynski, J. et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann. Oncol. 23, 2943–2947 (2012).
Schweizer, M. T. et al. The influence of prior abiraterone treatment on the clinical activity of docetaxel in men with metastatic castration-resistant prostate cancer. Eur. Urol. 66, 646–652 (2014).
de Bono, J. S. et al. Subsequent chemotherapy and treatment patterns after abiraterone acetate in patients with metastatic castration-resistant prostate cancer: post hoc analysis of COU-AA-302. Eur. Urol. http://dx.doi.org/10.1016/j.eururo.2016.06.033 (2016).
Chakraborty, P. S. et al. Metastatic poorly differentiated prostatic carcinoma with neuroendocrine differentiation: negative on 68Ga-PSMA PET/CT. Clin. Nucl. Med. 40, e163–e166 (2015).
Sheridan, T., Herawi, M., Epstein, J. I. & Illei, P. B. The role of P501S and PSA in the diagnosis of metastatic adenocarcinoma of the prostate. Am. J. Surg. Pathol. 31, 1351–1355 (2007).
Schelling, L. A. et al. Frequent TMPRSS2-ERG rearrangement in prostatic small cell carcinoma detected by fluorescence in situ hybridization: the superiority of fluorescence in situ hybridization over ERG immunohistochemistry. Hum. Pathol. 44, 2227–2233 (2013).
Mosquera, J. M. et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia 15, 1–10 (2013).
Williamson, S. R. et al. ERG-TMPRSS2 rearrangement is shared by concurrent prostatic adenocarcinoma and prostatic small cell carcinoma and absent in small cell carcinoma of the urinary bladder: evidence supporting monoclonal origin. Mod. Pathol. 24, 1120–1127 (2011).
Lotan, T. L. et al. ERG gene rearrangements are common in prostatic small cell carcinomas. Mod. Pathol. 24, 820–828 (2011).
Guo, C. C. et al. TMPRSS2-ERG gene fusion in small cell carcinoma of the prostate. Hum. Pathol. 42, 11–17 (2011).
Mulholland, D. J. et al. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 72, 1878–1889 (2012).
Ruscetti, M., Quach, B., Dadashian, E. L., Mulholland, D. J. & Wu, H. Tracking and functional characterization of epithelial–mesenchymal transition and mesenchymal tumor cells during prostate cancer metastasis. Cancer Res. 75, 2749–2759 (2015).
Ruscetti, M. et al. HDAC inhibition impedes epithelial-mesenchymal plasticity and suppresses metastatic, castration-resistant prostate cancer. Oncogene 35, 3781–3795 (2016).
Zhao, H. et al. Patient-derived tissue slice grafts accurately depict response of high-risk primary prostate cancer to androgen deprivation therapy. J. Transl Med. 11, 199 (2013).
Weinstein, M. H., Partin, A. W., Veltri, R. W. & Epstein, J. I. Neuroendocrine differentiation in prostate cancer: enhanced prediction of progression after radical prostatectomy. Hum. Pathol. 27, 683–687 (1996).
Sauer, C. G., Roemer, A. & Grobholz, R. Genetic analysis of neuroendocrine tumor cells in prostatic carcinoma. Prostate 66, 227–234 (2006).
Grasso, C. S. et al. Integrative molecular profiling of routine clinical prostate cancer specimens. Ann. Oncol. 26, 1110–1118 (2015).
Leong, K. G., Wang, B. E., Johnson, L. & Gao, W. Q. Generation of a prostate from a single adult stem cell. Nature 456, 804–808 (2008).
Simon, R. A. et al. CD44 expression is a feature of prostatic small cell carcinoma and distinguishes it from its mimickers. Hum. Pathol. 40, 252–258 (2009).
Lee, J. K. et al. N-Myc drives neuroendocrine prostate cancer initiated from human prostate epithelial cells. Cancer Cell 29, 536–547 (2016).
Goldstein, A. S., Huang, J., Guo, C., Garraway, I. P. & Witte, O. N. Identification of a cell of origin for human prostate cancer. Science 329, 568–571 (2010).
Stoyanova, T. et al. Prostate cancer originating in basal cells progresses to adenocarcinoma propagated by luminal-like cells. Proc. Natl Acad. Sci. USA 110, 20111–20116 (2013).
Beltran, H. et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 1, 487–495 (2011).
Tan, H. L. et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin. Cancer Res. 20, 890–903 (2014).
Kadakia, K. C. et al. Comprehensive serial molecular profiling of an “N of 1” exceptional non-responder with metastatic prostate cancer progressing to small cell carcinoma on treatment. J. Hematol. Oncol. 8, 109 (2015).
Epstein, J. I. et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am. J. Surg. Pathol. 38, 756–767 (2014).
Cerasuolo, M. et al. Neuroendocrine transdifferentiation in human prostate cancer cells: an integrated approach. Cancer Res. 75, 2975–2986 (2015).
Burchardt, T. et al. Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. J. Urol. 162, 1800–1805 (1999).
Zhang, X. Q. et al. Receptor protein tyrosine phosphatase alpha signaling is involved in androgen depletion-induced neuroendocrine differentiation of androgen-sensitive LNCaP human prostate cancer cells. Oncogene 22, 6704–6716 (2003).
Lin, D. et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 74, 1272–1283 (2014).
Ballas, N., Grunseich, C., Lu, D. D., Speh, J. C. & Mandel, G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 121, 645–657 (2005).
Zhang, X. et al. SRRM4 expression and the loss of REST activity may promote the emergence of the neuroendocrine phenotype in castration-resistant prostate cancer. Clin. Cancer Res. 21, 4698–4708 (2015).
Lapuk, A. V. et al. From sequence to molecular pathology, and a mechanism driving the neuroendocrine phenotype in prostate cancer. J. Pathol. 227, 286–297 (2012).
Li, Y. et al. SRRM4 drives neuroendocrine transdifferentiation of prostate adenocarcinoma under androgen receptor pathway inhibition. Eur. Urol. http://dx.doi.org/10.1016/j.eururo.2016.04.028 (2016).
Aggarwal, R. R. et al. Persistence of androgen receptor (AR) expression in patients (pts) with small cell prostate cancer (SCPC): Preliminary results from the SU2C/PCF/AACR West Coast Prostate Cancer Dream Team (WCDT). J. Clin. Oncol. 34 (Suppl. 2S), 288 (2016).
Puls, L. N., Eadens, M. & Messersmith, W. Current status of SRC inhibitors in solid tumor malignancies. Oncologist 16, 566–578 (2011).
Chu, I. et al. Src promotes estrogen-dependent estrogen receptor α proteolysis in human breast cancer. J. Clin. Invest. 117, 2205–2215 (2007).
Yu, E. Y. et al. Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology 77, 1166–1171 (2011).
Araujo, J. C. et al. Dasatinib combined with docetaxel for castration-resistant prostate cancer: results from a phase 1–2 study. Cancer 118, 63–71 (2012).
Spreafico, A. et al. A randomized phase II study of cediranib alone versus cediranib in combination with dasatinib in docetaxel resistant, castration resistant prostate cancer patients. Invest. New Drugs 32, 1005–1016 (2014).
Araujo, J. C. et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol. 14, 1307–1316 (2013).
Wang, X. D. et al. Identification of candidate predictive and surrogate molecular markers for dasatinib in prostate cancer: rationale for patient selection and efficacy monitoring. Genome Biol. 8, R255 (2007).
Siu, M. K. et al. Androgen receptor regulates SRC expression through microRNA-203. Oncotarget 7, 25726–25741 (2016).
Kumar, A. et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 22, 369–378 (2016).
Mulholland, D. J. et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell 19, 792–804 (2011).
Carver, B. S. et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19, 575–586 (2011).
Jia, S. et al. Opposing effects of androgen deprivation and targeted therapy on prostate cancer prevention. Cancer Discov. 3, 44–51 (2013).
Toren, P. et al. Combination AZD5363 with enzalutamide significantly delays enzalutamide-resistant prostate cancer in preclinical models. Eur. Urol. 67, 986–990 (2015).
Arora, V. K. et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155, 1309–1322 (2013).
Ndibe, C., Wang, C. G. & Sonpavde, G. Corticosteroids in the management of prostate cancer: a critical review. Curr. Treat. Options Oncol. 16, 6 (2015).
Isikbay, M. et al. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm. Cancer 5, 72–89 (2014).
Yemelyanov, A. et al. Differential targeting of androgen and glucocorticoid receptors induces ER stress and apoptosis in prostate cancer cells: a novel therapeutic modality. Cell Cycle 11, 395–406 (2012).
Pham, L. K. et al. Contextual effect of repression of bone morphogenetic protein activity in prostate cancer. Endocr. Relat. Cancer 20, 861–874 (2013).
Scher, H. I. et al. Impact of on-study corticosteroid use on efficacy and safety in the phase III AFFIRM study of enzalutamide (ENZA), an androgen receptor inhibitor [abstract]. J. Clin. Oncol. 31 (Suppl. 6), 6 (2013).
Morgan, C. J., Oh, W. K., Naik, G., Galsky, M. D. & Sonpavde, G. Impact of prednisone on toxicities and survival in metastatic castration-resistant prostate cancer: a systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Oncol. Hematol. 90, 253–261 (2014).
Montgomery, B. et al. Impact of baseline corticosteroids on survival and steroid androgens in metastatic castration-resistant prostate cancer: exploratory analysis from COU-AA-301. Eur. Urol. 67, 866–873 (2015).
Efstathiou, E. et al. Biological heterogeneity in localized high-risk prostate cancer (LHRPC) from a study of neoadjuvant abiraterone acetate plus leuprolide acetate (LHRHa) versus LHRHa [abstract]. J. Clin. Oncol. 33 (Suppl.), 5005 (2015).
Sartor, O., Parker, C. C. & de Bono, J. Reappraisal of glucocorticoids in castrate-resistant prostate cancer. Asian J. Androl. 16, 666 (2014).
Klokk, T. I. et al. Ligand-specific dynamics of the androgen receptor at its response element in living cells. Mol. Cell. Biol. 27, 1823–1843 (2007).
US National Library of Medicine. ClinicalTrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT02012296?term=NCT02012296&rank=1 (2016).
Omoto, Y. & Iwase, H. Clinical significance of estrogen receptor β in breast and prostate cancer from biological aspects. Cancer Sci. 106, 337–343 (2015).
Fixemer, T., Remberger, K. & Bonkhoff, H. Differential expression of the estrogen receptor beta (ERβ) in human prostate tissue, premalignant changes, and in primary, metastatic, and recurrent prostatic adenocarcinoma. Prostate 54, 79–87 (2003).
Mak, P. et al. Prostate tumorigenesis induced by PTEN deletion involves estrogen receptor beta repression. Cell Rep. 10, 1982–1991 (2015).
Lukacs, R. U., Memarzadeh, S., Wu, H. & Witte, O. N. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell 7, 682–693 (2010).
Goel, H. L. et al. VEGF/neuropilin-2 regulation of Bmi-1 and consequent repression of IGF-IR define a novel mechanism of aggressive prostate cancer. Cancer Discov. 2, 906–921 (2012).
Mak, P. et al. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell 17, 319–332 (2010).
Yuan, B. et al. A phosphotyrosine switch determines the antitumor activity of ERβ. J. Clin. Invest. 124, 3378–3390 (2014).
Jia, M., Dahlman-Wright, K. & Gustafsson, J. A. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 29, 557–568 (2015).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015).
Manson-Bahr, D. et al. Mutation detection in formalin-fixed prostate cancer biopsies taken at the time of diagnosis using next-generation DNA sequencing. J. Clin. Pathol. 68, 212–217 (2015).
Sole, X. et al. Biological convergence of cancer signatures. PLoS ONE 4, e4544 (2009).
Hoadley, K. A. et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 158, 929–944 (2014).
Honeth, G. et al. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 10, R53 (2008).
Foulkes, W. D., Smith, I. E. & Reis-Filho, J. S. Triple-negative breast cancer. N. Engl. J. Med. 363, 1938–1948 (2010).
Garay, J. P. & Park, B. H. Androgen receptor as a targeted therapy for breast cancer. Am. J. Cancer Res. 2, 434–445 (2012).
Niemeier, L. A., Dabbs, D. J., Beriwal, S., Striebel, J. M. & Bhargava, R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod. Pathol. 23, 205–212 (2010).
Gucalp, A. et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin. Cancer Res. 19, 5505–5512 (2013).
Proverbs-Singh, T., Feldman, J. L., Morris, M. J., Autio, K. A. & Traina, T. A. Targeting the androgen receptor in prostate and breast cancer: several new agents in development. Endocr. Relat. Cancer 22, R87–R106 (2015).
Collins, L. C. et al. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses' Health Study. Mod. Pathol. 24, 924–931 (2011).
Mrklic, I., Pogorelic, Z., Capkun, V. & Tomic, S. Expression of androgen receptors in triple negative breast carcinomas. Acta Histochem. 115, 344–348 (2013).
Thike, A. A. et al. Loss of androgen receptor expression predicts early recurrence in triple-negative and basal-like breast cancer. Mod. Pathol. 27, 352–360 (2014).
Safarpour, D., Pakneshan, S. & Tavassoli, F. A. Androgen receptor (AR) expression in 400 breast carcinomas: is routine AR assessment justified? Am. J. Cancer Res. 4, 353–368 (2014).
Barton, V. N. et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol. Cancer Ther. 14, 769–778 (2015).
Lehmann, B. D. et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 121, 2750–2767 (2011).
Peters, K. M. et al. Androgen receptor expression predicts breast cancer survival: the role of genetic and epigenetic events. BMC Cancer 12, 132 (2012).
Hickey, T. E. et al. Expression of androgen receptor splice variants in clinical breast cancers. Oncotarget 6, 44728–44744 (2015).
Barton, V. N. et al. Androgen receptor biology in triple negative breast cancer: a case for classification as AR+ or quadruple negative disease. Horm. Cancer 6, 206–213 (2015).
Louie, T. J. et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 364, 422–431 (2011).
Nichol, D. et al. Steering evolution with sequential therapy to prevent the emergence of bacterial antibiotic resistance. PLoS Comput. Biol. 11, e1004493 (2015).
Gatenby, R. A., Silva, A. S., Gillies, R. J. & Frieden, B. R. Adaptive therapy. Cancer Res. 69, 4894–4903 (2009).
Goldie, J. H. & Coldman, A. J. Quantitative model for multiple levels of drug resistance in clinical tumors. Cancer Treat. Rep. 67, 923–931 (1983).
Day, R. S. Treatment sequencing, asymmetry, and uncertainty: protocol strategies for combination chemotherapy. Cancer Res. 46, 3876–3885 (1986).
Goulart, C. P. et al. Designing antibiotic cycling strategies by determining and understanding local adaptive landscapes. PLoS ONE 8, e56040 (2013).
Kublin, J. G. et al. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J. Infect. Dis. 187, 1870–1875 (2003).
Martinez, J. A. et al. Comparison of antimicrobial cycling and mixing strategies in two medical intensive care units. Crit. Care Med. 34, 329–336 (2006).
Bennett, K. M. et al. Implementation of antibiotic rotation protocol improves antibiotic susceptibility profile in a surgical intensive care unit. J. Trauma 63, 307–311 (2007).
Cheng, L. et al. Evidence of independent origin of multiple tumors from patients with prostate cancer. J. Natl Cancer Inst. 90, 233–237 (1998).
Macintosh, C. A., Stower, M., Reid, N. & Maitland, N. J. Precise microdissection of human prostate cancers reveals genotypic heterogeneity. Cancer Res. 58, 23–28 (1998).
Liu, W. et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat. Med. 15, 559–565 (2009).
Madan, R. A., Pal, S. K., Sartor, O. & Dahut, W. L. Overcoming chemotherapy resistance in prostate cancer. Clin. Cancer Res. 17, 3892–3902 (2011).
Kantarjian, H. M. et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J. Clin. Oncol. 18, 547–561 (2000).
Thomas, D. A. et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood 104, 1624–1630 (2004).
Horning, S. J. et al. Assessment of the Stanford V regimen and consolidative radiotherapy for bulky and advanced Hodgkin's disease: Eastern Cooperative Oncology Group pilot study E1492. J. Clin. Oncol. 18, 972–980 (2000).
Goldie, J. H. & Coldman, A. J. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat. Rep. 63, 1727–1733 (1979).
Havemann, K. et al. Alternating versus sequential chemotherapy in small cell lung cancer. A randomized German multicenter trial. Cancer 59, 1072–1082 (1987).
Ettinger, D. S. et al. A randomized comparison of standard chemotherapy versus alternating chemotherapy and maintenance versus no maintenance therapy for extensive-stage small-cell lung cancer: a phase III study of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 8, 230–240 (1990).
Evans, W. K. et al. Superiority of alternating non-cross-resistant chemotherapy in extensive small cell lung cancer. A multicenter, randomized clinical trial by the National Cancer Institute of Canada. Ann. Intern. Med. 107, 451–458 (1987).
Wampler, G. L., Heim, W. J., Ellison, N. M., Ahlgren, J. D. & Fryer, J. G. Comparison of cyclophosphamide, doxorubicin, and vincristine with an alternating regimen of methotrexate, etoposide, and cisplatin/cyclophosphamide, doxorubicin, and vincristine in the treatment of extensive-disease small-cell lung carcinoma: a Mid-Atlantic Oncology Program study. J. Clin. Oncol. 9, 1438–1445 (1991).
Feld, R. et al. Canadian multicenter randomized trial comparing sequential and alternating administration of two non-cross-resistant chemotherapy combinations in patients with limited small-cell carcinoma of the lung. J. Clin. Oncol. 5, 1401–1409 (1987).
de Bono, J. S. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–1154 (2010).
Parker, C. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 369, 213–223 (2013).
Tran, C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009).
Loriot, Y. et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann. Oncol. 24, 1807–1812 (2013).
Noonan, K. L. et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann. Oncol. 24, 1802–1807 (2013).
Schrader, A. J. et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur. Urol. 65, 30–36 (2014).
Corn, P. G. et al. A multi-institutional randomized phase II study (NCT01505868) of cabazitaxel (CAB) plus or minus carboplatin (CARB) in men with metastatic castration-resistant prostate cancer (mCRPC) [abstract]. J. Clin. Oncol. 33 (Suppl.), 5010 (2015).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Schellhammer, P. F. et al. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology 81, 1297–1302 (2013).
Ross, R. W. et al. A whole-blood RNA transcript-based prognostic model in men with castration-resistant prostate cancer: a prospective study. Lancet Oncol. 13, 1105–1113 (2012).
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 (2014).
Gagneux, S. et al. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science 312, 1944–1946 (2006).
Imamovic, L. & Sommer, M. O. Use of collateral sensitivity networks to design drug cycling protocols that avoid resistance development. Sci. Transl Med. 5, 204ra132 (2013).
Munck, C., Gumpert, H. K., Wallin, A. I., Wang, H. H. & Sommer, M. O. Prediction of resistance development against drug combinations by collateral responses to component drugs. Sci. Transl Med. 6, 262ra156 (2014).
Lehar, J. et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 27, 659–666 (2009).
Gillet, J. P., Varma, S. & Gottesman, M. M. The clinical relevance of cancer cell lines. J. Natl Cancer Inst. 105, 452–458 (2013).
O'Neill, A. J. et al. Characterisation and manipulation of docetaxel resistant prostate cancer cell lines. Mol. Cancer 10, 126 (2011).
Zhang, B. et al. Telomere and microtubule targeting in treatment-sensitive and treatment-resistant human prostate cancer cells. Mol. Pharmacol. 82, 310–321 (2012).
van Soest, R. J. et al. Targeting the androgen receptor confers in vivo cross-resistance between enzalutamide and docetaxel, but not cabazitaxel, in castration-resistant prostate cancer. Eur. Urol. 67, 981–985 (2015).
Al Nakouzi, N. et al. Cabazitaxel remains active in patients progressing after docetaxel followed by novel androgen receptor pathway targeted therapies. Eur. Urol. 68, 228–235 (2015).
van Soest, R. J. et al. The influence of prior novel androgen receptor targeted therapy on the efficacy of cabazitaxel in men with metastatic castration-resistant prostate cancer. Eur. J. Cancer 51, 2562–2569 (2015).
Pezaro, C. J. et al. Activity of cabazitaxel in castration-resistant prostate cancer progressing after docetaxel and next-generation endocrine agents. Eur. Urol. 66, 459–465 (2014).
Mita, A. C. et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin. Cancer Res. 15, 723–730 (2009).
Zhu, Y. et al. Antiandrogens inhibit ABCB1 efflux and ATPase activity and reverse docetaxel resistance in advanced prostate cancer. Clin. Cancer Res. 21, 4133–4142 (2015).
Carracedo, A., Baselga, J. & Pandolfi, P. P. Deconstructing feedback-signaling networks to improve anticancer therapy with mTORC1 inhibitors. Cell Cycle 7, 3805–3809 (2008).
Carracedo, A. & Pandolfi, P. P. The PTEN–PI3K pathway: of feedbacks and cross-talks. Oncogene 27, 5527–5541 (2008).
Schwartz, S. et al. Feedback suppression of PI3Kα signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kβ. Cancer Cell 27, 109–122 (2015).
Acknowledgements
The authors want to thank M. Galsky, J. Sfakianos, A. Wolfe and M. Ruscetti for constructive advice. This work was supported in part by a grant from the Fondation de France to G.R. and an NIH grant (R01CA197910) to D.J.M.
Author information
Authors and Affiliations
Contributions
G.R. and D.J.M researched data and wrote the manuscript. All authors discussed the article's contents, revised and edited the manuscript before submission. B.C.L. and W.K.O. designed the PRINT clinical protocol.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Roubaud, G., Liaw, B., Oh, W. et al. Strategies to avoid treatment-induced lineage crisis in advanced prostate cancer. Nat Rev Clin Oncol 14, 269–283 (2017). https://doi.org/10.1038/nrclinonc.2016.181
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2016.181
This article is cited by
-
Analysis of genetic biomarkers, polymorphisms in ADME-related genes and their impact on pharmacotherapy for prostate cancer
Cancer Cell International (2023)
-
Neurokinin-1 receptor drives PKCɑ-AURKA/N-Myc signaling to facilitate the neuroendocrine progression of prostate cancer
Cell Death & Disease (2023)
-
Targeting signaling pathways in prostate cancer: mechanisms and clinical trials
Signal Transduction and Targeted Therapy (2022)
-
RETRACTED ARTICLE: Targeting KDM1B-dependent miR-215-AR-AGR2-axis promotes sensitivity to enzalutamide-resistant prostate cancer
Cancer Gene Therapy (2022)
-
Epithelial plasticity can generate multi-lineage phenotypes in human and murine bladder cancers
Nature Communications (2020)